In Vitro and In Vivo Evaluation of Nanostructured Biphasic Calcium Phosphate in Granules and Putty Configurations
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomaterial Composition
2.2. Physico-Chemical Characterization of Biomaterials
2.3. In Vitro Assay
2.4. In Vivo Analysis
2.4.1. Ethical Considerations
2.4.2. Animal Characterization and Location
2.4.3. Anesthesia and Surgery Procedures
2.4.4. Image Acquisition by Micro-CT and 3D Segmentation
2.4.5. Segmentation Protocol
2.4.6. Histological Processing
2.4.7. Histological and Histomorphometric Evaluation
2.4.8. Statistical Analysis
3. Results
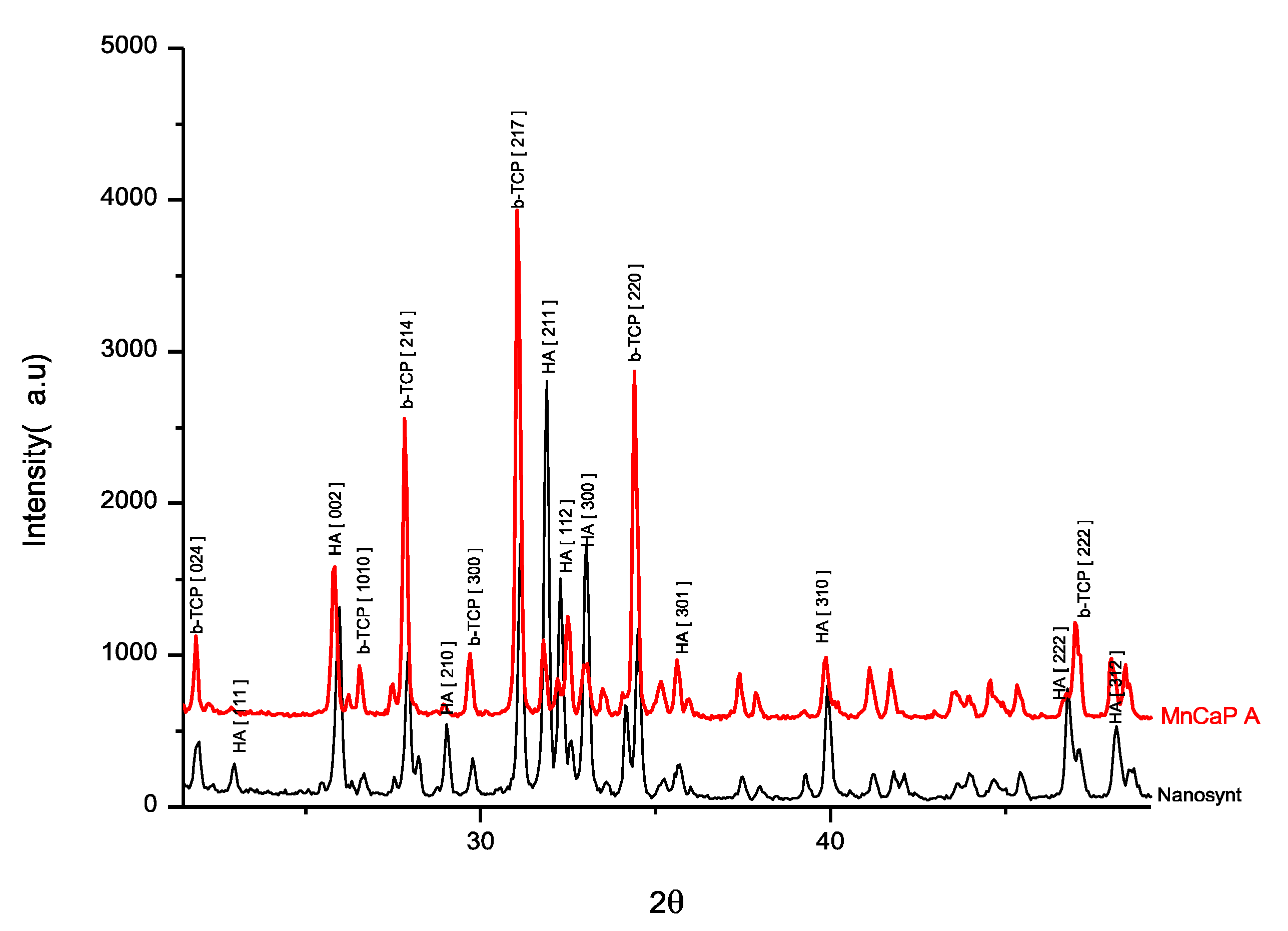
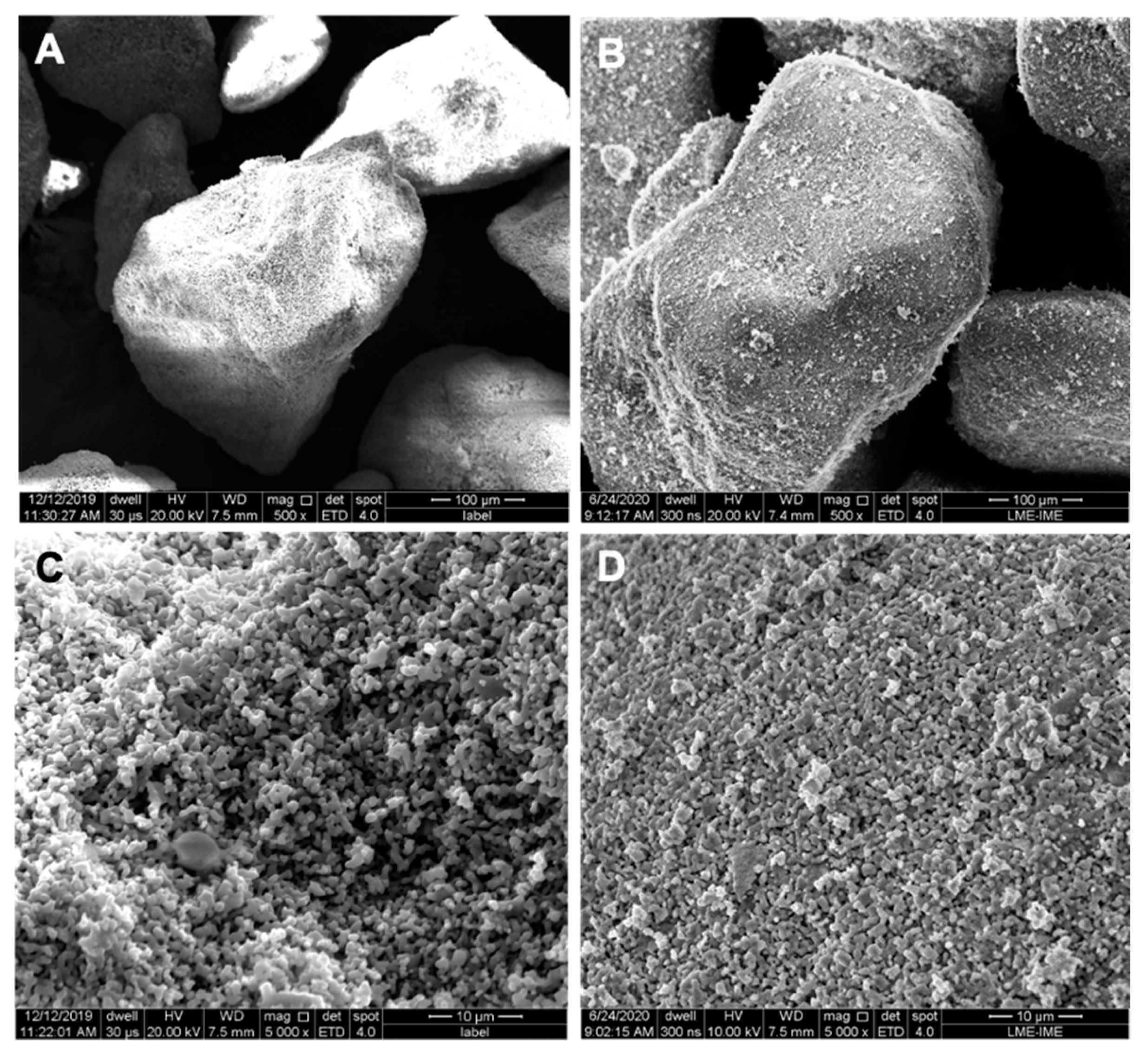
3.1. Biomaterial-Related Characterization
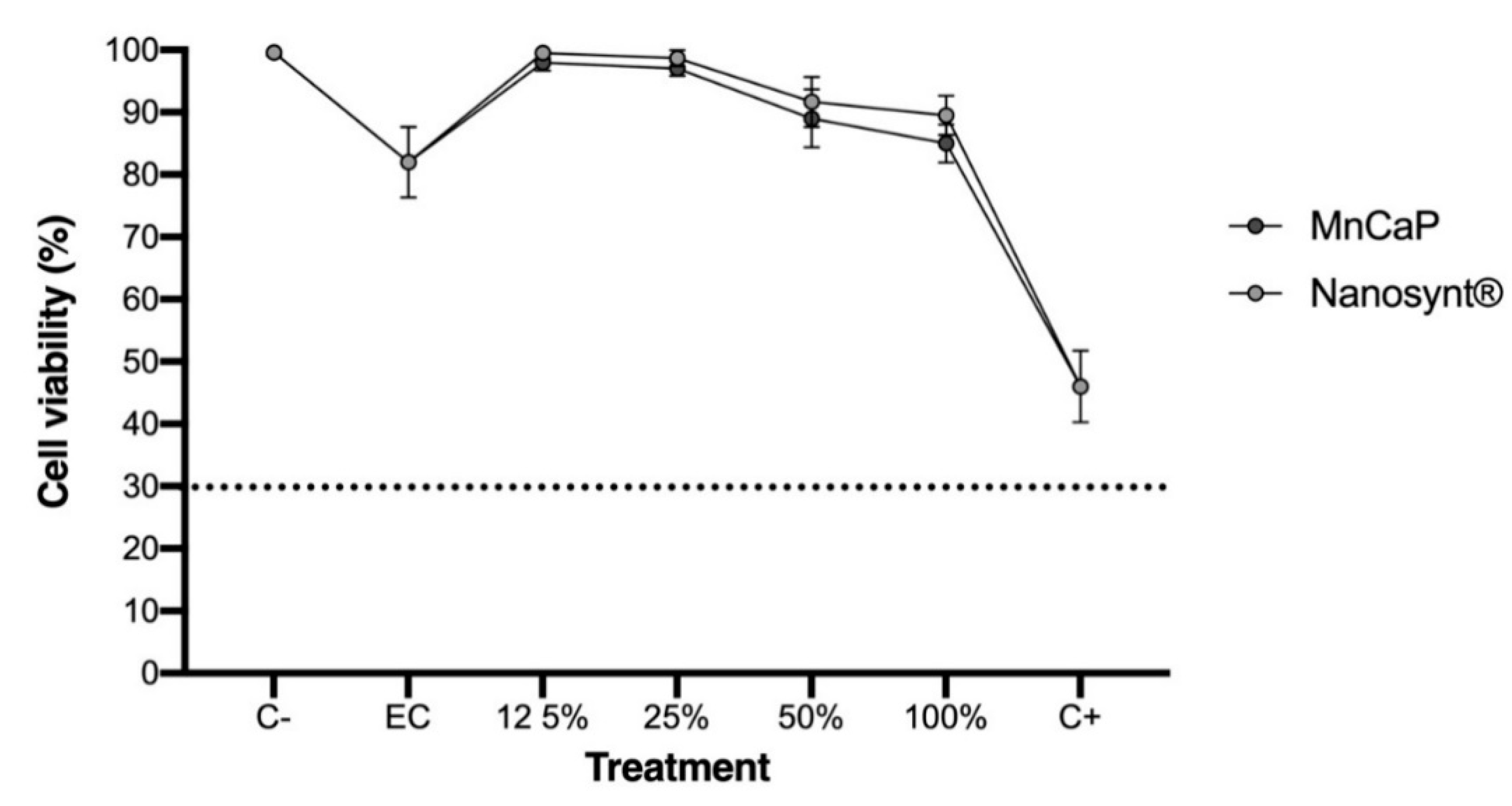
3.2. Cytocompatibility
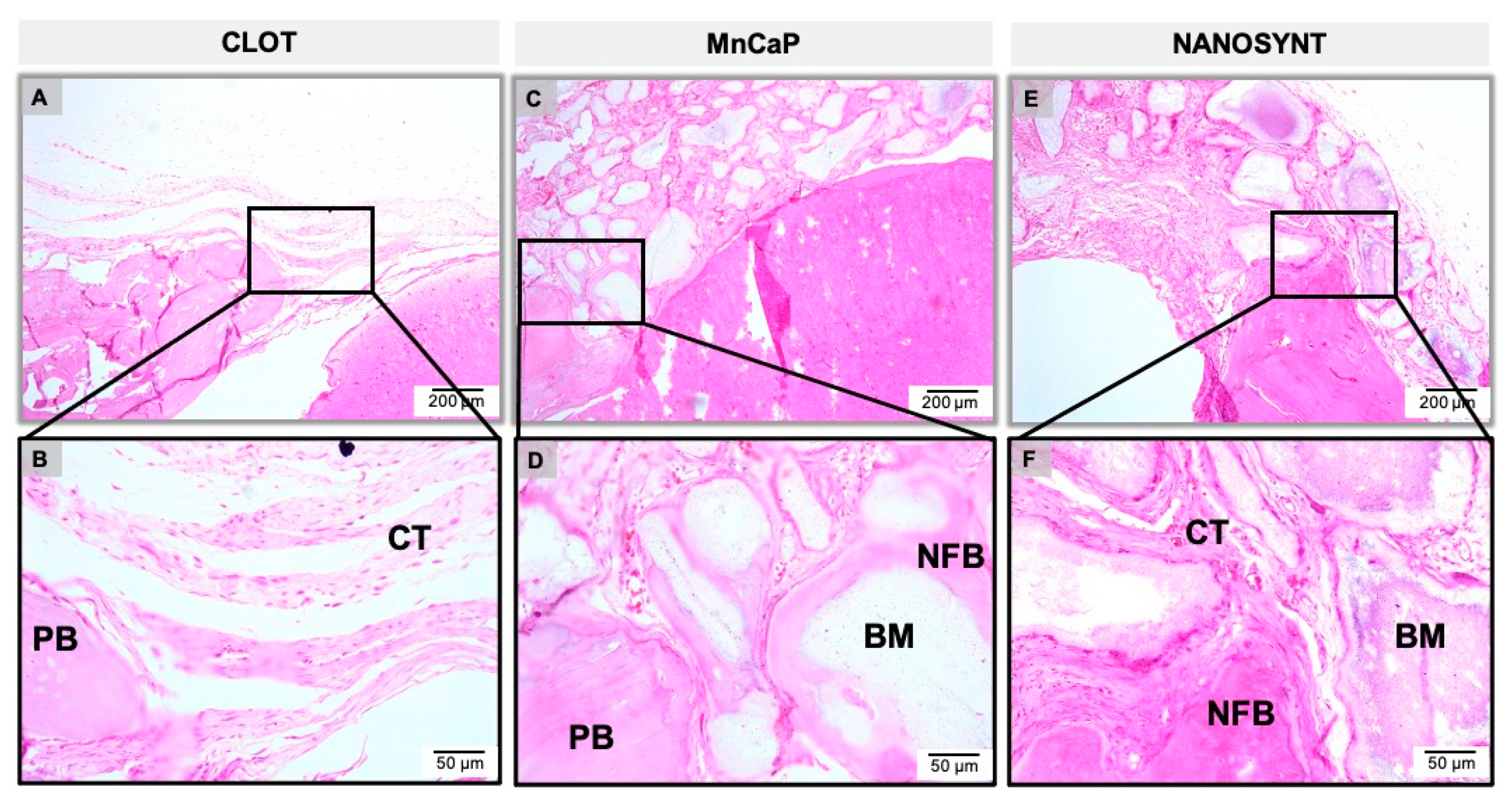
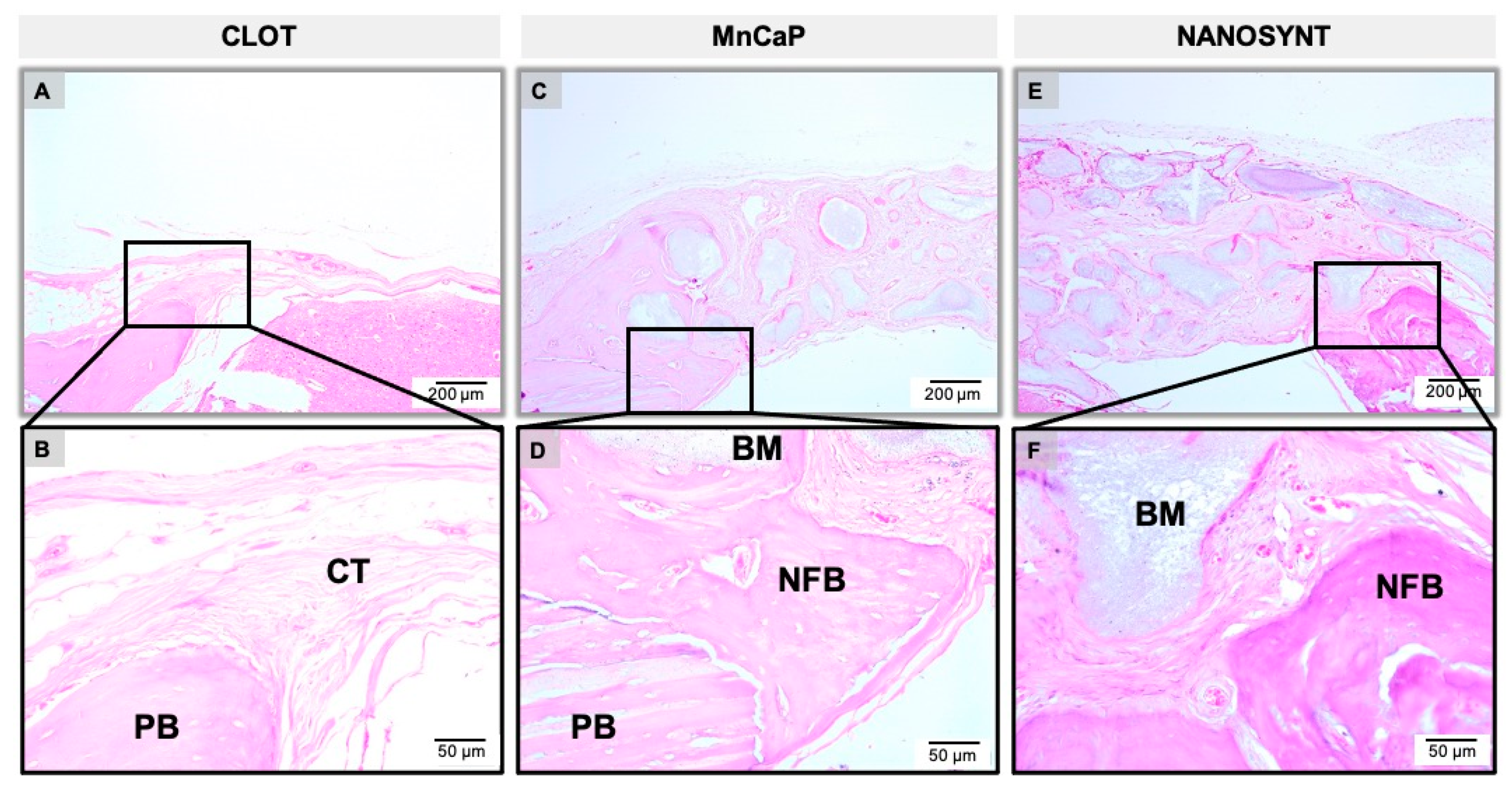
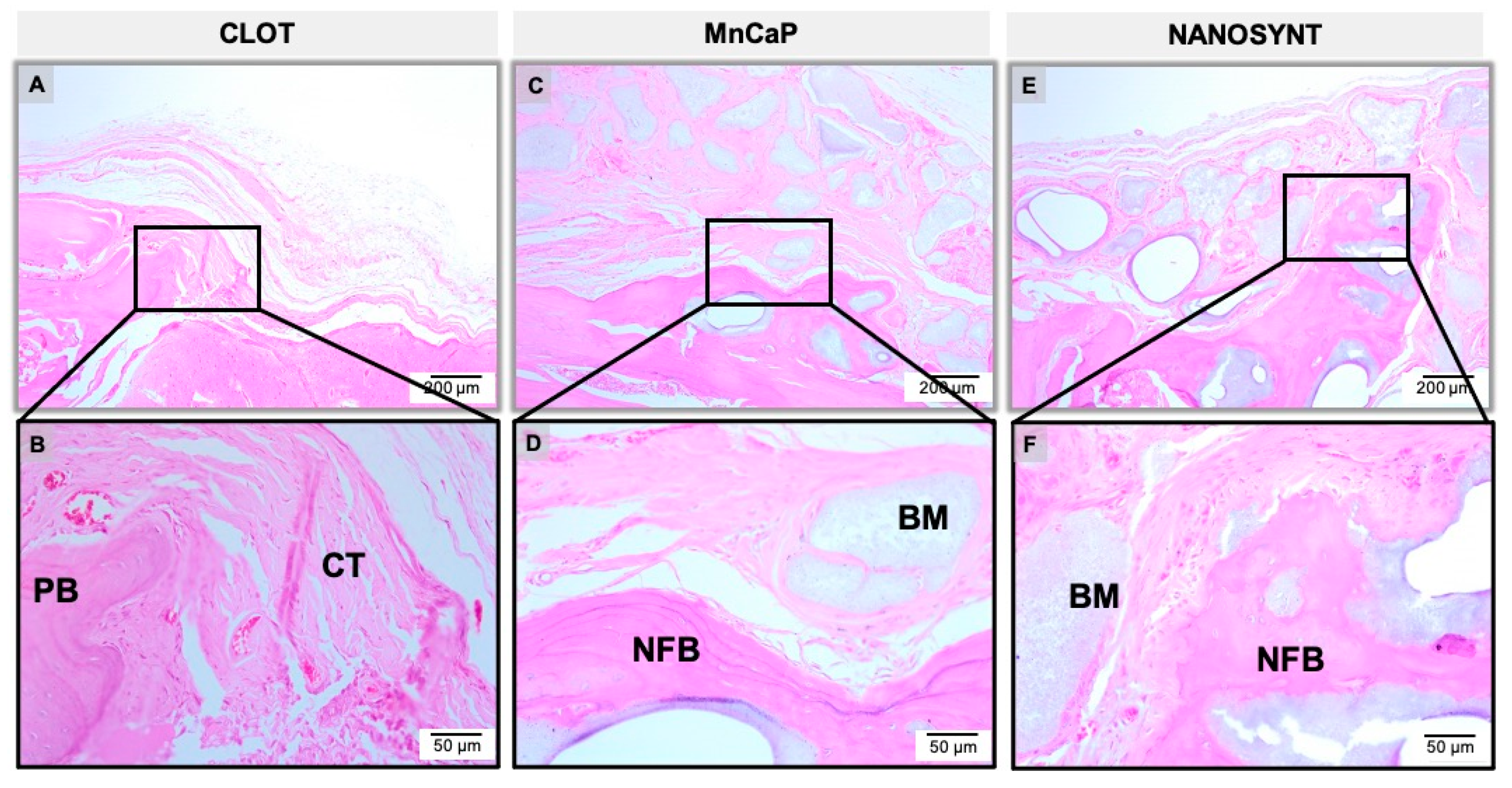
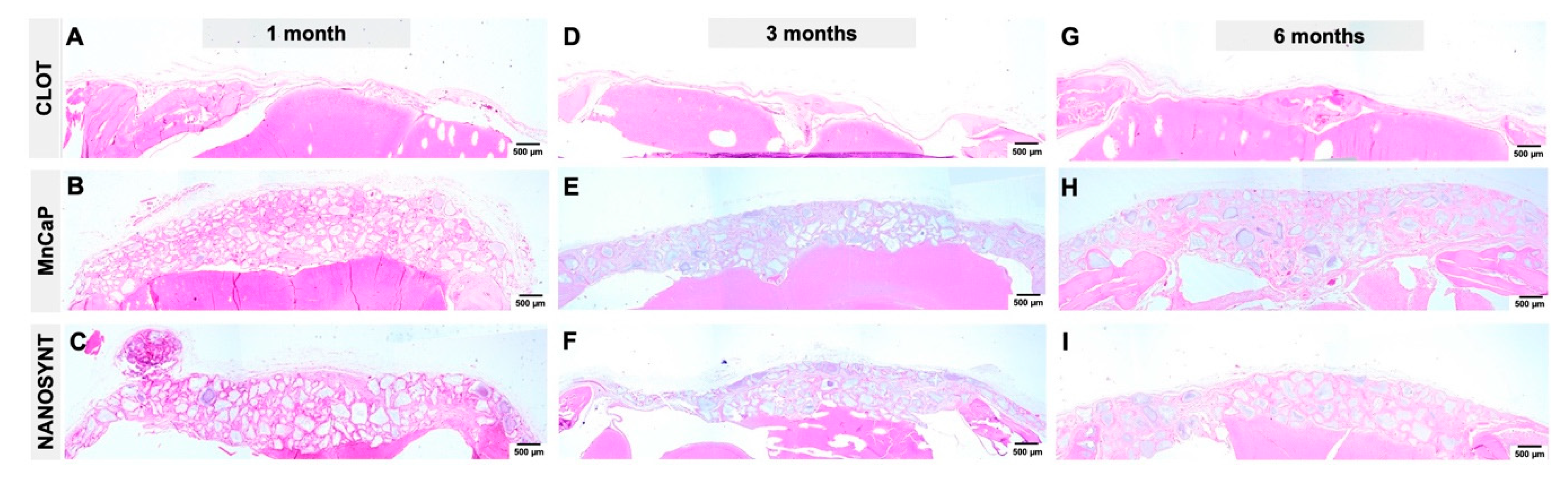
3.3. Descriptive Histology
3.3.1. One Month
3.3.2. Three Months
3.3.3. Six Months
3.4. Histomorphometric Results
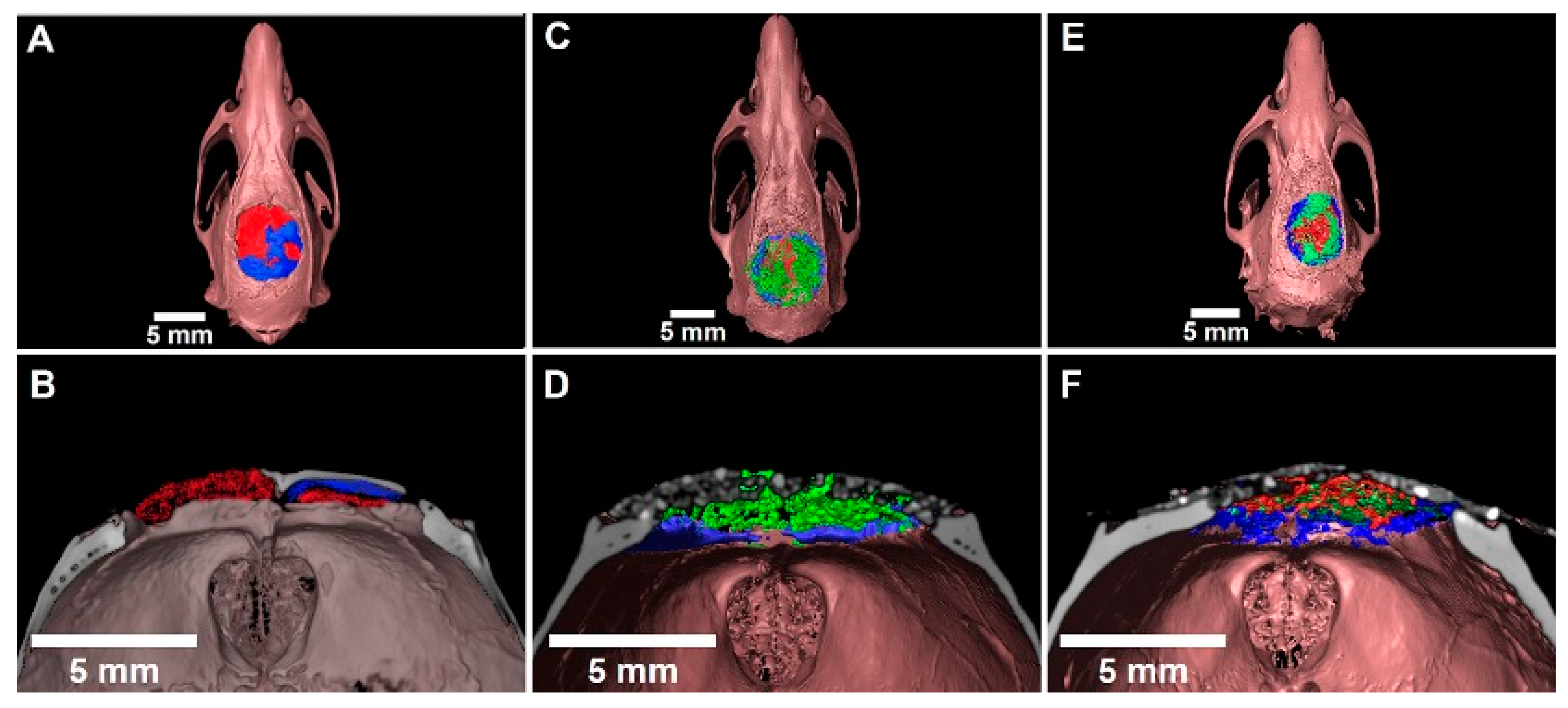
3.5. Three-Dimensional Evaluation by Micro-CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. J. Inj. 2005, 36, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Logeart-Avramoglou, D.; Anagnostou, F.; Bizios, R.; Petite, R. Engineering bone: Challenges and obstacles. J. Cell. Mol. Med. 2015, 9, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Miguel, F.M.; Cardoso, A.K.; Barbosa, A.A., Jr.; Marcantonio, E., Jr.; Goissis, G.; Rosa, F.P. Morphological assessment of the behavior of three-dimensional anionic collagen matrices in bone regeneration