Predictors of New Dementia Diagnoses in Elderly Individuals: A Retrospective Cohort Study Based on Prefecture-Wide Claims Data in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Data Source
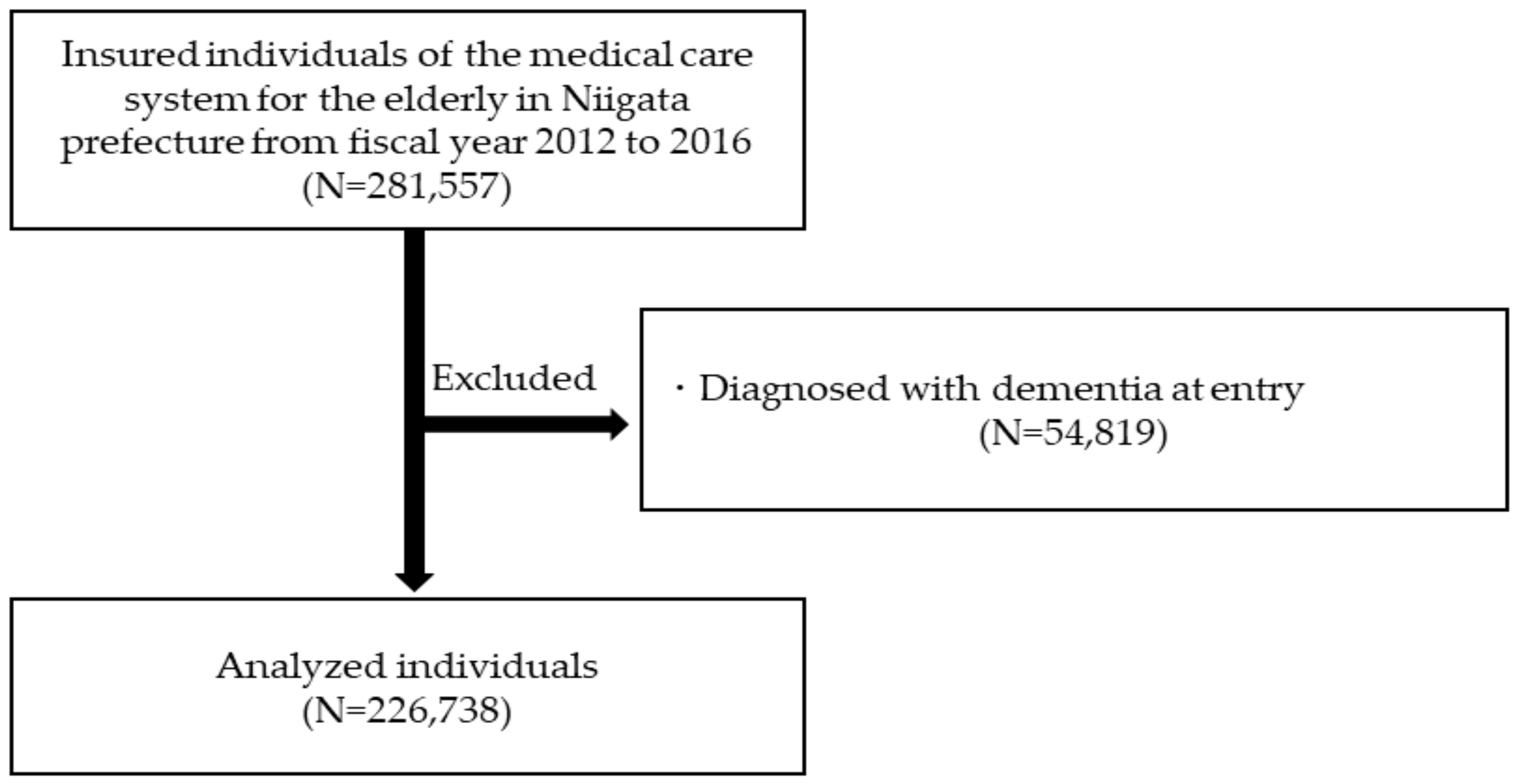
2.2. Study Population
2.3. Exposure Including Predictors
- Demographic measures: age, sex.
- Medical diagnosis: diabetes mellitus (ICD-10: E11–E14), ischemic heart disease (I20–I25), cerebrovascular disease (I60–I69), atrial fibrillation/flutter (I48) at baseline, and current depression diagnosis (F32, F33)/treatment with antidepressants.
