Long-Term Survival of Older Patients Hospitalized for COVID-19. Do Clinical Characteristics upon Admission Matter?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. In-Hospital Analysis
3.3. Post-Discharge Period Analysis
3.4. Factors Affecting Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Vura, N.V.R.K.; Gravenstein, S. COVID-19 in older adults. Aging Clin. Exp. Res. 2020, 32, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Gontijo Guerra, S.; Berbiche, D.; Vasiliadis, H.M. Measuring multimorbidity in older adults: Comparing different data sources. BMC Geriatr. 2019, 19, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, V.; Yuan, J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: A systematic review and meta-analysis. Int. J. Public Health 2020, 65, 533–546. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Iaccarino, G.; Grassi, G.; Borghi, C.; Ferri, C.; Salvetti, M.; Volpe Massimo, M. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension 2020, 76, 366–372. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Marzio, M.A.L.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bosworth, M. Updated Estimates of Coronavirus (COVID-19) Related Deaths by Disability Status, England: January 24th to November 20th 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronaviruscovid19relateddeathsbydisabilitystatusenglandandwales/24januaryto20november2020 (accessed on 10 October 2021).
- Mendes, A.; Serratrice, C.; Herrmann, F.R.; Genton, L.; Périvier, S.; Scheffler, M.; Fassier, T.; Huber, P.; Jacques, M.-C.; Prendki, V.; et al. Predictors of In-Hospital Mortality in Older Patients With COVID-19: The COVIDAge Study. J. Am. Med. Dir. Assoc. 2020, 21, 1546–1554.e3. [Google Scholar] [CrossRef]
- Neumann-Podczaska, A.; Chojnicki, M.; Karbowski, L.M.; Al-Saad, S.R.; Hashmi, A.A.; Chudek, J.; Tobis, S.; Kropinska, S.; Mozer-Lisewska, I.; Suwalska, A.; et al. Clinical Characteristics and Survival Analysis in a Small Sample of Older COVID-19 Patients with Defined 60-Day Outcome. Int. J. Environ. Res. Public Health 2020, 17, 8362. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1 (accessed on 10 October 2021).
- Sachdev, P.S.; Mohan, A.; Taylor, L.; Jeste, D.V. DSM-5 and Mental Disorders in Older Individuals: An Overview. Harv. Rev. Psychiatry 2015, 23, 320–328. [Google Scholar] [CrossRef]
