Precision Mapping of COVID-19 Vulnerable Locales by Epidemiological and Socioeconomic Risk Factors, Developed Using South Korean Data
Abstract
1. Introduction
2. Materials and Methods
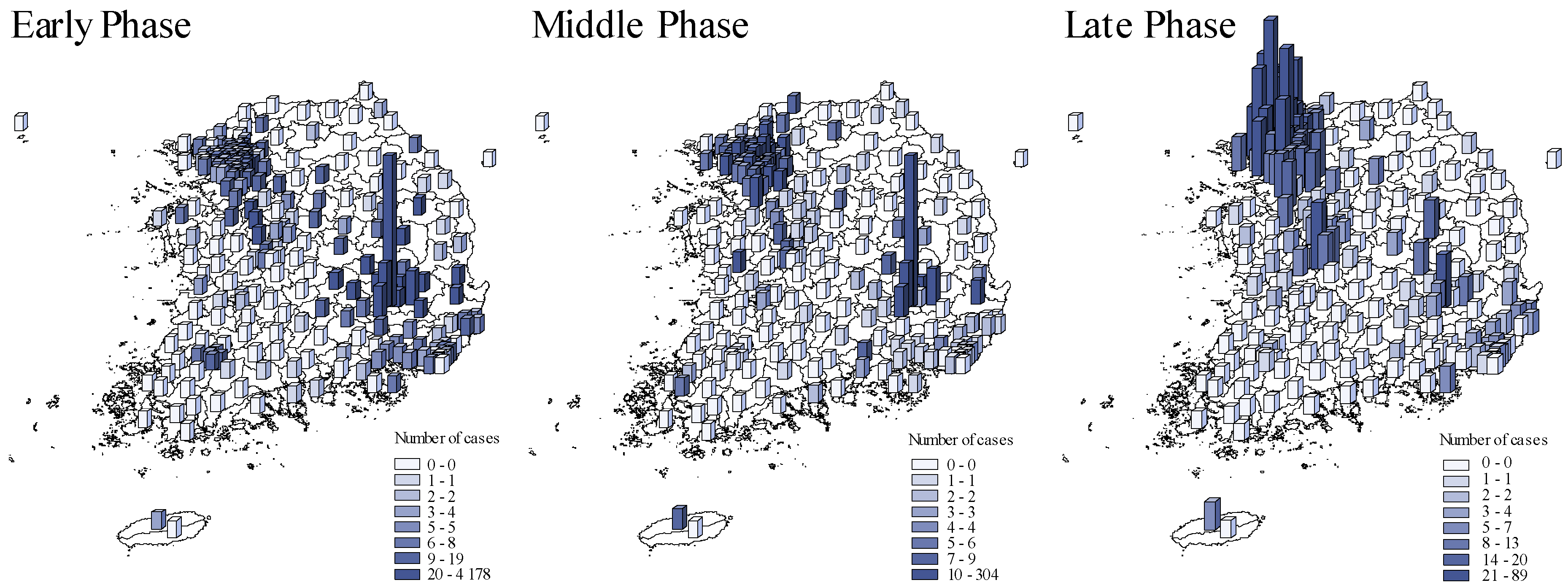
2.1. Study Design and Population
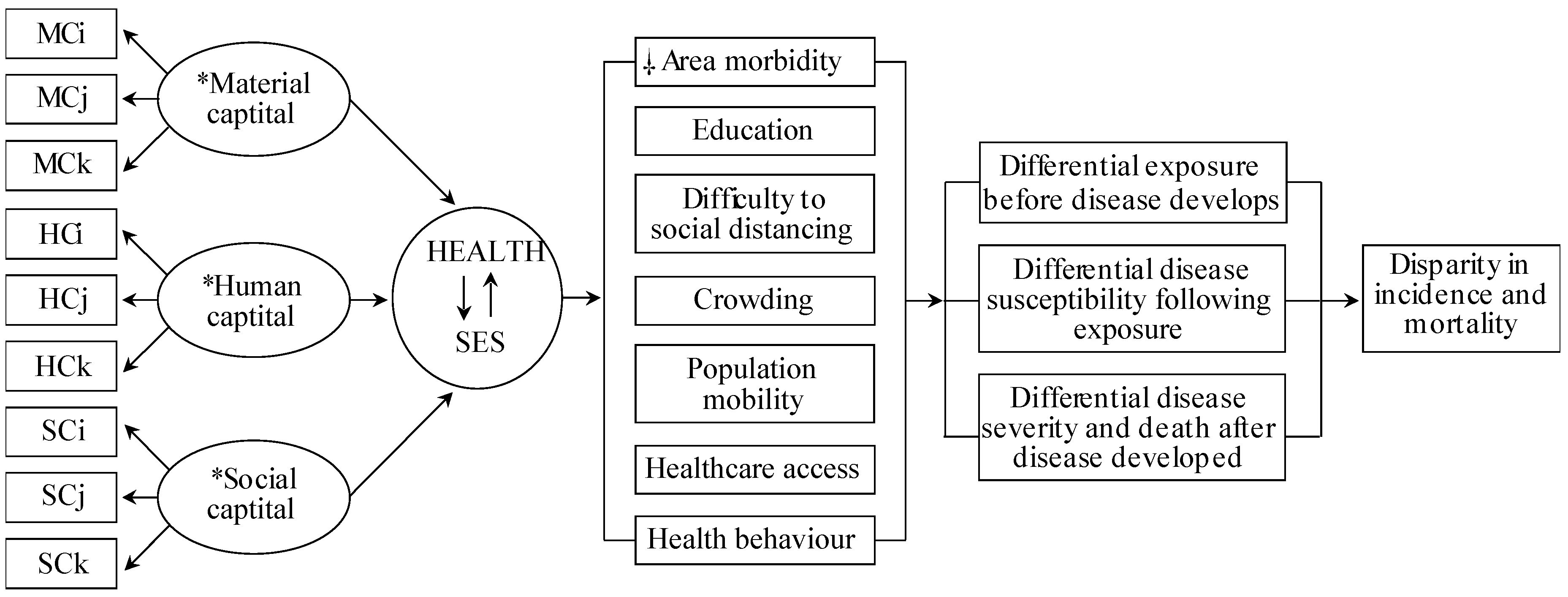
2.2. Conceptual Model
2.3. SES Measurement and Epidemiological Factors
The ratio of the 25th percentile and each theme’s maximum value was for healthcare access: 2.5; health behaviour: 