Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready to Eat Foods from Large Retailers and Canteens over a 2-Year Period in Northern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Description of RTE Food Samples
2.2. RTE Sample Analysis
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matera, A.; Altieri, G.; Ricciardi, A.; Zotta, T.; Condelli, N.; Galgano, F.; Genovese, F.; Di Renzo, G.C. Microbiological stability and overall quality of ready-to-heat meals based on traditional recipes of the Basilicata region. Foods 2020, 9, 406. [Google Scholar] [CrossRef]
- James, D.; Bowness, E.; Robin, T.; McIntyre, A.; Dring, C.; Desmarais, A.; Wittman, H. Dismantling and rebuilding the food system after COVID-19: Ten principles for redistribution and regeneration. J. Agric. Food Syst. Community Dev. 2021, 10, 1–23. [Google Scholar] [CrossRef]
- STATISTA. Ready-to-Eat Meals (Italy). Available online: https://www.statista.com/outlook/cmo/food/convenience-food/ready-to-eat-meals/italy (accessed on 5 July 2021).
- STATISTA. Italy: Frequency of Ready Meals Consumption by Age 2020. Available online: https://www.statista.com/statistics/1182258/frequency-of-ready-meals-consumption-by-age-italy/ (accessed on 5 July 2021).
- Kotzekidou, P. Microbiological examination of ready-to-eat foods and ready-to-bake frozen pastries from university canteens. Food Microbiol. 2013, 34, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Redmond, E.C.; Griffith, C.J.; Slader, J.; Humphrey, T.J. Microbiological and observational analysis of cross contamination risks during domestic food preparation. Br. Food J. 2004, 106, 581–597. [Google Scholar] [CrossRef]
- Balzaretti, C.M.; Marzano, M.A. Prevention of travel-related foodborne diseases: Microbiological risk assessment of food handlers and ready-to-eat foods in northern Italy airport restaurants. Food Control 2013, 29, 202–207. [Google Scholar] [CrossRef]
- Christison, C.A.; Lindsay, D.; von Holy, A. Microbiological survey of ready-to-eat foods and associated preparation surfaces in retail delicatessens, Johannesburg, South Africa. Food Control 2008, 19, 727–733. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Ryu, J.H. Produce Handling and Processing Practices. Emerg. Infect. Dis. 1997, 3, 459–465. [Google Scholar] [CrossRef]
- Gibbons, I.S.; Adesiyun, A.; Seepersadsingh, N.; Rahaman, S. Investigation for possible source(s) of contamination of ready-to-eat meat products with Listeria spp. and other pathogens in a meat processing plant in Trinidad. Food Microbiol. 2006, 23, 359–366. [Google Scholar] [CrossRef]
- Gilbreth, S.E.; Call, J.E.; Wallace, F.M.; Scott, V.N.; Chen, Y.; Luchansky, J.B. Relatedness of Listeria monocytogenes isolates recovered from selected ready-to-eat foods and listeriosis patients in the United States. Appl. Environ. Microbiol. 2005, 71, 8115–8122. [Google Scholar] [CrossRef]
- Farber, J.M. Microbiological aspects of modified-atmosphere packaging technology—A review. J. Food Prot. 1991, 54, 58–70. [Google Scholar] [CrossRef]
- Modzelewska-Kapituła, M.; Maj-Sobotka, K. The microbial safety of ready-to-eat raw and cooked sausages in Poland: Listeria monocytogenes and Salmonella spp. occurrence. Food Control 2013, 36, 212–216. [Google Scholar] [CrossRef]
- D’Aoust, J.Y.; Maurer, J. Salmonella species. In Food Microbiology. Fundamentals and Frontiers, 3rd ed.; Doyle, M.P., Beuchat, L.R., Eds.; ASM: Washington, DC, USA, 2007; pp. 187–236. [Google Scholar]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.; Wu, S.T.; den Bakker, H.C.; Cook, P.W.; Veenhuizen, D.R.; Hammons, S.R.; Singh, M.; Oliver, H.F. Listeria monocytogenes is prevalent in retail produce environments but Salmonella enterica is rare. Food Control 2020, 113, 107173. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Domínguez-Bernal, G.; González-Zorn, B.; Kreft, J.; Goebel, W. Pathogenicity islands and virulence evolution in Listeria. Microbes Infect. 2001, 3, 571–584. [Google Scholar] [CrossRef]
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the united states. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Crim, S.M.; Griffin, P.M.; Tauxe, R.; Marder, E.P.; Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K.; et al. Preliminary incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2014. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 495–499. [Google Scholar]
- WHO—World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 17 August 2021).