- Millán-Calenti, J.C.; Tubío, J.; Pita-Fernández, S.; Gonzalez-Abraldes, I.; Lorenzo, T.; Fernández-Arruty, T.; Maseda, A. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch. Gerontol. Geriatr. 2010, 50, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Putri, C.; Arisa, J.; Situmeang, R.F.V.; Kurniawan, A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2021, 93, 104299. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; De Matteis, G.; Santoro, M.; Sabia, L.; Simeoni, B.; Candelli, M.; Ojetti, V.; Franceschi, F. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥80 years. Geriatr. Gerontol. Int. 2020, 20, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, A.; Rozzini, R.; Guerini, F.; Boffelli, S.; Ranieri, P.; Minelli, G.; Bianchetti, L.; Trabucchi, M. Clinical Presentation of COVID19 in Dementia Patients. J. Nutr. Health Aging 2020, 24, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- España, P.P.; Bilbao, A.; García-Gutiérrez, S.; Lafuente, I.; Anton-Ladislao, A.; Villanueva, A.; Uranga, A.; Legarreta, M.J.; Aguirre, U.; Quintana, J.M.; et al. Predictors of mortality of COVID-19 in the general population and nursing homes. Intern. Emerg. Med. 2021, 16, 1487–1496. [Google Scholar] [CrossRef]
- Hessami, A.; Shamshirian, A.; Heydari, K.; Pourali, F.; Alizadeh-Navaei, R.; Moosazadeh, M.; Abrotan, S.; Shojaie, L.; Sedighi, S.; Shamshirian, D.; et al. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 46, 382–391. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Faghih Dinevari, M.; Somi, M.H.; Sadeghi Majd, E.; Abbasalizad Farhangi, M.; Nikniaz, Z. Anemia predicts poor outcomes of COVID-19 in hospitalized patients: A prospective study in Iran. BMC Infect. Dis. 2021, 21, 170. [Google Scholar] [CrossRef]
- Tao, Z.; Xu, J.; Chen, W.; Yang, Z.; Xu, X.; Liu, L.; Chen, R.; Xie, J.; Liu, M.; Wu, J.; et al. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2021, 93, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Aliberti, M.J.R.; Szlejf, C.; Avelino-Silva, V.I.; Suemoto, C.K.; Apolinario, D.; Dias, M.B.; Garcez, F.B.; Trindade, C.B.; Amaral, J.R.d.G.; de Melo, L.R.; et al. COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J. Am. Geriatr. Soc. 2021, 69, 1116–1127. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- Hägg, S.; Jylhävä, J.; Wang, Y.; Xu, H.; Metzner, C.; Annetorp, M.; Garcia-Ptacek, S.; Khedri, M.; Boström, A.-M.; Kadir, A.; et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with Coronavirus disease 2019 in geriatric care. J. Am. Med. Dir. Assoc. 2020, 21, 1555–1559.e2. [Google Scholar] [CrossRef]