1.2; crowding: 1.9; area morbidity: 1.3; education: 1.5; difficulty to social distancing: 3.0; and population mobility: 10.3.2.4. Statistics
2.4. Global Models
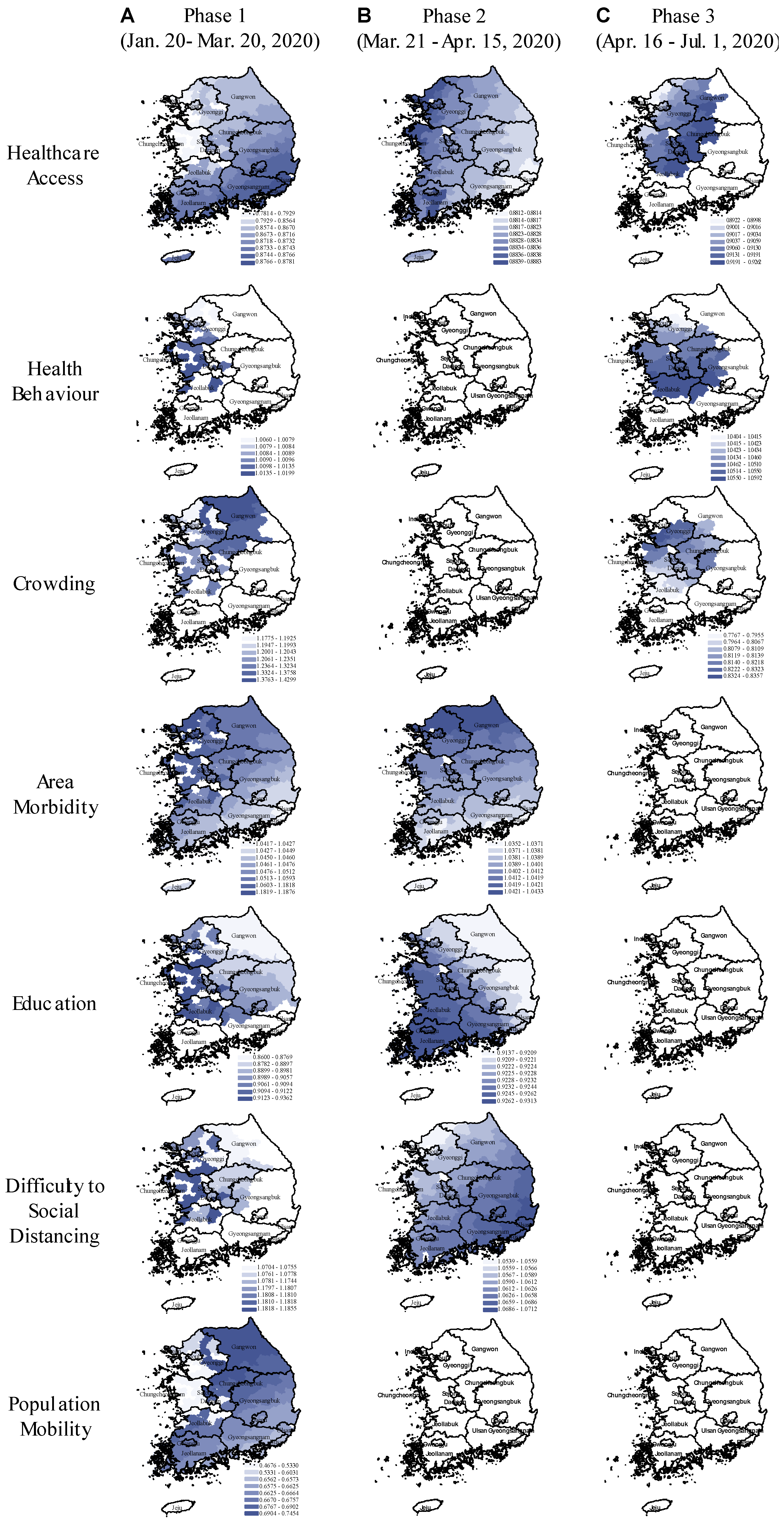
2.5. Local Spatial Models
3. Results
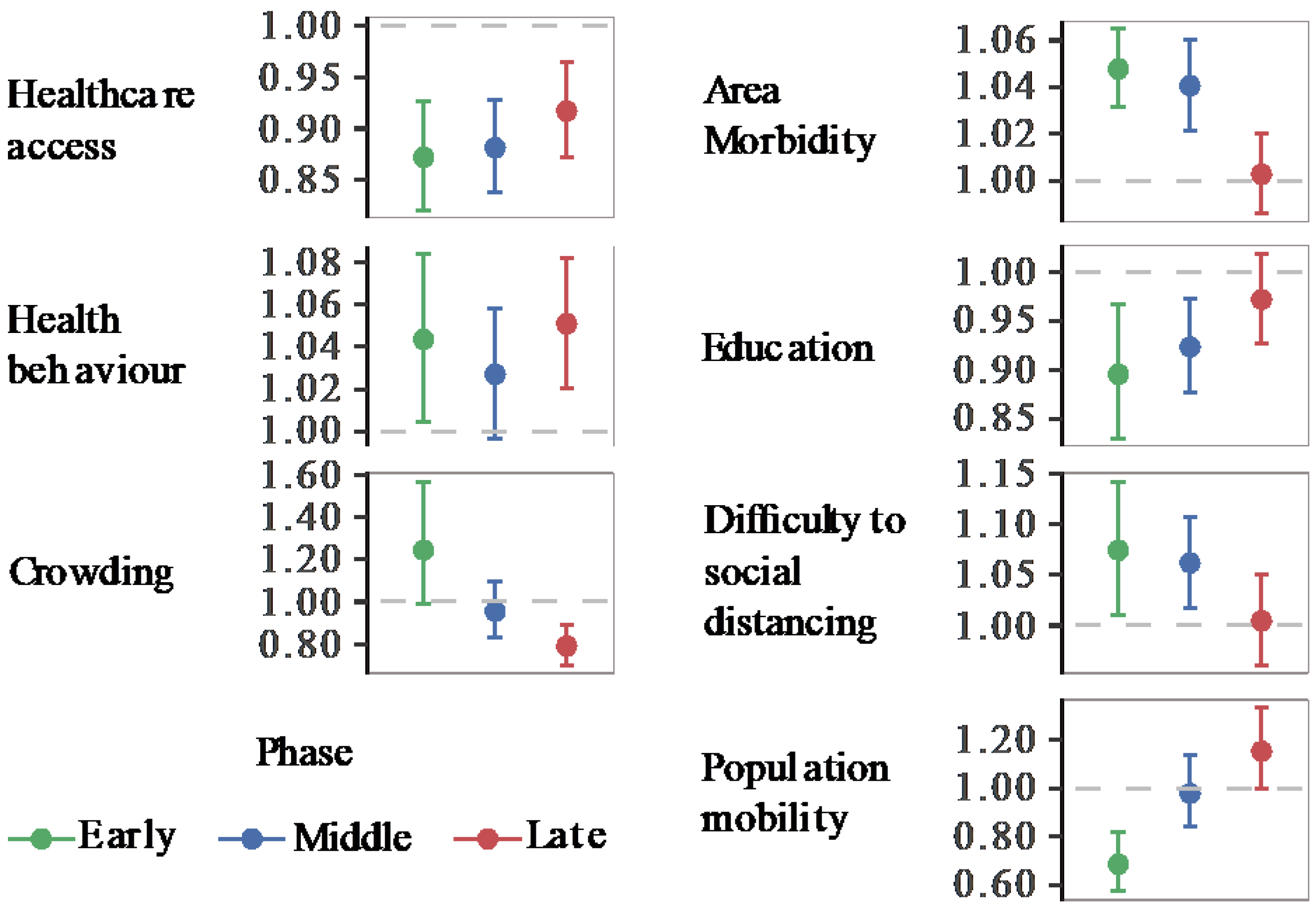
Global and Local Spatial Models
4. Discussion
5. Conclusions
- The completely remediated risk associated with area-morbidity and difficulty to social distancing is likely to be explained by the country’s emergency relief programs that targeted vulnerable individuals with socioeconomic disadvantages: Foreign workers, homeless, poor urban residents, disabled people, and elderly. The assistance programs provided free testing, financial support, food assistance, health check-up visits, as they acknowledged excess hardship in adhering to social distancing rules because of inability to afford unemployment.
- The observed overall protective effect of improved healthcare access and higher education in our study support the rationale behind the country’s primary anti-pandemic agenda to strengthen healthcare facilities for rapid diagnostic and therapeutic services, combined with actionable health promotion rules which reportedly gained high public compliance.
- However, we found risky health behaviour was a persistent risk factor during both major outbreaks in Daegu and Seoul. Elevated crowding associated risk coincided with the Seoul outbreak, as anticipated.
