Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECHO.CA.IL Prospective Birth Cohorts
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.1.1. Chemicals in Our Bodies Recruitment and Data Collection during Pregnancy
2.1.2. Illinois Kids Development Study Recruitment and Data Collection during Pregnancy
2.2. Demographics, Lifestyle, and Health Information
2.3. Per- and Polyfluoroalkyl Substances (PFAS) Measurement
2.4. Phenolic Compounds
2.5. Psychosocial Stress and Depression
2.6. Biomarkers of Chronic Stress Response
2.7. Birth Outcomes
2.8. Visual Recognition Memory (VRM) Outcomes at 7.5 Months
2.9. Additional Neurodevelopment Measures
2.10. Statistical Analysis
3. Results
3.1. Overall, Demographic Data
3.2. Stress
3.3. Birth Outcomes
3.4. VRM
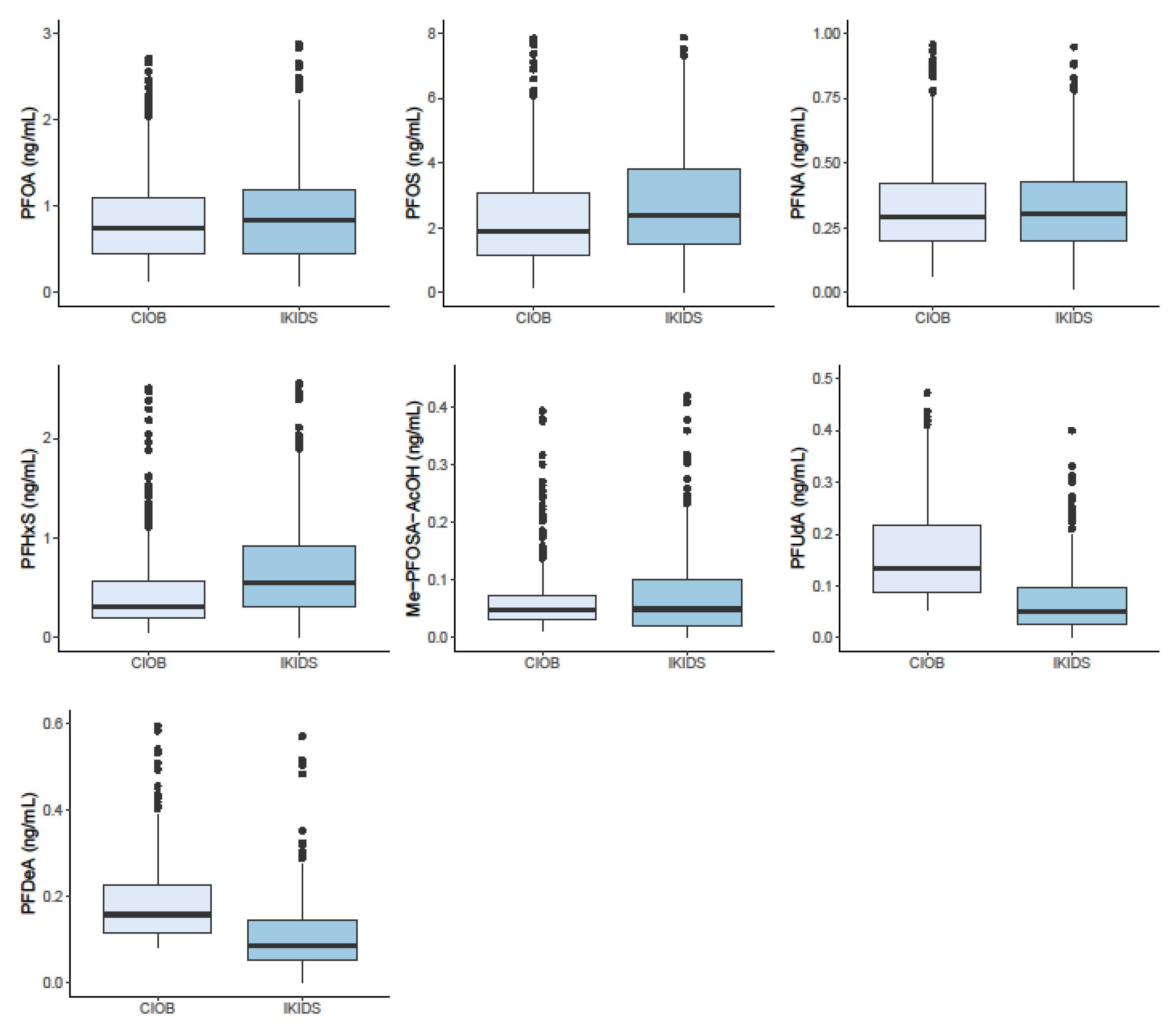
3.5. PFAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewinn, K.Z.; Stroud, L.R.; Molnar, B.E.; Ware, J.H.; Koenen, K.C.; Buka, S.L. Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. Int. J. Epidemiol. 2009, 38, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kim, M.S.; Lim, Y.H.; Lee, N.; Hong, Y.C. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res. 2018, 167, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.; Flachs, E.M.; Rimborg, S.; Glazer, C.H.; Giwercman, A.; Ramlau-Hansen, C.H.; Hougaard, K.S.; Høyer, B.B.; Hærvig, K.K.; Petersen, S.B.; et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod. Update 2016, 23, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wu, Y.; Zhu, Y.H.; Barry, J.; Ding, T.; Baio, G.; Muscat, R.; Todd, B.K.; Wang, F.-F.; Hardiman, P.J. The association between psychological stress and miscarriage: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Varshavsky, J.; Smith, A.; Wang, A.; Hom, E.; Izano, M.; Huang, H.; Padula, A.M.; Woodruff, T.J. Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reprod. Toxicol. 2019, 92, 14–56. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, H.M.; Morello-Frosch, R.; Sen, S.; Zeise, L.; Woodruff, T.J. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: Systematic-review of the human and animal evidence. PLoS ONE 2017, 12, e0176331. [Google Scholar] [CrossRef]
- Dole, N.; Savitz, D.A.; Hertz-Picciotto, I.; Siega-Riz, A.M.; McMahon, M.J.; Buekens, P. Maternal stress and preterm birth. Am. J. Epidemiol. 2003, 157, 14–24. [Google Scholar] [CrossRef]
- Alhusen, J.L.; Bower, K.M.; Epstein, E.; Sharps, P. Racial Discrimination and Adverse Birth Outcomes: An Integrative Review. J. Midwifery Womens Health 2016, 61, 707–720. [Google Scholar] [CrossRef]
- Staneva, A.; Bogossian, F.; Pritchard, M.; Wittkowski, A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth 2015, 28, 179–193. [Google Scholar] [CrossRef]
- Merced-Nieves, F.M.; Aguiar, A.; Dzwilewski, K.L.C.; Musaad, S.; Korrick, S.A.; Schantz, S.L. Association of prenatal maternal perceived stress with a sexually dimorphic measure of cognition in 4.5-month-old infants. Neurotoxicol. Teratol. 2020, 77, 106850. [Google Scholar] [CrossRef]