- Moug, S.; Carter, B.; Myint, P.K.; Hewitt, J.; McCarthy, K.; Pearce, L. Decision-Making in COVID-19 and Frailty. Geriatrics 2020, 5, 30. [Google Scholar] [CrossRef]
- De Smet, R.; Mellaerts, B.; Vandewinckele, H.; Lybeert, P.; Frans, E.; Ombelet, S.; Lemahieu, W.; Symons, R.; Ho, E.; Frans, J.; et al. Frailty and mortality in hospitalized older adults with COVID-19: Retrospective observational study. J. Am. Med. Dir. Assoc. 2020, 21, 928–932.e1. [Google Scholar] [CrossRef]
- Guillon, A.; Laurent, E.; Godillon, L.; Kimmoun, A.; Grammatico-Guillon, L. Long-term mortality of elderly patients after intensive care unit admission for COVID-19. Intensive Care Med. 2021, 47, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Li, Y.; Liu, F.; Zhou, Q.; Peng, Z. Association between thrombocytopenia and 180-day prognosis of COVID-19 patients in intensive care units: A two-center observational study. PLoS ONE 2021, 16, e0248671. [Google Scholar] [CrossRef]
- Laosa, O.; Pedraza, L.; Álvarez-Bustos, A.; Carnicero, J.A.; Rodriguez-Artalejo, F.; Rodriguez-Mañas, L. Rapid Assessment at Hospital Admission of Mortality Risk From COVID-19: The Role of Functional Status. J. Am. Med. Dir. Assoc. 2020, 21, 1798–1802.e2. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; De Matteis, G.; Della Polla, D.A.; Santoro, M.; Burzo, M.L.; Torelli, E.; Simeoni, B.; Russo, A.; Sandroni, C.; Gasbarrini, A.; et al. Predictors of in-hospital mortality AND death RISK STRATIFICATION among COVID-19 PATIENTS aged ≥ 80 YEARs OLD. Arch. Gerontol. Geriatr. 2021, 95, 104383. [Google Scholar] [CrossRef] [PubMed]
- Heras, E.; Garibaldi, P.; Boix, M.; Valero, O.; Castillo, J.; Curbelo, Y.; Gonzalez, E.; Mendoza, O.; Anglada, M.; Miralles, J.C.; et al. COVID-19 mortality risk factors in older people in a long-term care center. Eur. Geriatr. Med. 2020, 12, 601–607. [Google Scholar] [CrossRef]
- Lidoriki, I.; Frountzas, M.; Schizas, D. Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly? Med. Hypotheses 2020, 144, 109946. [Google Scholar] [CrossRef] [PubMed]
- Mlinac, M.E.; Feng, M.C. Assessment of Activities of Daily Living, Self-Care, and Independence. Arch. Clin. Neuropsychol. 2016, 31, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Wilber, S.T. Altered Mental Status in Older Patients in the Emergency Department. Clin. Geriatr. Med. 2013, 29, 101–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Total | In-Hospital 2 | Post-Discharge 3 | ||||