Supplementary Materials
Author Contributions
Funding
Exemption of Institutional Review Board and Ethics Committee Approval
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Lusignan, S.; Dorward, J.; Correa, A.; Jones, N.; Akinyemi, O.; Amirthalingam, G.; Andrews, N.; Byford, R.; Dabrera, G.; Elliot, A.; et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. Lancet Infect. Dis. 2020, 20, 1034–1042. [Google Scholar] [CrossRef]
- Quinn, S.C.; Kumar, S.; Freimuth, V.S.; Musa, D.; Casteneda-Angarita, N.; Kidwell, K. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am. J. Public Health 2011, 101, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S. Exploring the Determinants of Perceived Risk of Middle East Respiratory Syndrome (MERS) in Korea. Int. J. Environ. Res. Public Health 2018, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.A.; Krueger, A.B.; Steptoe, A.; Harter, J.K. The socioeconomic gradient in daily colds and influenza, headaches, and pain. Arch. Intern. Med. 2010, 170, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Manley, J.; Shrestha, V. Coronavirus Infections and Deaths by Poverty Status: The Effects of Social Distancing. J. Econ. Behav. Organ 2020. [Google Scholar] [CrossRef] [PubMed]
- Glanz, K.; Bishop, D.B. The role of behavioral science theory in development and implementation of public health interventions. Annu. Rev. Public Health 2010, 31, 399–418. [Google Scholar] [CrossRef]
- Zulman, D.M.; Vijan, S.; Omenn, G.S.; Hayward, R.A. The relative merits of population-based and targeted prevention strategies. Milbank Q. 2008, 86, 557–580. [Google Scholar] [CrossRef]
- Oakes, J.M.; Rossi, P.H. The measurement of SES in health research: Current practice and steps toward a new approach. Soc. Sci. Med. 2003, 56, 769–784. [Google Scholar] [CrossRef]
- Blumenshine, P.; Reingold, A.; Egerter, S.; Mockenhaupt, R.; Braveman, P.; Marks, J. Pandemic influenza planning in the United States from a health disparities perspective. Emerg Infect Dis 2008, 14, 709–715. [Google Scholar] [CrossRef]
- Jarman, B.; Townsend, P.; Carstairs, V. Deprivation indices. BMJ 1991, 303, 523. [Google Scholar] [CrossRef]
- Surgo Foundation, The COVID-19 Community Vulnerability Index (CCVI). 2020. Available online: https://precisionforcovid.org/ccvi (accessed on 15 September 2020).
- Mollalo, A.; Vahedi, B.; Rivera, K.M. GIS-based spatial modeling of COVID-19 incidence rate in the continental United States. Sci. Total Environ. 2020, 728, 138884. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.Q. On a Statistical Transmission Model in Analysis of the Early Phase of COVID-19 Outbreak. Stat. Biosci. 2020, 1–17. [Google Scholar] [CrossRef]
- da Silva, A.R.; Rodrigues, T.C.V. Geographically Weighted Negative Binomial Regression—incorporating overdispersion. Stat. Comput. 2014, 24, 769–783. [Google Scholar] [CrossRef]
- Ma, J. Estimating epidemic exponential growth rate and basic reproduction number. Infect. Dis. Model. 2020, 5, 129–141. [Google Scholar] [PubMed]
- Firth, J.A.; Hellewell, J.; Klepac, P.; Kissler, S.; Group, C.C.-W.; Kucharski, A.J.; Spurgin, L.G. Using a real-world network to model localized COVID-19 control strategies. Nat. Med. 2020, 26, 1616–1622. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention, Coronavirus Infectious Disease-19 Outbreak in Korea (Regular Briefing on July 1). 2020. Available online: http://ncov.mohw.go.kr/ (accessed on 1 September 2020).
- Kim, J. DS4C: Data Science for COVID-19 in South Korea. 2020. Available online: https://www.kaggle.com/kimjihoo/coronavirusdataset (accessed on 16 September 2020).
- Coleman, J.S. Foundations of Social Theory; Harvard University Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Covid-19 National Emergency Response Center; Case Management Team. Prevention, Coronavirus Disease-19: The First 7755 Cases in the Republic of Korea. Osong. Public Health Res. Perspect. 2020, 11, 85–90. [Google Scholar]
- Koh, D. Occupational risks for COVID-19 infection. Occup. Med. 2020, 70, 3–5. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.K.; Schwarz, D.; Ratcliffe, H.L.; Markuns, J.F.; Hirschhorn, L.R. National Response to COVID-19 in the Republic of Korea and Lessons Learned for Other Countries. Health Syst. Reform 2020, 6, e1753464. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Quinn, S.C.; Kumar, S. Health inequalities and infectious disease epidemics: A challenge for global health security. Biosecur. Bioterror. 2014, 12, 263–273. [Google Scholar] [CrossRef]
- Korea, S. KOSIS. KOrean Statistical Information System. Available online: http://kosis.kr/index/index.do (accessed on 15 August 2020).