in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Miguel, F.B.; Ade, A.B., Jr.; Paula, F.L.; Barreto, I.C.; Goissis, G.; Rosa, F.P. Regeneration of critical bone defects with anionic collagen matrix as scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Costa Mendes, L.; Sauvigné, T.; Guiol, J. Morbidity of autologous bone harvesting in implantology: Literature review from 1990 to 2015. Rev. Stomatol. Chir. Maxillofac. Chir. Orale 2016, 117, 388–402. [Google Scholar]
- Jakoi, A.M.; Iorio, J.A.; Cahill, P.J. Autologous bone graft harvesting: A review of grafts and surgical techniques. Musculoskelet. Surg. 2015, 99, 171–178. [Google Scholar] [CrossRef]
- Mehta, S.; Blagg, R.; Willcockson, J.; Gociman, B.; Yamashiro, D.; Siddiqi, F. Cost-Effectiveness Analysis of Demineralized Bone Matrix and rhBMP-2 versus Autologous Iliac Crest Bone Grafting in Alveolar Cleft Patients. Plast. Reconstr. Surg. 2018, 142, 737–743. [Google Scholar] [CrossRef]
- Azi, M.L.; Aprato, A.; Santi, I.; Kfuri, M., Jr.; Masse, A.; Joeris, A. Autologous bone graft in the treatment of post-traumatic bone defects: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2016, 17, 465. [Google Scholar] [CrossRef]
- Macedo, R.M.; Lacerda, S.A.; Thomazini, J.A.; Brentegani, L.G. Bone integration behavior of hydroxyapatite/b-tricalcium phosphate graft implanted in dental alveoli: A histomorphometric and scanning electron microscopy study. Implant Dent. 2014, 23, 710–715. [Google Scholar] [CrossRef]