- CeIRSA—Centro Iterdipartimentale di Ricerca e Documentazione sulla Sicurezza Alimentare. Available online: https://www.ceirsa.org/matrice_alim.php#inizio (accessed on 17 August 2021).
- Koskar, J.; Kramarenko, T.; Meremäe, K.; Kuningas, M.; Sõgel, J.; Mäesaar, M.; Anton, D.; Lillenberg, M.; Roasto, M. Prevalence and numbers of listeria monocytogenes in various ready-to-eat foods over a 5-year period in Estonia. J. Food Prot. 2019, 82, 597–604. [Google Scholar] [CrossRef]
- Ministero della Salute. Listeriosi Di Origine Alimentare: Valutazione Del Rischio Di Esposizione Per Il Consumatore. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=3049 (accessed on 5 July 2021).
- Gormley, F.J.; Little, C.L.; Grant, K.A.; de Pinna, E.; McLauchlin, J. The microbiological safety of ready-to-eat specialty meats from markets and specialty food shops: A UK wide study with a focus on Salmonella and Listeria monocytogenes. Food Microbiol. 2010, 27, 243–249. [Google Scholar] [CrossRef]
- Yang, X.; Huang, J.; Wu, Q.; Zhang, J.; Liu, S.; Guo, W.; Cai, S.; Yu, S. Prevalence, antimicrobial resistance and genetic diversity of Salmonella isolated from retail ready-to-eat foods in China. Food Control 2016, 60, 50–56. [Google Scholar] [CrossRef]
- Kramarenko, T.; Nurmoja, I.; Kärssin, A.; Meremäe, K.; Hörman, A.; Roasto, M. The prevalence and serovar diversity of Salmonella in various food products in Estonia. Food Control 2014, 42, 43–47. [Google Scholar] [CrossRef]
- Kurpas, M.; Wieczorek, K.; Osek, J. Ready-to-eat meat products as a source of Listeria monocytogenes. J. Vet. Res. 2018, 62, 49–55. [Google Scholar] [CrossRef]
- Effimia, E. Prevalence of Listeria monocytogenes and Salmonella spp. in Ready-to-Eat Foods in Kefalonia, Greece. J. Bacteriol. Parasitol. 2015, 6, 5. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, A.; Carrasco, E.; García, R.M.; Zurera, G. Understanding and modelling bacterial transfer to foods: A review. Trends Food Sci. Technol. 2008, 19, 131–144. [Google Scholar] [CrossRef]
- Cabedo, L.; Picart I Barrot, L.; Teixidó I Canelles, A. Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J. Food Prot. 2008, 71, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Gowda, C.; Hadley, C.; Aiello, A.E. The association between food insecurity and inflammation in the US adult population. Am. J. Public Health 2012, 102, 1579–1586. [Google Scholar] [CrossRef]