- Wichern, R.A.J.D.W. Applied Multivariate Statistical Analysis, 4th ed.; Pearson: London, UK, 1998. [Google Scholar]
- da Silva, A.R.; Fotheringham, A.S. The Multiple Testing Issue in Geographically Weighted Regression. Geogr. Anal. 2016, 48, 233–247. [Google Scholar] [CrossRef]
- Her, M. How Is COVID-19 Affecting South Korea? What Is Our Current Strategy? Disaster. Med. Public Health Prep. 2020, 14, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ma, Z.; Peppelenbosch, M.P.; Pan, Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health 2020, 8, e480. [Google Scholar] [CrossRef]
- Bavel, J.J.V.; Baicker, K.; Boggio, P.S.; Capraro, V.; Cichocka, A.; Cikara, M.; Crockett, M.J.; Crum, A.J.; Douglas, K.M.; Druckman, J.N.; et al. Using social and behavioural science to support COVID-19 pandemic response. Nat. Hum. Behav. 2020, 4, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Bruine de Bruin, W.; Bennett, D. Relationships Between Initial COVID-19 Risk Perceptions and Protective Health Behaviors: A National Survey. Am. J. Prev. Med. 2020, 59, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.S.; Lu, X.; Yuan, Y.; Xu, G.; Jia, J.; Christakis, N.A. Population flow drives spatio-temporal distribution of COVID-19 in China. Nature 2020, 582, 389–394. [Google Scholar] [CrossRef]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020, 18, 20. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef]
- Tanne, J.H.; Hayasaki, E.; Zastrow, M.; Pulla, P.; Smith, P.; Rada, A.G. Covid-19: How doctors and healthcare systems are tackling coronavirus worldwide. BMJ 2020, 368, m1090. [Google Scholar] [CrossRef]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef]
- Lancet, T. Redefining vulnerability in the era of COVID-19. Lancet 2020, 395, 1089. [Google Scholar] [CrossRef]
- Omori, R.; Mizumoto, K.; Chowell, G. Changes in testing rates could mask the novel coronavirus disease (COVID-19) growth rate. Int. J. Infect. Dis. 2020, 94, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.E.; Rozhnova, G.; Bootsma, M.C.J.; van Boven, M.; van de Wijgert, J.; Bonten, M.J.M. Impact of delays on effectiveness of contact tracing strategies for COVID-19: A modelling study. Lancet Public Health 2020, 5, e452–e459. [Google Scholar] [CrossRef]
- Kinoshita, R.; Anzai, A.; Jung, S.M.; Linton, N.M.; Miyama, T.; Kobayashi, T.; Hayashi, K.; Suzuki, A.; Yang, Y.; Akhmetzhanov, A.R.; et al. Containment, Contact Tracing and Asymptomatic Transmission of Novel Coronavirus Disease (COVID-19): A Modelling Study. J. Clin. Med. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.A.; Satchell, L.P.; Fido, D.; Latzman, R.D. Functional Fear Predicts Public Health Compliance in the COVID-19 Pandemic. Int. J. Ment. Health Addict. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dryhurst, S.; Schneider, C.R.; Kerr, J.; Freeman, A.L.J.; Recchia, G.; van der Bles, A.M.; Spiegelhalter, D.; van der Linden, S. Risk perceptions of COVID-19 around the world. J. Risk Res. 2020, 23, 994–1006. [Google Scholar] [CrossRef]
- Wise, T.; Zbozinek, T.D.; Michelini, G.; Hagan, C.C.; Mobbs, D. Changes in risk perception and self-reported protective behaviour during the first week of the COVID-19 pandemic in the United States. R. Soc. Open Sci. 2020, 7, 200742. [Google Scholar] [CrossRef]