- Prescriptions: antihypertensive drugs (ATC-code: C02, C03, C07, C08, C09), antihyperlipidemia drugs (C10), antidepressants (N06A), antipsychotics (N05A), anxiolytics (N05B), hypnotics (N05C), and antithrombotics (B01AC).
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Study Cohort
3.2. Characteristics of Incident Dementia Cases
3.3. Logistic Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International: London, UK, 2018; Available online: https://www.alz.co.uk/research/world-report-2018 (accessed on 29 October 2020).
- Ministry of Health, Labour and Welfare. Long-Term Care Insurance System of Japan. Available online: https://www.mhlw.go.jp/english/policy/care-welfare/care-welfare-elderly/ (accessed on 29 October 2020).
- Ohara, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Iwaki, T.; Kitazono, T.; Kanba, S.; Kiyohara, Y.; Ninomiya, T. Trends in dementia prevalence, incidence, and survival rate in a japanese community. Neurology 2017, 88, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Stephan, B.C.; Tang, E.; Muniz-Terrera, G. Composite risk scores for predicting dementia. Curr. Opin. Psychiatry 2016, 29, 174–180. [Google Scholar] [CrossRef]
- Tang, E.Y.; Harrison, S.L.; Errington, L.; Gordon, M.F.; Visser, P.J.; Novak, G.; Dufouil, C.; Brayne, C.; Robinson, L.; Launer, L.J.; et al. Current developments in dementia risk prediction modelling: An updated systematic review. PLoS ONE 2015, 10, e0136181. [Google Scholar] [CrossRef]
- Walters, K.; Hardoon, S.; Petersen, I.; Iliffe, S.; Omar, R.Z.; Nazareth, I.; Rait, G. Predicting dementia risk in primary care: Development and validation of the dementia risk score using routinely collected data. BMC Med. 2016, 14, 6. [Google Scholar] [CrossRef]
- Hessler, J.B.; Ander, K.H.; Brönner, M.; Etgen, T.; Förstl, H.; Poppert, H.; Sander, D.; Bickel, H. Predicting dementia in primary care patients with a cardiovascular health metric: A prospective population-based study. BMC Neurol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Kuźma, E.; Lourida, I.; Moore, S.F.; Levine, D.A.; Ukoumunne, O.C.; Llewellyn, D.J. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers Dement. 2018, 14, 1416–1426. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front. Aging Neurosci. 2013, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Chui, H.C.; Zheng, L.; Reed, B.R.; Vinters, H.V.; Mack, W.J. Vascular risk factors and alzheimer’s disease: Are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimers Res. Ther. 2012, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the national alzheimer’s coordinating centre. Brain 2013, 136, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F., 3rd. Late-life depression and risk of vascular dementia and alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef]
- Reynolds, C.F., 3rd; Cuijpers, P.; Patel, V.; Cohen, A.; Dias, A.; Chowdhary, N.; Okereke, O.I.; Dew, M.A.; Anderson, S.J.; Mazumdar, S.; et al. Early intervention to reduce the global health and economic burden of major depression in older adults. Annu. Rev. Public Health 2012, 33, 123–135. [Google Scholar] [CrossRef]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S.; et al. Advances in the prevention of alzheimer’s disease and dementia. J. Intern. Med. 2014, 275, 229–250. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Schneider, A.L.; Albert, M.; Alonso, A.; Bandeen-Roche, K.; Coker, L.; Coresh, J.; Knopman, D.; Power, M.C.; Rawlings, A.; et al. Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014, 71, 1218–1227. [Google Scholar] [CrossRef]