- Calafat, A.M.; Wong, L.Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the national health and nutrition examination survey (NHANES) 2003-2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to Bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Henare, K.; Thorstensen, E.B.; Ponnampalam, A.P.; Mitchell, M.D. Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 2010, 202, 393.e1–393.e7. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, Q.Q.; Chu, C.; Wang, S.Z.; Tang, Y.T.; Appleton, A.A.; Qiu, R.L.; Yang, B.Y.; Hu, L.W. High trans-placental transfer of perfluoroalkyl substances alternatives in the matched maternal-cord blood serum: Evidence from a birth cohort study. Sci. Total Environ. 2020, 705, 1–7. [Google Scholar] [CrossRef]
- Chu, C.; Zhou, Y.; Li, Q.Q.; Bloom, M.S.; Lin, S.; Yu, Y.J.; Chen, D.; Yu, H.Y.; Hu, L.W.; Yang, B.Y.; et al. Are perfluorooctane sulfonate alternatives safer? New insights from a birth cohort study. Environ. Int. 2020, 135, 105365. [Google Scholar] [CrossRef]
- Chen, M.H.; Ha, E.H.; Wen, T.W.; Su, Y.N.; Lien, G.W.; Chen, C.Y.; Chen, P.C.; Hsieh, W.S. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS ONE 2012, 7, e42474. [Google Scholar] [CrossRef]
- Mustieles, V.; Zhang, Y.; Yland, J.; Braun, J.M.; Williams, P.L.; Wylie, B.J.; Attaman, J.A.; Ford, J.B.; Azevedo, A.; Calafat, A.M.; et al. Maternal and paternal preconception exposure to phenols and preterm birth. Environ. Int. 2020, 137, 105523. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.H.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Räikkönen, K.; King, S.; et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef]
- Forns, J.; Verner, M.A.; Iszatt, N.; Nowack, N.; Bach, C.C.; Vrijheid, M.; Costa, O.; Andiarena, A.; Sovcikova, E.; Høyer, B.B.; et al. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: A meta-analysis of nine European population-based studies. Environ. Health Perspect. 2020, 128, 057002. [Google Scholar] [CrossRef]
- Grobman, W.A.; Parker, C.B.; Willinger, M.; Wing, D.A.; Silver, R.M.; Wapner, R.J.; Simhan, H.N.; Parry, S.; Mercer, B.M.; Haas, D.M.; et al. Racial disparities in adverse pregnancy outcomes and psychosocial stress. Obstet. Gynecol. 2018, 131, 328–335. [Google Scholar] [CrossRef]
- Eick, S.M.; Goin, D.E.; Izano, M.A.; Cushing, L.; DeMicco, E.; Padula, A.M.; Woodruff, T.J.; Morello-Frosch, R. Relationships between psychosocial stressors among pregnant women in San Francisco: A path analysis. PLoS ONE 2020, 15, e0234579. [Google Scholar] [CrossRef] [PubMed]
- Hobel, C.J.; Goldstein, A.; Barrett, E.S. Psychosocial stress and pregnancy outcome. Clin. Obstet. Gynecol. 2008, 51, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; O’Keeffe, G.W.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Webster, T.F.; Mora, A.M.; Harris, M.H.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci Technol. 2015, 49, 11849–11858. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, P.; Thomsen, C.; Casas, M.; de Bont, J.; Haug, L.S.; Maitre, L.; Papadopoulou, E.; Sakhi, A.K.; Slama, R.; Saulnier, P.J.; et al. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int. J. Hyg. Environ. Health 2019, 222, 864–872. [Google Scholar] [CrossRef]