- Milicevic, V.; Colavita, G.; Castrica, M.; Ratti, S.; Baldi, A.; Balzaretti, C.M. Risk assessment in the recovery of food for social solidarity purposes: Preliminary data. Ital. J. Food Saf. 2016, 5, 6187. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Bittencourt, G.M.; Oliveira, C.A.F. Listeria monocytogenes in Brazilian foods: Occurrence, risks to human health and their prevention. Curr. Res. Nutr. Food Sci. 2019, 7, 320–330. [Google Scholar] [CrossRef]
- Dobrucka, R.; Cierpiszewski, R. Active and Intelligent Packaging Food—Research and Development—A Review Renata Dobrucka *, Ryszard Cierpiszewski Department of Industrial Products Quality and Ecology, Faculty of Commodity Science. Pol. J. Food Nutr. Sci. 2014, 64, 7–15. [Google Scholar] [CrossRef]
- Castrica, M.; Panseri, S.; Siletti, E.; Borgonovo, F.; Chiesa, L.; Balzaretti, C.M. Evaluation of smart portable device for food diagnostics: A preliminary study on Cape Hake fillets (M. Capensis and M. Paradoxus). J. Chem. 2019, 2019, 2904724. [Google Scholar] [CrossRef]
- Castrica, M.; Chiesa, L.M.; Nobile, M.; De Battisti, F.; Siletti, E.; Pessina, D.; Panseri, S.; Balzaretti, C.M. Rapid safety and quality control during fish shelf-life by using a portable device. J. Sci. Food Agric. 2021, 101, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Garofalo, C.; Clementi, F.; Tavoletti, S.; Aquilanti, L. Bioluminescence ATP monitoring for the routine assessment of food contact surface cleanliness in a university canteen. Int. J. Environ. Res. Public Health 2014, 11, 10824–10837. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, C.; Mammina, C.; Giammanco, S.; Giammanco, M.; Guardia, M.L.; Casuccio, A. Knowledge, attitudes and self-reported practices of food service staff in nursing homes and long-term care facilities. Food Control 2010, 21, 1367–1373. [Google Scholar] [CrossRef]
- Santana, N.G.; Almeida, R.C.C.; Ferreira, J.S.; Almeida, P.F. Microbiological quality and safety of meals served to children and adoption of good manufacturing practices in public school catering in Brazil. Food Control 2009, 20, 255–261. [Google Scholar] [CrossRef]
- Veiros, M.B.; Proença, R.P.C.; Santos, M.C.T.; Kent-Smith, L.; Rocha, A. Food safety practices in a Portuguese canteen. Food Control 2009, 20, 936–941. [Google Scholar] [CrossRef]
- EFSA. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, e06007. [Google Scholar]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the UK: London, UK, 2016.
| ID Groups | Description of Ready-to-Eat Groups | No. of Samples per Parameter | No. of Samples Per Sampling Site | ||||