- Leventis, M.D.; Fairbairn, P.; Kakar, A.; Leventis, A.D.; Margaritis, V.; Lückerath, W.; Horowitz, R.A.; Rao, B.H.; Lindner, A.; Nagursky, H. Minimally invasive alveolar ridge preservation utilizing an in situ hardening b-tricalcium phosphate bone substitute: A multicenter case series. Int. J. Dent. 2016, 2016, 5406736. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, 9–19. [Google Scholar] [CrossRef]
- Schmidt, L.D.; Hadad, H.; Vasconcelos, I.R.; Colombo, L.T.; Silva, R.C.; Santos, A.F.P.; Cervantes, L.C.C.; Poli, P.P.; Signorino, F.; Maiorana, C.; et al. Critical defect healing assessment in rat calvaria filled with injectable calcium phosphate cement. J. Funct. Biomater. 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Valiense, H.; Barreto, M.; Resende, R.F.; Alves, A.T.; Rossi, A.M.; Mavropoulos, E.; Granjeiro, J.M.; Calasans-Maia, M.D. In vitro and in vivo evaluation of strontium-containig nanostructured carbonated hydroxyapatite/sodium alginate for sinus lift in rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.F.; Fernandes, G.V.; Santos, S.R.; Rossi, A.M.; Lima, I.; Granjeiro, J.M.; Calasans-Maia, M.D. Long-term biocompatibility evaluation of 0.5% zinc containing hydroxyapatite in rabbits. J. Mater. Sci. Mater. Med. 2013, 24, 1455–1463. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Harel, N.; Moses, O.; Palti, A.; Ormianer, Z. Long term results of implants immediately placed into extraction sockets grafed with β-tricalcium phosphate: A retrospective study. J. Oral Maxillofac. Surg. 2013, 71, e63–e68. [Google Scholar] [CrossRef]
- Murakami, S.; Miyaji, H.; Nishida, E.; Kawamoto, K.; Miyata, S.; Takita, H.; Akasaka, T.; Fugetsu, B.; Iwanaga, T.; Hongo, H.; et al. Dose effects of beta-tricalcium phosphate nanoparticles on biocompatibility and bone conductive ability of three-dimensional collagen scaffolds. Dent. Mater. J. 2017, 36, 573–583. [Google Scholar] [CrossRef]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation on hydroxyapatite and biphasic calcium phosphate ceramics: A case report. Int. J. Periodontics Dent. Restaurador. 