- Padula, A.M.; Monk, C.; Brennan, P.A.; Borders, A.; Barrett, E.S.; McEvoy, C.T.; Foss, S.; Desai, P.; Alshawabkeh, A.; Wurth, R.; et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—implications for research on perinatal outcomes in the ECHO program. J. Perinatol. 2020, 40, 10–24. [Google Scholar] [CrossRef]
- Dzwilewski, K.L.C.; Merced-Nieves, F.M.; Aguiar, A.; Korrick, S.A.; Schantz, S.L. Characterization of performance on an automated visual recognition memory task in 7.5-month-old infants. Neurotoxicol. Teratol. 2020, 81, 106904. [Google Scholar] [CrossRef]
- Merced-Nieves, F.M.; Dzwilewski, K.L.C.; Aguiar, A.; Lin, J.; Schantz, S. Associations of prenatal maternal stress with measures of cognition in 7.5-month-old infants. Dev. Psychobiol. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Entringer, S.; Epel, E.S.; Lin, J.; Buss, C.; Shahbaba, B.; Blackburn, E.H.; Simhan, H.N.; Wadhwa, P.D. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet Gynecol. 2013, 208, 134.e1–134.e7. [Google Scholar] [CrossRef]
- Moog, N.K.; Buss, C.; Entringer, S.; Shahbaba, B.; Gillen, D.L.; Hobel, C.J.; Wadhwa, P.D. Maternal Exposure to Childhood Trauma Is Associated during Pregnancy with Placental-Fetal Stress Physiology. Biol. Psychiatry 2016, 79, 831–839. [Google Scholar] [CrossRef]
- Squires, J.; Bricker, D.D.; Twombly, E. Ages & Stages Questionnaires; Brookes, P.H., Ed.; Paul H. Brookes Publishing Co.: Balitmore, MD, USA, 2009. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Cushing, L.J.; Jesdale, B.M.; Schwartz, J.M.; Guo, W.; Guo, T.; Wang, M.; Harwani, S.; Petropoulou, S.-S.E.; Duong, W.; et al. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ. Sci. Technol. 2016, 50, 12464–12472. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Hom Thepaksorn, E.K.; Izano, M.A.; Cushing, L.J.; Wang, Y.; Smith, S.C.; Gao, S.; Park, J.-S.; Padula, A.M.; DeMicco, E.; et al. Associations between prenatal maternal exposure to per- and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environ. Health 2020, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.T.; Nouli, E.; Xekoukoulotakis, N.P.; Mantzavinos, D. Effect of key operating parameters on phenols degradation during H2O2-assisted TiO2 photocatalytic treatment of simulated and actual olive mill wastewaters. Appl. Catal. B Environ. 2007, 73, 11–22. [Google Scholar] [CrossRef]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal. Chem. 2005, 77, 5407–5413. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Kupst, M.J.; Butt, Z.; Stoney, C.M.; Griffith, J.W.; Salsman, J.M.; Folkman, S.; Cella, D. Assessment of stress and self-efficacy for the NIH Toolbox for Neurological and Behavioral Function. Anxiety Stress Coping 2015, 28, 531–544. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of Postnatal Depression: Development of the 10-item Edinburgh Postnatal Depression scale. Br. J. Psychiatry. 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Weissman, M.M.; Sholomskas, D.; Pottenger, M.; Prusoff, B.A.; Locke, B.Z. Assessing depressive symptoms in five psychiatric populations: A validation study. Am. J. Epidemiol. 1977, 106, 203–214. [Google Scholar] [CrossRef]
- Matthey, S.; Henshaw, C.; Elliott, S.; Barnett, B. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale—Implications for clinical and research practice. Arch. Womens Ment. Health. 2006, 9, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.W.; Hunt, L.P. Psychosocial stress in pregnancy and its relation to low birth weight. Br Med J (Clin Res Ed). Br. Med. J. Publ. Group 1984, 288, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Dohrenwend, B.P. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychol. Bull. 2006, 132, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Epel, E.S.; Lin, J.; Wilhelm, F.H.; Wolkowitz, O.M.; Cawthon, R.; Adler, N.E.; Dolbier, C.; Mendes, W.B.; Blackburn, E.H. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 2006, 31, 277–287. [Google Scholar] [CrossRef]
- Scarr, S. Effects of birth weight on later intelligence. Biodemogr. Soc. Biol. 1982, 29, 230–237. [Google Scholar] [CrossRef]
- Bhutta, A.T.; Cleves, M.A.; Casey, P.H.; Cradock, M.M.; Anand, K.J.S. Cognitive and Behavioral Outcomes of school-aged children who were born preterm: A meta-analysis. JAMA 2007, 288, 728–737. [Google Scholar] [CrossRef]
- Aarnoudse-Moens, C.S.H.; Weisglas-Kuperus, N.; Van Goudoever, J.B.; Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009, 124, 717–728. [Google Scholar] [CrossRef]
- World Health Organization. Preterm Birth; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Talge, N.M.; Mudd, L.M.; Sikorskii, A.; Basso, O. United states birth weight reference corrected for implausible gestational age estimates. Pediatrics 2014, 133, 844–853. [Google Scholar] [CrossRef]
- .Rose, S.A.; Feldman, J.F.; Jankowski, J.J. Attention and recognition memory in the 1st year of life: A longitudinal study of preterm and full-term infants. Dev. Psychol. 2001, 37, 135–151. [Google Scholar] [CrossRef]
- Fagan, J.F., III. Infants’ recognition memory for faces. J. Exp. Child. Psychol. 1972, 14, 453–476. [Google Scholar] [CrossRef]
- Cornell, E.H. Infants’ recognition memory, forgetting, and savings. J. Exp. Child. Psychol. 1979, 28, 359–374. [Google Scholar] [CrossRef]
- Achenbach, T.M. Manual for the Child Behavior Checklist/4-18 and 1991 Profile; University of Vermont—Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Centers for Disease Control. NHANES 2013–2014. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013 (accessed on 1 September 2020).