|---|---|---|---|---|---|---|---|
| Non-Survivors | Survivors | p-Value | Non-Survivors | Survivors | p-Value | ||
| Age | 77.5 ± 10.0 (77; 60–101) | 79.7 ± 8.9 (79; 63–99) | 76.6 ± 10.3 (75; 60–101) | p < 0.01 | 80.2 ± 11.1 (79.5; 61–101) | 76.0 ± 10.1 (74; 60–99) | 0.06 |
| Gender | |||||||
| Male | 131 (40.7%) | 46 (48.4%) | 85 (37.4%) | 0.07 | 9 (30.0%) | 76 (38.6%) | 0.37 |
| Female | 191 (59.3%) | 49 (51.6%) | 142 (62.6%) | 21 (70.0%) | 121 (61.4%) | ||
| Symptoms | |||||||
| ● Subfebrile Temperature | 94 (32.8%) | 26 (33.8%) | 68 (33.4%) | 0.82 | 7 (25.0%) | 60 (33.5%) | 0.39 |
| ● Fever | 33 (11.5%) | 10 (13.0%) | 23 (11.1%) | 0.63 | 0 (0.0%) | 23 (12.6%) | 0.95 |
| ● Cough | 66 (26.4%) | 12 (18.5%) | 54 (29.2%) | 0.09 | 3 (13.6%) | 51 (31.3%) | 0.14 |
| ● Dyspnea | 54 (20.5%) | 18 (25.0%) | 36 (18.8%) | 0.26 | 3 (12.5%) | 33 (19.6%) | 0.58 |
| ● Dysosmia | 2 (1.3%) | 1 (2.9%) | 1 (0.8%) | 0.34 | 0 (0.0%) | 1 (1.0%) | 0.25 |
| ● Dysgeusia | 5 (3.3%) | 1 (2.9%) | 4 (3.3%) | 0.91 | 0 (0.0%) | 4 (3.8%) | 1.00 |
| ● Myalgia | 15 (6.3%) | 5 (7.8%) | 10 (5.8%) | 0.79 | 0 (0.0%) | 10 (6.6%) | 0.45 |
| Cognitive function | |||||||
| ● Cognitively intact | 172 (53.9%) | 33 (34.7%) | 139 (62.1%) | p < 0.0001 | 12 (41.4%) | 127 (65.1%) | <0.05 |
| ● Mild impairment | 117 (36.7%) | 42 (44.2%) | 75 (33.5%) | 13 (44.8%) | 62 (31.8%) | ||
| ● Severe impairment | 30 (9.4%) | 20 (21.1%) | 10 (4.5%) | 4 (13.8%) | 6 (3.1%) | ||
| Functional capacity 1 | |||||||
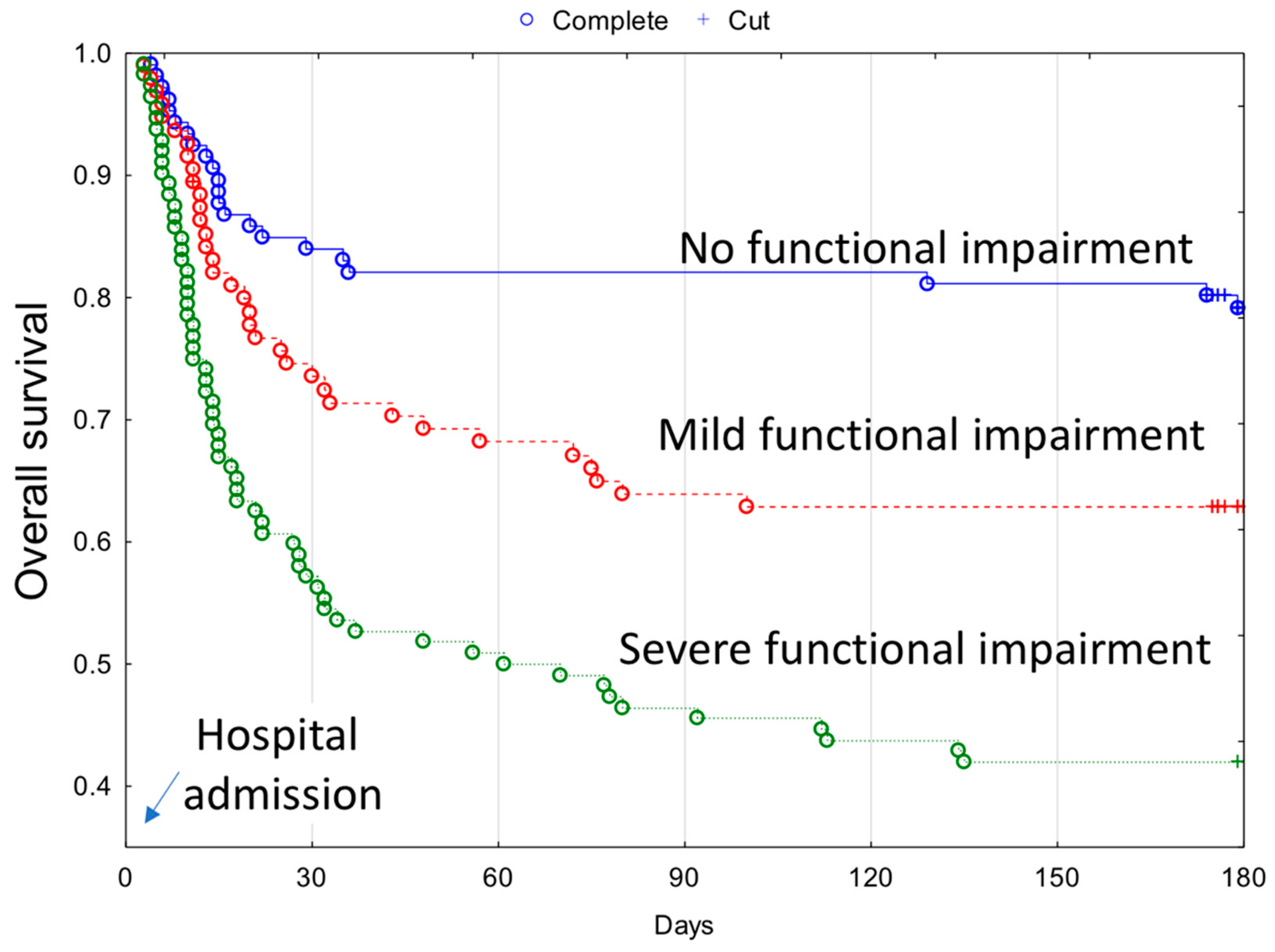
| ● No functional impairment | 110 (34.5%) | 18 (19.0%) | 92 (41.1%) | p < 0.0001 | 4 (13.8%) | 88 (45.1%) | <0.001 |
| ● Mild functional impairment | 97 (30.4%) | 29 (30.5%) | 68 (30.3%) | 8 (27.6%) | 60 (30.8%) | ||
| ● Severe functional impairment | 112 (35.1%) | 48 (50.5%) | 64 (28.6%) | 17 (58.6%) | 47 (24.1%) | ||
| Comorbidities | |||||||
| Cardiovascular diseases | 265 (82.3%) | 84 (88.4%) | 181 (79.7%) | 0.63 | 23 (76.7%) | 158 (80.2%) | 0.65 |
| ● Hypertension | 199 (62.1%) | 57 (60.0%) | 142 (63.4%) | 0.57 | 15 (50.0%) | 127 (65.5%) | 0.10 |
| ● Heart diseases | 189 (59.3%) | 67 (70.5%) | 122 (54.5%) | <0.01 | 20 (66.7%) | 102 (52.6%) | 0.15 |
| Diabetes | 99 (31.0%) | 40 (42.1%) | 59 (26.3%) | <0.01 | 8 (26.7%) | 51 (26.3%) | 0.97 |
| Respiratory diseases | 30 (9.4%) | 13 (13.7%) | 17 (7.6%) | 0.13 | 5 (16.7%) | 12 (6.2%) | 0.10 |
| Renal dysfunction | 49 (15.4%) | 22 (23.2%) | 27 (12.1%) | <0.05 | 5 (16.7%) | 22 (11.3%) | 0.59 |