- Rouch, L.; Cestac, P.; Hanon, O.; Cool, C.; Helmer, C.; Bouhanick, B.; Chamontin, B.; Dartigues, J.F.; Vellas, B.; Andrieu, S. Antihypertensive drugs, prevention of cognitive decline and dementia: A systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs 2015, 29, 113–130. [Google Scholar] [CrossRef]
- Ohara, T.; Doi, Y.; Ninomiya, T.; Hirakawa, Y.; Hata, J.; Iwaki, T.; Kanba, S.; Kiyohara, Y. Glucose tolerance status and risk of dementia in the community: The hisayama study. Neurology 2011, 77, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Greenslade, N.; Paudyal, P.; Bremner, S.; Smith, H.E.; Banerjee, S.; Sadhwani, S.; Rooney, P.; Oliver, S.; Cassell, J. Predicting dementia from primary care records: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0194735. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.S.; Hanna, M.; Kim, D.; Perfetto, E.M. Predicting Diagnosis of Alzheimer’s Disease and Related Dementias Using Administrative Claims. J. Manag. Care Spec. Pharm. 2018, 24, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| n = 226,738 | n = 82,145 | n = 144,593 | ||||
| Baseline age, mean (SD) | 80.81 | (4.67) | 80.02 | (4.18) | 81.26 | (4.87) |
| Comorbidities, n (%) | ||||||
| Cerebrovascular disease | 61,979 | (27.3%) | 22,889 | (27.9%) | 39,090 | (27.0%) |
| Diabetes mellitus | 63,406 | (28.0%) | 26,202 | (31.9%) | 37,204 | (25.7%) |
| Ischemic heart disease | 37,955 | (16.7%) | 14,838 | (18.1%) | 23,117 | (16.0%) |
| Atrial fibrillation | 17,520 | (7.7%) | 8866 | (10.8%) | 8654 | (6.0%) |
| Depression | 16,845 | (7.4%) | 3828 | (4.7%) | 13,017 | (9.0%) |
| Hypertension | 154,132 | (68.0%) | 54,477 | (66.3%) | 99,655 | (68.9%) |
| Dyslipidemia | 98,723 | (43.5%) | 28,848 | (35.1%) | 69,875 | (48.3%) |
| Prescriptions, n (%) | ||||||
| Antidepressants | 10,272 | (4.5%) | 2248 | (2.7%) | 8024 | (5.5%) |
| Antipsychotics | 7344 | (3.2%) | 2084 | (2.5%) | 5260 | (3.6%) |
| Anxiolytics | 53,707 | (23.7%) | 13,195 | (16.1%) | 40,512 | (28.0%) |
| Hypnotics | 18,704 | (8.2%) | 4428 | (5.4%) | 14,276 | (9.9%) |
| Antihypertensive drugs | 148,663 | (65.6%) | 52,575 | (64.0%) | 96,088 | (66.5%) |
| Dyslipidemia drugs | 68,662 | (30.3%) | 17,735 | (21.6%) | 50,927 | (35.2%) |
| Type of Dementia | Alzheimer’s Disease | Vascular Dementia | Unspecified Dementia | All-Cause Dementia |
|---|---|---|---|---|
| Dementia incidence, n | 19,093 | 604 | 5875 | 26,092 |
| Age, n (%) | ||||
| 75–79 y | 6591 (34.5%) | 201 (33.3%) | 1603 (27.3%) | 8627 (33.1%) |
| 80–84 y | 7447 (39.0%) | 211 (34.9%) | 1990 (33.9%) | 9835 (37.7%) |
| ≥85 y | 5055 (26.5%) | 192 (31.8%) | 2282 (38.8%) | 7630 (29.2%) |
| Prescriptions, n (%) | ||||
| Any anti-dementia drugs | 14,912 (78.1%) | 128 (21.2%) | 1110 (18.9%) | 16,330 (62.6%) |
| Anticholinesterases | 12,851 (67.3%) | 98 (16.2%) | 886 (15.1%) | 14,006 (53.7%) |
| Memantine | 3670 (19.2%) | 41 (6.8%) | 318 (5.4%) | 4050 (15.5%) |
| Predictors | Age- and Sex- Adjusted Model | Multivariable-Adjusted Model a | ||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Age (per 1-year increase) | 1.06 (1.06–1.07) b | <0.001 | 1.06 (1.06–1.07) | <0.001 |
| Female | 1.19 (1.16–1.22) c | <0.001 | 1.18 (1.14–1.21) | <0.001 |
| Cerebrovascular disease | 1.19 (1.16–1.23) | <0.001 | 1.15 (1.11–1.18) | <0.001 |
| Diabetes mellitus | 1.02 (0.99–1.05) | 0.152 | 1.03 (1.00–1.06) | 0.075 |