- Woods, S.M.; Melville, J.L.; Guo, Y.; Fan, M.Y.; Gavin, A. Psychosocial stress during pregnancy. Am. J. Obstet. Gynecol. 2010, 202, 61.e1–61.e7. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, S.L.; Eliot, M.N.; Kelsey, K.T.; Calafat, A.M.; Ehrlich, S.; Lanphear, B.P.; Chen, A.; Braun, J.M. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ. Res. 2018, 165, 247–257. [Google Scholar] [CrossRef]
- Castorina, R.; Bradman, A.; Sjödin, A.; Fenster, L.; Jones, R.S.; Harley, K.G.; Eisen, E.; Eskenazi, B. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environ. Sci Technol. 2011, 45, 6553–6560. [Google Scholar] [CrossRef]
- Park, S.K.; Peng, Q.; Ding, N.; Mukherjee, B.; Harlow, S.D. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ. Res. 2019, 175, 186–199. [Google Scholar] [CrossRef]
- Dzwilewski, K.L.C.; Woodbury, M.L.; Aguiar, A.; Korrick, S.A.; Merced-Nieves, F.S.S. Associations of prenatal exposure to phthalates with measures of cognition in 7.5-month-old infants. 2020, submitted.
- Grigoriadis, S.; Graves, L.; Peer, M.; Mamisashvili, L.; Tomlinson, G.; Vigod, S.N.; Dennis, C.-L.; Steiner, M.; Brown, C.; Cheung, A.; et al. A systematic review and meta-analysis of the effects of antenatal anxiety on postpartum outcomes. Arch. Womens Ment. Health. 2018, 22, 543–556. [Google Scholar] [CrossRef]
- Lee, E.H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs. Res. 2012, 6, 121–127. [Google Scholar] [CrossRef]

| Demographics | CIOB (n = 822) | IKIDS (n = 565) | Total (n = 1387) |
|---|---|---|---|
| n (%) or Mean (SD) | n (%) or Mean (SD) | n (%) or Mean (SD) | |
| Maternal Age (years) | |||
| <25 | 83 (10%) | 48 (8%) | 131 (9%) |
| 25–29 | 106 (13%) | 182 (32%) | 288 (21%) |
| 30–34 | 301 (37%) | 252 (45%) | 553 (40%) |
| ≥35 | 315 (38%) | 83 (15%) | 398 (29%) |
| Missing | 17 (2.1%) | 0 (0%) | 17 (1.2%) |
| Pre-pregnancy BMI (kg/m2) | |||
| Underweight (<18.5 kg/m2) | 23 (3%) | 14 (2%) | 37 (3%) |
| Normal (18.5–24.9 kg/m2) | 364 (44%) | 286 (51%) | 650 (47%) |
| Overweight (25–29.9 kg/m2) | 164 (20%) | 123 (22%) | 287 (21%) |
| Obese (≥30 kg/m2) | 105 (13%) | 138 (24%) | 243 (18%) |
| Missing | 166 (20.2%) | 4 (0.7%) | 170 (12.3%) |
| Maternal Education | |||
| <College degree | 281 (34%) | 112 (20%) | 393 (28%) |
| College degree | 192 (23%) | 197 (35%) | 389 (28%) |
| Graduate degree | 290 (35%) | 256 (45%) | 546 (39%) |
| Missing | 59 (7.2%) | 0 (0%) | 59 (4.3%) |
| Maternal Race/Ethnicity | |||
| White | 313 (38%) | 453 (80%) | 766 (55%) |
| Black | 50 (6%) | 31 (5%) | 81 (6%) |
| Asian/Pacific Islander | 141 (17%) | 31 (5%) | 172 (12%) |
| Hispanic | 282 (34%) | 15 (3%) | 297 (21%) |
| Other/multiracial | 25 (3%) | 35 (6%) | 60 (4%) |
| Missing | 11 (1.3%) | 0 (0%) | 11 (0.8%) |
| Infant Sex | |||