- Irigoyen-Camacho, M.E.; Velazquez-Alva, M.C.; Zepeda-Zepeda, M.A.; Cabrer-Rosales, M.F.; Lazarevich, I.; Castano-Seiquer, A. Effect of Income Level and Perception of Susceptibility and Severity of COVID-19 on Stay-at-Home Preventive Behavior in a Group of Older Adults in Mexico City. Int. J. Environ. Res. Public Health 2020, 17, 20. [Google Scholar] [CrossRef]
- Baldassarre, A.; Giorgi, G.; Alessio, F.; Lulli, L.G.; Arcangeli, G.; Mucci, N. Stigma and Discrimination (SAD) at the Time of the SARS-CoV-2 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 17. [Google Scholar] [CrossRef]
- Park, Y.J.; Choe, Y.J.; Park, O.; Park, S.Y.; Kim, Y.M.; Kim, J.; Kweon, S.; Woo, Y.; Gwack, J.; Kim, S.S.; et al. Contact Tracing during Coronavirus Disease Outbreak, South Korea, 2020. Emerg. Infect. Dis. 2020, 26, 2465–2468. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yuh, W.T.; Yang, J.M.; Cho, Y.S.; Yoo, I.K.; Koh, H.Y.; Marshall, D.; Oh, D.; Ha, E.K.; Han, M.Y.; et al. Nationwide Results of COVID-19 Contact Tracing in South Korea: Individual Participant Data From an Epidemiological Survey. JMIR Med. Inf. 2020, 8, e20992. [Google Scholar] [CrossRef] [PubMed]
- Logie, C.H.; Turan, J.M. How Do We Balance Tensions Between COVID-19 Public Health Responses and Stigma Mitigation? Learning from HIV Research. Aids Behav. 2020, 24, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 3 January 2021).
| Health/SE themes | Estimates | Relative Risk (95% CI) | p-Value |
|---|---|---|---|
| Healthcare access | −0.13 | 0.88 (0.84–0.92) | <0.0001 |
| Health behaviour | 0.04 | 1.04 (1.01–1.07) | 0.019 |
| Crowding | 0.05 | 1.05 (0.89–1.25) | 0.545 |
| Area morbidity | 0.04 | 1.04 (1.03–1.06) | <0.0001 |
| Education | −0.09 | 0.91 (0.86–0.97) | 0.002 |
| Difficulty to social distancing | 0.06 | 1.06 (1.01–1.12) | 0.017 |
| Population mobility | −0.22 | 0.80 (0.69–0.93) | 0.003 |
| Dispersion a | 2.49 | ||
| AIC | 1850 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinstein, B.; da Silva, A.R.; Kouzoukas, D.E.; Bose, T.; Kim, G.J.; Correa, P.A.; Pondugula, S.; Lee, Y.; Kim, J.; Carpenter, D.O. Precision Mapping of COVID-19 Vulnerable Locales by Epidemiological and Socioeconomic Risk Factors, Developed Using South Korean Data. Int. J. Environ. Res. Public Health 2021, 18, 604. https://doi.org/10.3390/ijerph18020604
Weinstein B, da Silva AR, Kouzoukas DE, Bose T, Kim GJ, Correa PA, Pondugula S, Lee Y, Kim J, Carpenter DO. Precision Mapping of COVID-19 Vulnerable Locales by Epidemiological and Socioeconomic Risk Factors, Developed Using South Korean Data. International Journal of Environmental Research and Public Health. 2021; 18(2):604. https://doi.org/10.3390/ijerph18020604
Chicago/Turabian StyleWeinstein, Bayarmagnai, Alan R. da Silva, Dimitrios E. Kouzoukas, Tanima Bose, Gwang Jin Kim, Paola A. Correa, Santhi Pondugula, YoonJung Lee, Jihoo Kim, and David O. Carpenter. 2021. "Precision Mapping of COVID-19 Vulnerable Locales by Epidemiological and Socioeconomic Risk Factors, Developed Using South Korean Data" International Journal of Environmental Research and Public Health 18, no. 2: 604. https://doi.org/10.3390/ijerph18020604
APA StyleWeinstein, B., da Silva, A. R., Kouzoukas, D. E., Bose, T., Kim, G. J., Correa, P. A., Pondugula, S., Lee, Y., Kim, J., & Carpenter, D. O. (2021). Precision Mapping of COVID-19 Vulnerable Locales by Epidemiological and Socioeconomic Risk Factors, Developed Using South Korean Data. International Journal of Environmental Research and Public Health, 18(2), 604. https://doi.org/10.3390/ijerph18020604