|---|---|---|---|---|---|---|---|
| L. monocytogenes | Salmonella spp. | ||||||
| L. monocytogenes | Salmonella spp. | Large retailers | Canteens | Large retailers | Canteens | ||
| Group A | Multi-ingredient preparations composed of fully cooked food, ready for immediate consumption or with minimum further handling before consumption (e.g., pasta, pizza, burgers, vegetables, ready meals after regeneration, whole pies, sausage rolls, quiches, roast meats, and chicken portions). | 2442 | 5367 | 143 | 2299 | 167 | 5200 |
| Group B | Multi-ingredient preparations composed of cooked and uncooked food or preparation consisting only of raw ingredients (e.g., seafood sauces, roast beef with raw rocket, mixed salads, julienned carrots, sliced fennel, chopped lettuce, radicchio, and pre-prepared fruit salads). | 554 | 1364 | 228 | 326 | 361 | 1003 |
| Parameters | Culture Techniques | Incubation | Culture Media and Agar | Biochemical Confirmation Test | Serological Testing | Reference Method | |
|---|---|---|---|---|---|---|---|
| Time (h) | Temp. (°C) | ||||||
| L. monocytogenes detection | Enrichment | 24 | 30 | Listeria ½ Fraser 1 | Microgen™ Listeria-ID 3 | AFNOR BRD 07/04-09/98 | |
| Plate | 24–48 | 37 | RAPID’ L. mono 2 | ||||
| Salmonella spp. detection | Pre-enrichment | 18 | 37 | buffered peptone water 1 | API 20 E NE 4 | Poly A-S + Vi 5 Poly H 5 | ISO 6579:2017 |
| Selective enrichment | 24 | 41.5 | Rappaport-Vassiliadis broth (RVS) 1 | ||||
| 24 | 37 | Muller-Kauffmann Tetrathionate-Novobiocin broth (MKTTn) 1 | |||||
| Plate | 24 | 37 | Xylose lysine Desoxycholate Agar (XLD) 1 | ||||
| 24 | 37 | Brilliant Green Agar (BGA) 1 | |||||
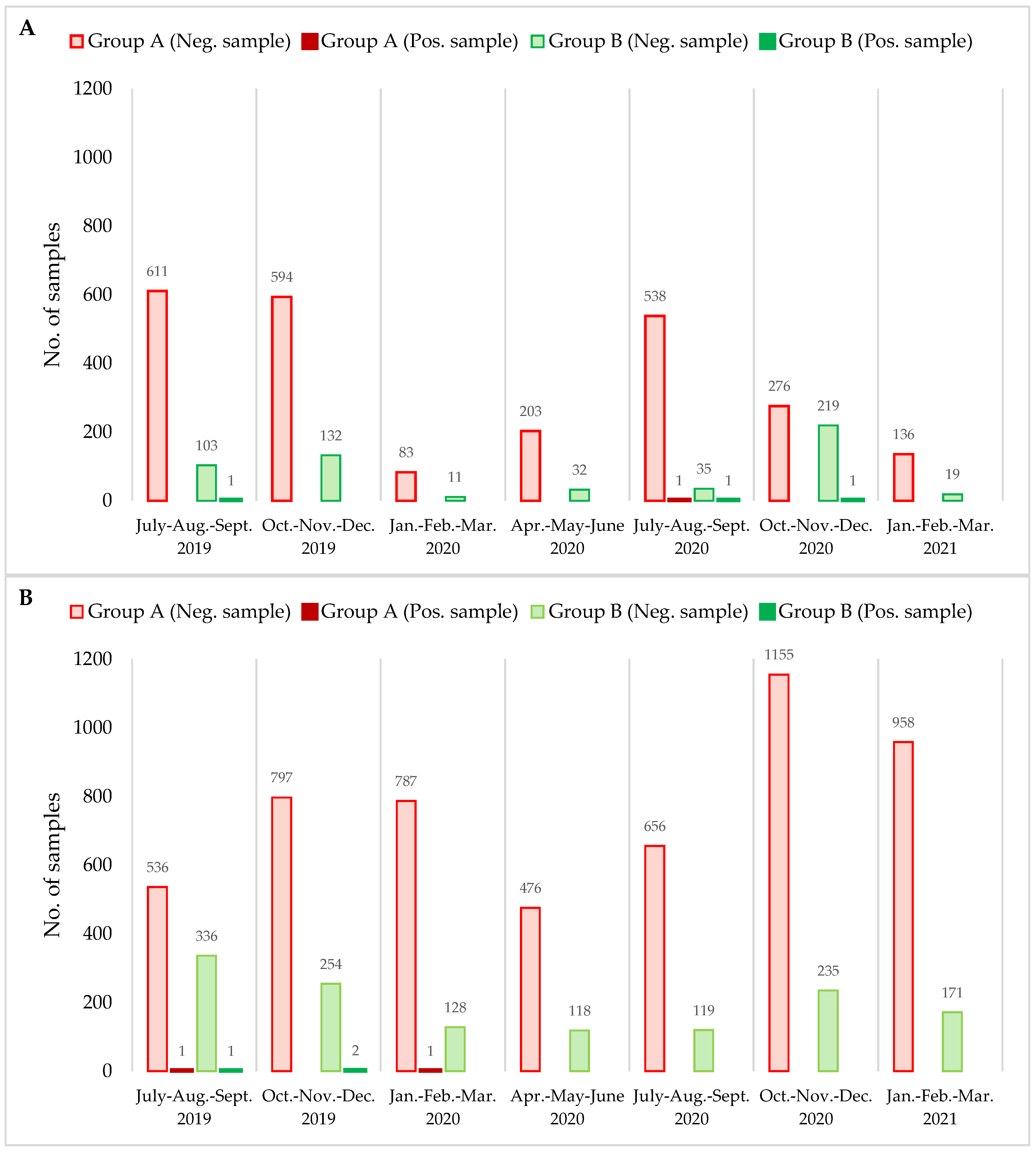
| No. Positive/Total No. of Samples (% Positive) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID Groups | July–Aug.–Sept. 2019 | Oct.–Nov.–Dec. 2019 | Jan.–Feb.–Mar. 2020 | Apr.–May–June 2020 | July–Aug.–Sept. 2020 | Oct.–Nov.–Dec. 2020 | Jan.–Feb.–Mar. 2021 | Total | CI95 of % positive 1 |
| Group A | 0/611 (0) | 0/594 (0) | 0/83 (0) | 0/203 (0) | 1 a/539 (0.18) | 0/276 (0) | 0/136 (0) | 1/2442 (0.04) | 0.01–0.25 |
| Group B | 1 b/104 (0.96) | 0/132 (0) | 0/11 (0) | 0/32 (0) | 1 c/36 (2.77) | 1 d/220 (0.45) | 0/19 (0) | 3/554 (0.54) | 0.18–1.58 |
| Total | 1/715 (0.13) | 0/726 (0) | 0/94 (0) | 0/235 (0) | 2/575 (0.34) | 1/496 (0.20) | 0/155 (0) | 4/2996 (0.13) | |
| CI95 (%) 1 | 0.02–0.79 | 0.00–0.53 | 0.00–3.93 | 0.00–1.61 | 0.10–1.26 | 0.04–1.13 | 0.00–2.42 | 0.05–0.34 | |