| Male | 373 (45%) | 254 (45%) | 627 (45%) |
| Female | 391 (48%) | 273 (48%) | 664 (48%) |
| Missing | 58 (7.1%) | 38 (6.7%) | 96 (6.9%) |
| Parity | |||
| 1+ Births | 339 (41%) | 339 (60%) | 678 (49%) |
| No prior births | 366 (45%) | 226 (40%) | 592 (43%) |
| Missing | 117 (14.2%) | 0 (0%) | 117 (8.4%) |
| Smoking Status | |||
| Not current smoker | 766 (93%) | 556 (98%) | 1322 (95%) |
| Current smoker | 10 (1%) | 9 (2%) | 19 (1%) |
| Missing | 46 (5.6%) | 0 (0%) | 46 (3.3%) |
| Marital Status | |||
| Married or living together | 639 (78%) | 535 (95%) | 1174 (85%) |
| Single | 78 (9%) | 30 (5%) | 108 (8%) |
| Missing | 105 (12.8%) | 0 (0%) | 105 (7.6%) |
| Psychosocial Stressors | |||
| Perceived stress (range 0–85) | 50 (8.7) | 45 (11) | 48 (10) |
| Missing | 72 (8.8%) | 9 (1.6%) | 81 (5.8%) |
| Clinical Levels of Depression | |||
| No | 662 (81%) | 529 (94%) | 1191 (86%) |
| Yes | 50 (6%) | 25 (4%) | 75 (5%) |
| Missing | 110 (13.4%) | 11 (1.9%) | 121 (8.7%) |
| Stressful life events (range 0–5) | 1.0 (1.1) | 0.32 (0.66) | 0.77 (1.0) |
| Missing | 57 (6.9%) | 118 (20.9%) | 175 (12.6%) |
| VRM and Birth Outcomes | CIOB (n = 822) | IKIDS (n = 565) | Total (n = 1387) |
|---|---|---|---|
| n (%) or Mean (SD) | n (%) or Mean (SD) | n (%) or Mean (SD) | |
| Delivery method | |||
| Vaginal | 542 (66%) | 361 (64%) | 903 (65%) |
| C-section | 135 (16%) | 138 (24%) | 273 (20%) |
| Missing | 145 (17.6%) | 66 (11.7%) | 211 (15.2%) |
| Gestational age (weeks) | 39 (2.0) | 39 (1.5) | 39 (1.8) |
| Missing | 126 (15.3%) | 38 (6.7%) | 164 (11.8%) |
| Preterm birth | |||
| Yes | 58 (7%) | 25 (4%) | 83 (6%) |
| No | 638 (78%) | 502 (89%) | 1140 (82%) |
| Missing | 126 (15.3%) | 38 (6.7%) | 164 (11.8%) |
| Birth weight (grams) | 3400 (580) | 3500 (430) | 3400 (530) |
| Missing | 84 (10.2%) | 116 (20.5%) | 200 (14.4%) |
| Low birth weight | |||
| Yes | 44 (5%) | 4 (1%) | 48 (3%) |
| No | 694 (84%) | 445 (79%) | 1139 (82%) |
| Missing | 84 (10.2%) | 116 (20.5%) | 200 (14.4%) |
| Birth weight z-score | 0.002 (1.0) | 0.16 (0.93) | 0.063 (0.98) |
| Missing or incomplete | 140 (17.0%) | 124 (21.9%) | 264 (19.0%) |
| Novelty preference (proportion) 1 | 56 (6.6) | 57 (6.8) | 57 (6.8) |
| Time to reach familiarization (seconds) 1 | 60 (53) | 52 (21) | 54 (31) |
| Average run duration (seconds) 1 | 4.6 (2.2) | 4.4 (2.4) | 4.3 (6.6) |
| Demographics | Gestational Age (Weeks) | Birth Weight Z-Score | ||||
|---|---|---|---|---|---|---|
| n | β | 95% CI | n | β | 95% CI | |
| Maternal Age (years) | ||||||
| <25 | 99 | −0.21 | (−0.63, 0.21) | 91 | −0.18 | (−0.42, 0.06) |
| 25–29 | 251 | Ref | Ref | 216 | Ref | Ref |
| 30–34 | 508 | −0.02 | (−0.3, 0.25) | 458 | 0.15 | (−0.01, 0.31) |
| ≥35 | 365 | 0.07 | (−0.22, 0.36) | 358 | 0.12 | (−0.04, 0.29) |