| Liver dysfunction | 10 (3.1%) | 4 (4.2%) | 6 (2.7%) | 0.71 | 2 (6.7%) | 4 (2.1%) | 0.40 |
| Cancer | 30 (9.4%) | 11 (11.6%) | 19 (8.5%) | 0.39 | 5 (16.7%) | 14 (7.2%) | 0.17 |
| Stroke | 63 (19.8%) | 27 (28.4%) | 36 (16.1%) | <0.05 | 7 (23.3%) | 29 (15.0%) | 0.32 |
| Parameter | Total | In-Hospital 1 | Post-Discharge 2 | ||||
|---|---|---|---|---|---|---|---|
| Non-Survivors | Survivors | p-Value | Non-Survivors | Survivors | p-Value | ||
| White Blood Cells (×103/µL) 4.0–11.0 | 7.3 ± 3.5 (6.8; 1.7–29.0) | 8.4 ± 4.6 (7.5; 1.7–29.0) | 6.8 ± 2.9 (6.5; 1.7–17.1) | p < 0.01 | 7.8 ± 3.3 (7.6; 3.2–17.1) | 6.7 ± 2.8 (6.2; 1.7–14.8) | 0.07 |
| Hemoglobin (g/dL) Female 12–16 | 11.9 ± 1.7 (12.1; 6.7–15.3) | 11.4 ± 2.0 (11.3; 7.3–15.2) | 12.0 ± 1.6 (12.3; 6.7–15.3) | p < 0.05 | 11.6 ± 2.0 (12.7; 8.1–14.0) | 12.1 ± 1.5 (12.2; 6.7–15.3) | 0.54 |
| Hemoglobin (g/dL) Male 14–18 | 12.5 ± 2.0 (12.7; 6.4–17.3) | 11.5 ± 2.1 (11.7; 6.5–15.4) | 13.0 ± 1.8 (13.1; 6.4–17.3) | p < 0.001 | 11.6 ± 2.6 (12.1; 6.4–15.6) | 13.1 ± 1.7 (13.2; 9.1–17.3) | p < 0.05 |
| Platelets (×103/µL) 130–440 | 243.3 ± 109.2 (221.5; 35.0–867.0) | 236.2 ± 124.6 (204.5; 35.0–867.0) | 246.4 ± 102.1 (227.5; 61.0–665.0) | 0.18 | 257.2 ± 91.9 (263.0; 85.0–465.0) | 244.7 ± 103.7 (226.0; 61.0–665.0) | 0.28 |
| Lymphocytes (×103/µL) 1.0–4.0 | 1.4 ± 0.8 (1.2; 0.0–8.1) | 1.3 ± 1.1 (1.1; 0.3–8.1) | 1.4 ± 0.7 (1.2; 0.0–3.8) | 0.07 | 1.4 ± 0.7 (1.2; 0.5–2.6) | 1.4 ± 0.7 (1.2; 0–3.8) | 0.74 |
| Neutrophils (×103/µL) 1.5–7.7 | 4.9 ± 2.9 (4.3; 0.1–16.9) | 6.0 ± 3.7 (5.1; 0.1–16.9) | 4.6 ± 2.4 (4.1; 0.7–12.9) | p < 0.01 | 4.9 ± 2.3 (4.1; 2.4–11.1) | 4.5 ± 2.5 (4.0; 0.7–12.9) | 0.40 |
| Urea (mmol/L) Female 3.6–7.1 | 8.1 ± 6.9 (6.1; 2.0–45.2) | 10.6 ± 8.0 (9.0; 2.0–31.8) | 7.3 ± 6.4 (5.8; 2.5–45.2) | p < 0.05 | 10.1 ± 11.4 (6.8; 3.1–45.2) | 6.9 ± 5.1 (5.7; 2.5–37.9) | 0.17 |
| Urea (mmol/L) Male 3.2–8.9 | 9.6 ± 9.5 (6.9; 1.4–69.1) | 13.4 ± 13.8 (9.7; 3.8–69.1) | 7.3 ± 3.7 (6.3; 1.4–20.4) | p < 0.01 | 10.9 ± 5.6 (9.7; 5.1–20.4) | 6.8 ± 3.1 (6.1; 1.4–15.7) | p < 0.05 |
| Lactate Dehydrogenase (U/L) 125–220 | 326.5 ± 159.8 (289.5; 5.3–1332.0) | 388.8 ± 215.3 (346.0; 5.3–1332.0) | 300.9 ± 122.0 (279.0; 133.0–856.0) | p < 0.001 | 288.6 ± 126.8 (262.0; 138.0–762.0) | 302.8 ± 121.6 (283.0; 133.0–856.0) | 0.46 |
| CRP (mg/L) 0–5 | 70.9 ± 76.3 (44.9; 0.0–455.0) | 102.3 ± 92.8 (71.8; 2.4–455.0) | 57.4 ± 63.5 (34.3; 0.0–290.5) | p < 0.001 | 54.2 ± 54.5 (36.0; 0.0–176.1) | 57.9 ± 64.9 (34.0; 0.0–290.5) | 0.76 |