| Ischemic heart disease | 1.00 (0.97–1.04) | 0.857 | 0.99 (0.95–1.02) | 0.430 |
| Atrial fibrillation | 0.98 (0.93–1.02) | 0.321 | 0.97 (0.92–1.02) | 0.233 |
| Depression or use of antidepressants at baseline | 1.56 (1.50–1.63) | <0.001 | 1.38 (1.31–1.44) | <0.001 |
| Use of antipsychotics at baseline | 1.71 (1.61–1.82) | <0.001 | 1.40 (1.31–1.49) | <0.001 |
| Use of anxiolytics at baseline | 1.15 (1.11–1.18) | <0.001 | 1.00 (0.96–1.03) | 0.851 |
| Use of hypnotics at baseline | 1.33 (1.27–1.38) | <0.001 | 1.17 (1.11–1.24) | <0.001 |
| Use of antihypertensive drugs at baseline | 0.94 (0.91–0.97) | <0.001 | 0.90 (0.88–0.93) | <0.001 |
| Use of dyslipidemia drugs at baseline | 0.94 (0.91–0.96) | <0.001 | 0.92 (0.89–0.95) | <0.001 |
| Use of antithrombotics at baseline | 1.12 (1.09–1.15) | <0.001 | 1.06 (1.02–1.10) | 0.001 |
| 75–79 y | 80–84 y | ≥85 y | |
|---|---|---|---|
| Predictors | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age (per 1-year increase) | 1.14 (1.12–1.16) | 1.08 (1.06–1.10) | 0.99 (0.99–1.00) |
| Female | 1.19 (1.14–1.25) | 1.23 (1.17–1.29) | 1.12 (1.06–1.19) |
| Cerebrovascular disease | 1.25 (1.18–1.32) | 1.13 (1.08–1.19) | 1.06 (1.00–1.13) |
| Diabetes mellitus | 1.12 (1.06–1.17) | 0.98 (0.93–1.03) | 0.97 (0.91–1.03) |
| Ischemic heart disease | 0.99 (0.92–1.05) | 1.01 (0.95–1.07) | 0.96 (0.90–1.03) |
| Atrial fibrillation | 1.06 (0.97–1.16) | 0.95 (0.87–1.02) | 0.87 (0.79–0.96) |
| Depression or use of antidepressants at baseline | 1.47 (1.36–1.59) | 1.38 (1.28–1.49) | 1.21 (1.10–1.32) |
| Use of antipsychotics at baseline | 1.48 (1.33–1.64) | 1.40 (1.25–1.56) | 1.31 (1.15–1.49) |
| Use of anxiolytics at baseline | 1.03 (0.97–1.10) | 0.95 (0.89–1.01) | 0.97 (0.91–1.05) |
| Use of hypnotics at baseline | 1.17 (1.07–1.28) | 1.19 (1.10–1.30) | 1.16 (1.04–1.29) |
| Use of antihypertensive drugs at baseline | 0.89 (0.85–0.94) | 0.89 (0.85–0.93) | 0.90 (0.85–0.95) |
| Use of dyslipidemia drugs at baseline | 0.87 (0.83–0.92) | 0.92 (0.88–0.97) | 0.96 (0.91–1.02) |
| Use of antithrombotics at baseline | 1.09 (1.02–1.15) | 1.05 (0.99–1.11) | 1.01 (0.94–1.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakaoku, Y.; Takahashi, Y.; Tominari, S.; Nakayama, T. Predictors of New Dementia Diagnoses in Elderly Individuals: A Retrospective Cohort Study Based on Prefecture-Wide Claims Data in Japan. Int. J. Environ. Res. Public Health 2021, 18, 629. https://doi.org/10.3390/ijerph18020629
Nakaoku Y, Takahashi Y, Tominari S, Nakayama T. Predictors of New Dementia Diagnoses in Elderly Individuals: A Retrospective Cohort Study Based on Prefecture-Wide Claims Data in Japan. International Journal of Environmental Research and Public Health. 2021; 18(2):629. https://doi.org/10.3390/ijerph18020629
Chicago/Turabian StyleNakaoku, Yuriko, Yoshimitsu Takahashi, Shinjiro Tominari, and Takeo Nakayama. 2021. "Predictors of New Dementia Diagnoses in Elderly Individuals: A Retrospective Cohort Study Based on Prefecture-Wide Claims Data in Japan" International Journal of Environmental Research and Public Health 18, no. 2: 629. https://doi.org/10.3390/ijerph18020629
APA StyleNakaoku, Y., Takahashi, Y., Tominari, S., & Nakayama, T. (2021). Predictors of New Dementia Diagnoses in Elderly Individuals: A Retrospective Cohort Study Based on Prefecture-Wide Claims Data in Japan. International Journal of Environmental Research and Public Health, 18(2), 629. https://doi.org/10.3390/ijerph18020629