1986, 6, 22–33. [Google Scholar]
- Dalcusi, G.; Legeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biophasic calcim phosfate ceramics in vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar]
- Uzeda, M.J.; de Brito Resende, R.F.; Sartoretto, S.C.; Alves, A.T.N.N.; Granjeiro, J.M.; Calasans-Maia, M.D. Randomized clinical trial for the biological evaluation of two nanostructured biphasic calcium phosphate biomaterials as a bone substitute. Clin. Implant Dent. Relat. Res. 2017, 19, 802–811. [Google Scholar] [CrossRef]
- Kim, S.-S.; Park, M.S.; Jeon, O.; Choi, C.Y.; Kim, B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Maté Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Gomez-Moreno, G.; Martınez, C.P.-A.; Mazon, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part A: Synthesis, characterization of the materials, and SEM analysis. Clin. Oral Implants Res. 2016, 27, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed]
- Maté Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Gomez-Moreno, G.; Gehrke, S.; Mazon, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity, and crystallinity on tissue reaction in vivo. Part B: A comparative study with biphasic synthetic biomaterials. Clin. Oral Implants Res. 2018, 29, 1077–1084. [Google Scholar] [CrossRef]
- Calasans-Maia, M.D.; Melo, B.R.; Alves, A.T.N.N.; Resende, R.F.B.R.; Louro, R.S.L.; Sartoretto, S.C.; Granjeiro, J.M. Cytocompatibility and biocompatibility of nanostructured carbonated hydroxyapatite spheres for bone repair. J. Appl. Oral Sci. 2015, 23, 599–608. [Google Scholar] [CrossRef]