| PCT (ng/mL) 0–0.1 | 0.7 ± 4.4 (0.1; 0.0–49.1) | 1.7 ± 7.1 (0.1; 0.0–49.1) | 0.3 ± 2.2 (0.0; 0.0–30.3) | p < 0.001 | 0.4 ± 0.8 (0.0; 0.0–4.1) | 0.3 ± 2.4 (0.0; 0.0–30.3) | 0.11 |
| IL-6 (pg/mL) 1.5–7.0 | 71.5 ± 154.0 (28.4; 0.0–1592.0) | 147.3 ± 253.4 (60.6; 0.0–1592.0) | 39.9 ± 60.5 (18.3; 0.0–500.0) | p < 0.001 | 40.0 ± 36.3 (24.2; 0.0–127.1) | 39.9 ± 63.7 (17.7; 0.0–500.0) | 0.21 |
| Parameter | Cut-Off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| WBC (×103/µL) | >10.0 | 33.3 | 100.0 |
| Neutrophils (×103/µL) | >5.0 | 66.7 | 80.0 |
| CRP (mg/L) | >108.0 | 55.6 | 89.2 |
| Il-6 (pg/mL) | >29.0 | 72.7 | 82.1 |
| Factor | Feature | In-Hospital Mortality | Post-Discharge Mortality | Overall 180-Day Mortality | |||
|---|---|---|---|---|---|---|---|
| Univariable Model | Multivariable Model | Univariable Model | Multivariable Model | Univariable Model | Multivariable Model | ||
| Age | ≥75 | 1.64 (1.07–2.51); p < 0.05 | - | 1.51 (0.72–3.17); p = 0.27 | - | 1.65 (1.14–2.38); p < 0.01 | - |
| Sex | M | 1.02 (1.00–1.04); p < 0.05 | 1.02 (1.00–1.04); p < 0.05 | 0.62 (0.28–1.41); p = 0.26 | - | 1.07 (0.39–2.96); p = 0.89 | - |
| Functional impairment | Mild | 1.46 (0.80–2.66); p = 0.22 | - | 2.90 (0.87–9.65); p = 0.08 | 2.90 (0.87–9.65); p = 0.08 | 2.02 (1.19–3.45); p < 0.01 | 1.96 (1.10–3.48); p < 0.05 |
| Severe | 2.49 (1.44–4.29); p < 0.01 | - | 7.02 (2.36–20.90); p < 0.001 | 7.02 (2.36–20.90); p < 0.001 | 3.80 (3.34–6.17); p < 0.001 | 2.92 (1.68–5.06); p < 0.001 | |
| Cognitive impairment | Mild | 1.73 (1.08–2.76); p < 0.05 | - | 2.20 (1.00–4.84); p < 0.05 | - | 2.05 (1.38–3.06); p < 0.001 | - |
| Severe | 4.29 (2.45–7.49); p < 0.001 | 4.42 (2.59–7.53); p < 0.001 | 5.19 (1.67–16.11); p < 0.01 | - | 5.10 (3.09–8.43); p < 0.001 | 3.10 (1.84–5.25); p < 0.001 | |
| Diabetes | - | 1.55 (1.02–2.34); p < 0.05 | - | 1.10 (0.49–2.48); p = 0.82 | - | 1.57 (1.09–2.25); p < 0.05 | - |
| Heart disease | - | 1.86 (1.19–2.92); p < 0.01 | 2.17 (1.34–3.54); p < 0.01 | 1.68 (0.78–3.60); p = 0.19 | - | 1.78 (1.22–2.61); p < 0.01 | 1.99 (1.32–2.99); p < 0.01 |
| History of kidney disease | - | 1.37 (0.84–2.27); p = 0.21 | - | 1.57 (0.60–4.13); p = 0.36 | - | 1.66 (1.08–2.57); p < 0.05 | - |
| eGFR | <60 | 1.86 (1.22–2.82); p < 0.01 | - | 0.86 (0.38–1.94); p = 0.71 | - | 1.46 (1.01–2.09); p < 0.05 | - |
| Past stroke | - | 1.50 (0.96–3.33); p = 0.08 | - | 1.64 (0.70–3.86); p = 0.25 | - | 1.75 (1.19–2.58); p < 0.01 | - |