| No. Positive/Total No. of Samples (% Positive) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID Groups | July–Aug.–Sept. 2019 | Oct.–Nov.– Dec. 2019 | Jan.–Feb.– Mar. 2020 | Apr.–May–June 2020 | July–Aug.– Sept. 2020 | Oct.–Nov.– Dec. 2020 | Jan.–Feb.– Mar. 2021 | Total | CI95 of % positive 1 |
| Group A | 1 a/537 (0.18) | 0/797 (0) | 1 b/788 (0.12) | 0/476 (0) | 0/656 (0) | 0/1.155 (0) | 0/958 (0) | 2/5367 (0.03) | 0.01–0.14 |
| Group B | 1 c/337 (0.29) | 2 d/256 (0.78) | 0/128 (0) | 0/118 (0) | 0/119 (0) | 0/235 (0) | 0/171 (0) | 3/1364 (0.21) | 0.07–0.64 |
| Total | 2/874 (0.22) | 2/1053 (0.18) | 1/916 (0.10) | 0/594 (0) | 0/775 (0) | 0/1390 (0) | 0/1129 (0) | 5/6731 (0.07) | |
| CI95 (%) 1 | 0.06–0.83 | 0.05–0.69 | 0.02–0.62 | 0.00–0.64 | 0.00–0.49 | 0.00–0.28 | 0.00–0.34 | 0.03–0.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrica, M.; Andoni, E.; Intraina, I.; Curone, G.; Copelotti, E.; Massacci, F.R.; Terio, V.; Colombo, S.; Balzaretti, C.M. Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready to Eat Foods from Large Retailers and Canteens over a 2-Year Period in Northern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10568. https://doi.org/10.3390/ijerph182010568
Castrica M, Andoni E, Intraina I, Curone G, Copelotti E, Massacci FR, Terio V, Colombo S, Balzaretti CM. Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready to Eat Foods from Large Retailers and Canteens over a 2-Year Period in Northern Italy. International Journal of Environmental Research and Public Health. 2021; 18(20):10568. https://doi.org/10.3390/ijerph182010568
Chicago/Turabian StyleCastrica, Marta, Egon Andoni, India Intraina, Giulio Curone, Emma Copelotti, Francesca Romana Massacci, Valentina Terio, Silvia Colombo, and Claudia Maria Balzaretti. 2021. "Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready to Eat Foods from Large Retailers and Canteens over a 2-Year Period in Northern Italy" International Journal of Environmental Research and Public Health 18, no. 20: 10568. https://doi.org/10.3390/ijerph182010568
APA StyleCastrica, M., Andoni, E., Intraina, I., Curone, G., Copelotti, E., Massacci, F. R., Terio, V., Colombo, S., & Balzaretti, C. M. (2021). Prevalence of Listeria monocytogenes and Salmonella spp. in Different Ready to Eat Foods from Large Retailers and Canteens over a 2-Year Period in Northern Italy. International Journal of Environmental Research and Public Health, 18(20), 10568. https://doi.org/10.3390/ijerph182010568