| Pre-pregnancy BMI (kg/m2) | ||||||
| Underweight (<18.5 kg/m2) | 35 | −0.53 | (−1.11, 0.06) | 31 | 0.02 | (−0.34, 0.37) |
| Normal (18.5–24.9 kg/m2) | 624 | Ref | Ref | 578 | Ref | Ref |
| Overweight (25–29.9 kg/m2) | 274 | −0.10 | (−0.34, 0.15) | 254 | 0.19 | (0.05, 0.34) |
| Obese (≥30 kg/m2) | 231 | −0.46 | (−0.72, −0.20) | 204 | 0.33 | (0.18, 0.49) |
| Maternal Education | ||||||
| <College degree | 307 | −0.44 | (−0.70, −0.19) | 283 | −0.09 | (−0.24, 0.07) |
| College degree | 371 | Ref | Ref | 341 | Ref | Ref |
| Graduate degree | 523 | 0.09 | (−0.13, 0.32) | 477 | 0 | (−0.13, 0.14) |
| Race/Ethnicity | ||||||
| White | 722 | Ref | Ref | 647 | Ref | Ref |
| Black | 70 | −0.77 | (−1.21, −0.33) | 64 | −0.67 | (−0.92, −0.43) |
| Asian/Pacific Islander | 160 | −0.14 | (−0.44, 0.17) | 153 | −0.44 | (−0.61, −0.27) |
| Hispanic | 206 | −0.41 | (−0.69, −0.13) | 202 | −0.18 | (−0.33, −0.03) |
| Multi-Racial/Other | 57 | 0.25 | (−0.23, 0.73) | 50 | −0.37 | (−0.65, −0.10) |
| Infant Sex | ||||||
| Male | 582 | Ref | Ref | 547 | Ref | Ref |
| Female | 629 | −0.01 | (−0.21, 0.19) | 576 | −0.02 | (−0.14, 0.09) |
| Parity | ||||||
| 1+ prior births | 565 | Ref | Ref | 522 | Ref | Ref |
| No prior births | 650 | −0.06 | (−0.26, 0.13) | 594 | 0.33 | (0.22, 0.44) |
| Current Smoker | ||||||
| No | 1212 | Ref | Ref | 1212 | Ref | Ref |
| Yes | 11 | 0.50 | (−0.57, 1.57) | 11 | −0.35 | (−0.93, 0.24) |
| Marital Status | ||||||
| Married or living together | 1083 | Ref | Ref | 989 | Ref | Ref |
| Single | 86 | −0.27 | (−0.65, 0.10) | 81 | −0.48 | (−0.70, −0.26) |
| Demographics | Novelty Preference (Proportion) | Time to Reach Familiarization (Seconds) | Average Run Duration (Seconds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | β | 95% CI | n | β | 95% CI | n | β | 95% CI | |
| Maternal Age (years) | |||||||||
| <25 | 18 | 0.24 | (−3.24, 3.72) | 18 | 2.41 | (−13.61, 18.43) | 18 | −0.01 | (−3.39, 3.38) |
| 25–29 | 89 | Ref | Ref | 89 | Ref | Ref | 89 | Ref | Ref |
| 30–34 | 156 | 0.01 | (−1.78, 1.79) | 156 | 3.10 | (−5.13, 11.34) | 156 | 0.04 | (−1.70, 1.78) |
| ≥35 | 70 | -0.03 | (−2.18, 2.13) | 70 | 2.12 | (−7.78, 12.02) | 70 | 0.43 | (−1.67, 2.52) |
| Pre-pregnancy BMI (kg/m2) | |||||||||
| Underweight (<18.5 kg/m2) | 8 | 0.75 | (−4.08, 5.58) | 8 | 4.50 | (−13.66, 22.66) | 8 | 1.47 | (−3.34, 6.27) |
| Normal (18.5–24.9 kg/m2) | 168 | Ref | Ref | 168 | Ref | Ref | 168 | Ref | Ref |
| Overweight (25–29.9 kg/m2) | 73 | −1.2 | (−3.07, 0.67) | 73 | 2.09 | (−4.95, 9.12) | 73 | −0.39 | (−2.25, 1.47) |
| Obese (≥30 kg/m2) | 69 | −0.61 | (−2.52, 1.3) | 69 | 3.39 | (−3.78, 10.57) | 69 | −0.44 | (−2.34, 1.46) |
| Maternal Education | |||||||||
| <College degree | 50 | −0.85 | (−3.11, 1.41) | 50 | 1.57 | (−8.82, 11.95) | 50 | 0.77 | (−1.44, 2.98) |