| Anemia | - | 2.48 (1.55–3.99); p < 0.001 | 2.21 (1.33–3.68); p < 0.01 | 1.20 (0.57–2.52); p = 0.63 | - | 2.17 (1.47–3.20); p < 0.001 | 1.97 (1.30–2.99); p < 0.01 |
| WBC | >10.0 | 2.51 (1.48–4.29); p < 0.001 | - | 1.66 (0.58–4.80); p = 0.35 | - | 1.96 (1.22–3.13); p < 0.01 | - |
| Neutrophiles | >5.0 | 1.99 (1.18–3.36); p = 0.01 | - | 1.09 (0.41–2.89); p = 0.87 | - | 1.62 (1.03–2.55); p < 0.05 | - |
| CRP | >100 | 1.86 (1.21–2.85); p < 0.01 | - | 1.11 (0.47–2.61); p = 0.81 | - | 1.52 (1.04–2.22); p < 0.05 | - |
| IL6 | ≥30 | 3.81 (2.34–6.22); p < 0.001 | 3.49 (2.13–5.74); p < 0.001 | 1.65 (0.79–3.47); p = 0.19 | - | 3.09 (2.08–4.58); p < 0.001 | 3.18 (2.12–4.77); p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnicki, M.; Neumann-Podczaska, A.; Seostianin, M.; Tomczak, Z.; Tariq, H.; Chudek, J.; Tobis, S.; Mozer-Lisewska, I.; Suwalska, A.; Tykarski, A.; et al. Long-Term Survival of Older Patients Hospitalized for COVID-19. Do Clinical Characteristics upon Admission Matter? Int. J. Environ. Res. Public Health 2021, 18, 10671. https://doi.org/10.3390/ijerph182010671
Chojnicki M, Neumann-Podczaska A, Seostianin M, Tomczak Z, Tariq H, Chudek J, Tobis S, Mozer-Lisewska I, Suwalska A, Tykarski A, et al. Long-Term Survival of Older Patients Hospitalized for COVID-19. Do Clinical Characteristics upon Admission Matter? International Journal of Environmental Research and Public Health. 2021; 18(20):10671. https://doi.org/10.3390/ijerph182010671
Chicago/Turabian StyleChojnicki, Michał, Agnieszka Neumann-Podczaska, Mikołaj Seostianin, Zofia Tomczak, Hamza Tariq, Jerzy Chudek, Sławomir Tobis, Iwona Mozer-Lisewska, Aleksandra Suwalska, Andrzej Tykarski, and et al. 2021. "Long-Term Survival of Older Patients Hospitalized for COVID-19. Do Clinical Characteristics upon Admission Matter?" International Journal of Environmental Research and Public Health 18, no. 20: 10671. https://doi.org/10.3390/ijerph182010671
APA StyleChojnicki, M., Neumann-Podczaska, A., Seostianin, M., Tomczak, Z., Tariq, H., Chudek, J., Tobis, S., Mozer-Lisewska, I., Suwalska, A., Tykarski, A., Merks, P., Kropińska, S., Sobieszczańska, M., Romanelli, F., & Wieczorowska-Tobis, K. (2021). Long-Term Survival of Older Patients Hospitalized for COVID-19. Do Clinical Characteristics upon Admission Matter? International Journal of Environmental Research and Public Health, 18(20), 10671. https://doi.org/10.3390/ijerph182010671