- Sartoretto, S.; Gemini-Piperni, S.; Silva, R.A.; Calasans, M.D.; Rucci, N.; Santos, T.M.P.; Lima, I.B.C.; Rossi, A.M.; Alves, G.G.; Granjeiro, J.M.; et al. Apoptosis-associated speck-like preotein containing a caspase-1 recruitment domain (ASC) contributes to osteoblast differentiation and osteogenesis. J. Cell Physiol. 2019, 234, 4140–4153. [Google Scholar] [CrossRef]
- Jones, A.C.; Milthorpe, B.; Averdunk, H.; Limayer, A.; Sendent, T.J.; Sakellariou, A. Analysis of 3D bone ingrowth into polymer scaffolds via micro-computer tomography imaging. Biomaterials 2004, 25, 4947–4954. [Google Scholar] [CrossRef]
- Gauthier, O.; Müller, R.; Von Stechow, D.; Lamy, B.; Weiss, P.; Bouler, J.M.; Aguado, E.; Daculsi, G. In vivo regeneration with injectable calcium phosphate biomaterial: A three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials 2005, 26, 5444–5453. [Google Scholar] [CrossRef]
- Kilkenny, C.; Brown, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Di Chiro, G. Beam hardening in X-ray reconstructive tomography. Phys. Med. Biol. 1976, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Manjón, J.V.; Carbonell-Caballero, J.; Lull, J.J.; García-Martí, G.; Martí-Bonmatí, L.; Robles, M. MRI denoising using non-local means. Med. Image Anal. 2008, 12, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Bleau, A.; Leon, L.J. Watershed-based segmentation and region merging. Comput. Vis. Image Underst. 2000, 77, 317–370. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Calasans-Maia, M.D.; Alves, A.T.N.N.; Resende, R.F.B.; Fernandes, C.J.C.; Padilha, P.M.; Rossi, A.M.; Teti, A.; Granjeiro, J.M.; Zambuzzi, W.F. The role of apoptosis associated speck-like protein containing a caspase-1 recruitment domain (ASC) in response to bone substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110965. [Google Scholar] [CrossRef] [PubMed]
- Bohning, B.P.; Davenport, W.D.; Jeansonne, B.G. The effect of guided tissue regeneration on the healing of osseous defect in rat calvaria. J. Endod. 1999, 25, 81–84. [Google Scholar] [CrossRef]
- Bosch, C.; Melsen, B.; Vargervik, K. Importance of the critical-size bone defect in testing bone-regenerating materials. J. Craniofac. Surg. 1998, 9, 310–316. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for cranio-mandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar]
- Luvizuto, E.R.; Tangl, S.; Zanoni, G.; Okamoto, T.; Sonada, C.K.; Gruber, R.; Okamoto, R. The effect of BMP-2 on the osteoconductive properties of β-tricalcium phosphate in rat calvaria defects. Biomaterials 2011, 32, 3855–3861. [Google Scholar] [CrossRef]
- Martinez-Zelaya, V.R.; Zarranz, L.; Herrera, H.Z.; Alves, A.T.; Uzeda, M.J.; Mavropoulos, H.; Mello, A.; Granjeiro, J.M.; Calasans-Maia, M.D.; Rossi, A.M. In vitro and in vivo evaluations of nanocrystalline Zn-doped carbonated hydroxyapatite/alginate microspheres: Zinc and calcium bioavailability and bone regeneration. Int. J. Nanomed. 2019, 10, 3471–3490. [Google Scholar] [CrossRef] [PubMed]