| College degree | 118 | Ref | Ref | 118 | Ref | Ref | 118 | Ref | Ref |
| Graduate degree | 165 | 0.61 | (−1.00, 2.23) | 165 | −6.25 | (−13.67, 1.17) | 165 | 0.10 | (−1.48, 1.67) |
| Race/Ethnicity | |||||||||
| White | 244 | Ref | Ref | 244 | Ref | Ref | 244 | Ref | Ref |
| Black | 15 | −0.35 | (−3.93, 3.23) | 15 | 3.72 | (−12.56, 20) | 15 | −0.49 | (−3.95, 2.97) |
| Asian/Pacific Islander | 31 | −0.22 | (−2.78, 2.35) | 31 | −4.88 | (−16.55, 6.8) | 31 | −0.97 | (−3.46, 1.51) |
| Hispanic | 22 | 1.32 | (−1.68, 4.32) | 22 | 19.48 | (5.85, 33.1) | 22 | −3.36 | (−6.26, −0.47) |
| Multiracial/other | 21 | −0.59 | (−3.65, 2.47) | 21 | −4.22 | (−18.14, 9.7) | 21 | −0.71 | (−3.66, 2.25) |
| Infant Sex | |||||||||
| Male | 156 | Ref | Ref | 156 | Ref | Ref | 156 | Ref | Ref |
| Female | 176 | 0.42 | (−1.06, 1.90) | 176 | 0.16 | (−6.64, 6.96) | 176 | −0.39 | (−1.61, 0.83) |
| Parity | |||||||||
| 1+ prior births | 150 | Ref | Ref | 150 | Ref | Ref | 150 | Ref | Ref |
| No prior births | 177 | 1.06 | (−0.44, 2.55) | 177 | −0.92 | (−6.48, 4.63) | 177 | −0.51 | (−1.96, 0.95) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eick, S.M.; Enright, E.A.; Geiger, S.D.; Dzwilewski, K.L.C.; DeMicco, E.; Smith, S.; Park, J.-S.; Aguiar, A.; Woodruff, T.J.; Morello-Frosch, R.; et al. Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECHO.CA.IL Prospective Birth Cohorts. Int. J. Environ. Res. Public Health 2021, 18, 742. https://doi.org/10.3390/ijerph18020742
Eick SM, Enright EA, Geiger SD, Dzwilewski KLC, DeMicco E, Smith S, Park J-S, Aguiar A, Woodruff TJ, Morello-Frosch R, et al. Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECHO.CA.IL Prospective Birth Cohorts. International Journal of Environmental Research and Public Health. 2021; 18(2):742. https://doi.org/10.3390/ijerph18020742
Chicago/Turabian StyleEick, Stephanie M., Elizabeth A. Enright, Sarah D. Geiger, Kelsey L. C. Dzwilewski, Erin DeMicco, Sabrina Smith, June-Soo Park, Andrea Aguiar, Tracey J. Woodruff, Rachel Morello-Frosch, and et al. 2021. "Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECHO.CA.IL Prospective Birth Cohorts" International Journal of Environmental Research and Public Health 18, no. 2: 742. https://doi.org/10.3390/ijerph18020742
APA StyleEick, S. M., Enright, E. A., Geiger, S. D., Dzwilewski, K. L. C., DeMicco, E., Smith, S., Park, J.-S., Aguiar, A., Woodruff, T. J., Morello-Frosch, R., & Schantz, S. L. (2021). Associations of Maternal Stress, Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFAS), and Demographic Risk Factors with Birth Outcomes and Offspring Neurodevelopment: An Overview of the ECHO.CA.IL Prospective Birth Cohorts. International Journal of Environmental Research and Public Health, 18(2), 742. https://doi.org/10.3390/ijerph18020742