- Cuozzo, R.C.; Sartoretto, S.C.; Resende, R.F.B.; Alves, A.T.N.N.; Mavropoulos, E.; Silva, M.H.P.; Calasans-Maia, M.D. Biological evaluation of zinc-containing calcium alginate-hydroxyapatite composite microspheres for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Souza, C.A.; Rossi, A.L.; Mavropoulos, E.; Hausen, M.A.; Tanaka, M.N.; Calasans-Maia, M.D.; Granjeiro, J.M.; Rocha-Leão, M.H.M.; Rossi, A.M. Chlorexidine-loaded hydroxyapatite microspheres as an antimicrobial delivery system and its effect on in vivo osteo-conductive properties. J. Mater. Sci. Mater. Med. 2015, 26, 166. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Seo, Y.S.; Lee, G.J.; You, J.S.; Klim, S.G. A Comparative Study with Biphasic Calcium Phosphate to Deproteinized Bovine Bone in Maxillary Sinus Augmentation: A Prospective Randomized and Controlled Clinical Trial. Int. J. Oral Maxillofac. Implants 2019, 34, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Schimidlin, P.R.; Nicholls, F.; Kruse, A.; Zwahlen, R.A.; Weber, F.E. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin. Oral Implants Res. 2013, 24, 149–157. [Google Scholar] [CrossRef]
- Lindgren, C.; Hallman, M.; Sennerby, L.; Sammons, R. Back-scattered electron imaging and elemental analysis of retrieved bone tissue following sinus augmentation with deproteinized bovine bone or biphasic calcium phosphate. Clin. Oral Implants Res. 2010, 21, 924–930. [Google Scholar] [CrossRef]
- Levengood, S.L.; Murphy, W.L. Biomaterials for high-throughput stem cell culture. Curr. Stem Cell Res. Ther. 2010, 5, 261–267. [Google Scholar] [CrossRef]
- Fellah, B.H.; Weiss, P.; Gauthier, O.; Rouillon, T.; Pilet, P.; Daculsi, G.; Layrolle, P. Bone repair using a new injectable self-crosslinkable bone substitute. J. Orthop. Res. 2006, 24, 628–635. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, J.R.B.; Sartoretto, S.C.; Alves, A.T.N.N.; Mourão, C.F.A.B.; Martinez-Zelaya, V.R.; Uzeda, M.J.; Granjeiro, J.M.; Montemezzi, P.; Calasans-Maia, M.D.; Calasans-Maia, J.A. In Vitro and In Vivo Evaluation of Nanostructured Biphasic Calcium Phosphate in Granules and Putty Configurations. Int. J. Environ. Res. Public Health 2021, 18, 533. https://doi.org/10.3390/ijerph18020533
Nascimento JRB, Sartoretto SC, Alves ATNN, Mourão CFAB, Martinez-Zelaya VR, Uzeda MJ, Granjeiro JM, Montemezzi P, Calasans-Maia MD, Calasans-Maia JA. In Vitro and In Vivo Evaluation of Nanostructured Biphasic Calcium Phosphate in Granules and Putty Configurations. International Journal of Environmental Research and Public Health. 2021; 18(2):533. https://doi.org/10.3390/ijerph18020533
Chicago/Turabian StyleNascimento, Jhonathan R. B., Suelen C. Sartoretto, Adriana T. N. N. Alves, Carlos F. A. B. Mourão, Victor R. Martinez-Zelaya, Marcelo J. Uzeda, José M. Granjeiro, Pietro Montemezzi, Monica D. Calasans-Maia, and José A. Calasans-Maia. 2021. "In Vitro and In Vivo Evaluation of Nanostructured Biphasic Calcium Phosphate in Granules and Putty Configurations" International Journal of Environmental Research and Public Health 18, no. 2: 533. https://doi.org/10.3390/ijerph18020533
APA StyleNascimento, J. R. B., Sartoretto, S. C., Alves, A. T. N. N., Mourão, C. F. A. B., Martinez-Zelaya, V. R., Uzeda, M. J., Granjeiro, J. M., Montemezzi, P., Calasans-Maia, M. D., & Calasans-Maia, J. A. (2021). In Vitro and In Vivo Evaluation of Nanostructured Biphasic Calcium Phosphate in Granules and Putty Configurations. International Journal of Environmental Research and Public Health, 18(2), 533. https://doi.org/10.3390/ijerph18020533






