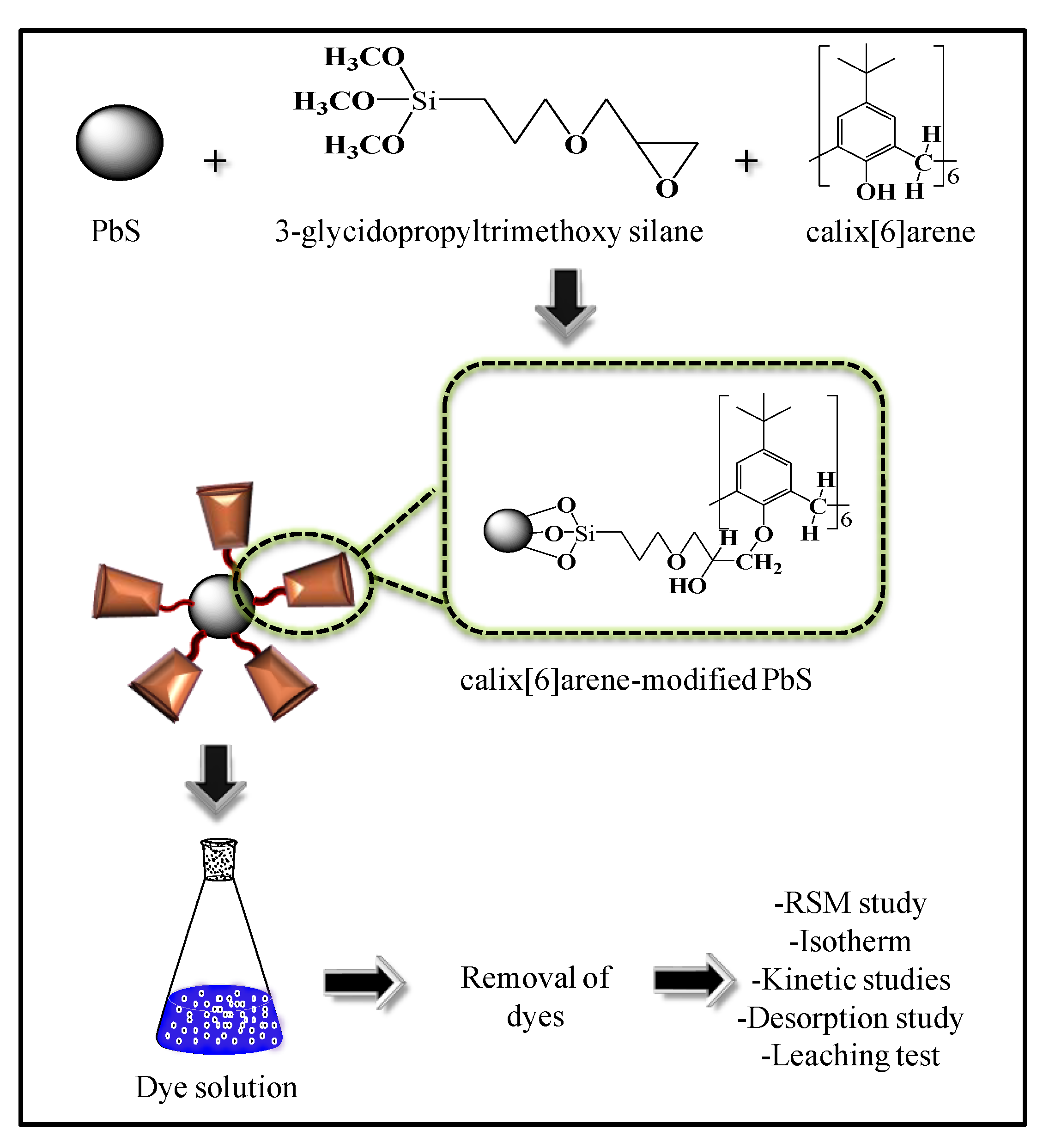
1. Introduction
Globally, various industries such as textile, printing, and leather industries have contributed to major sources of coloured wastewater that can cause severe water pollution [
1,
2]. More than 7 × 10
5 tons/year of dyes produced using toxic, nonbiodegradable, mutagenic, and carcinogenic materials are reportedly released into the environment in wastewater without any proper treatment. Surprisingly, most of the dyes can be present in water at very minute concentrations (~1 ppm), which is sufficient to cause an aesthetic problem [
3,
4]. Methylene blue (MB) is one of the common aromatic–cationic dyes that are widely used in various applications, including cell staining, antimicrobial chemotherapy, phototherapy, and cancer research [
5,
6,
7,
8]. Although MB is useful in medical applications, the exposure to MB in large doses (>7.0 mg/kg) can cause harm to humans, such as skin damage, a burning sensation in the eyes, high blood pressure, digestive disease, mental confusion, nausea, abdominal pain, and methemoglobinemia [
9]. Therefore, the removal of MB dye from wastewater is a critical step prior to its release into the environment.
Many technological innovations and techniques have been introduced to tackle worldwide water pollution challenges. Some common techniques are flocculation–coagulation, membrane separation, chemical and electrochemical oxidation, biodegradation, and adsorption [
10,
11,
12,
13,
14,
15]. Among those aforementioned techniques, adsorption is a compelling choice for the removal of dyes due to its multiple benefits, such as simplicity in design and operation, efficiency in treatment, low cost, good applicability, and high adsorption capacity [
16,
17]. Additionally, adsorption has been established as one of the top control techniques adopted by the United States Environmental Protection Agency (USEPA) [
18].
Nanomaterials are now overtaking other types of adsorbents. They have fascinated scientists and been extensively explored to improve the efficiency and adsorption capacities of contaminant removal from wastewater. It is an ideal adsorbent due a variety of factors, such as high surface area, low cost, ready availability, and faster adsorption. Some established adsorbents in the dye adsorption process are carbon nanotubes (CNTs), graphene, ferric oxide (Fe
3O
4), titanium oxide (TiO
2), magnesium oxide (MgO), manganese oxide (MnO), and zinc oxide (ZnO) [
19,
20,
21,
22]. Besides, metal sulphide-loaded nanomaterials (e.g., activated carbon) are also gaining attention as an adsorbent is due to their efficiency in wastewater treatment. However, only a few studies have explored metal sulphide modification as an alternative adsorbent in wastewater treatment systems [
23]. For example, Jeong et al. synthesised a nanocrystalline mackinawite (FeS) for the removal of mercuric ions [
24]. To the best of the authors’ knowledge, no study has yet analysed lead sulphide (PbS) as an adsorbent in the removal of MB dye.
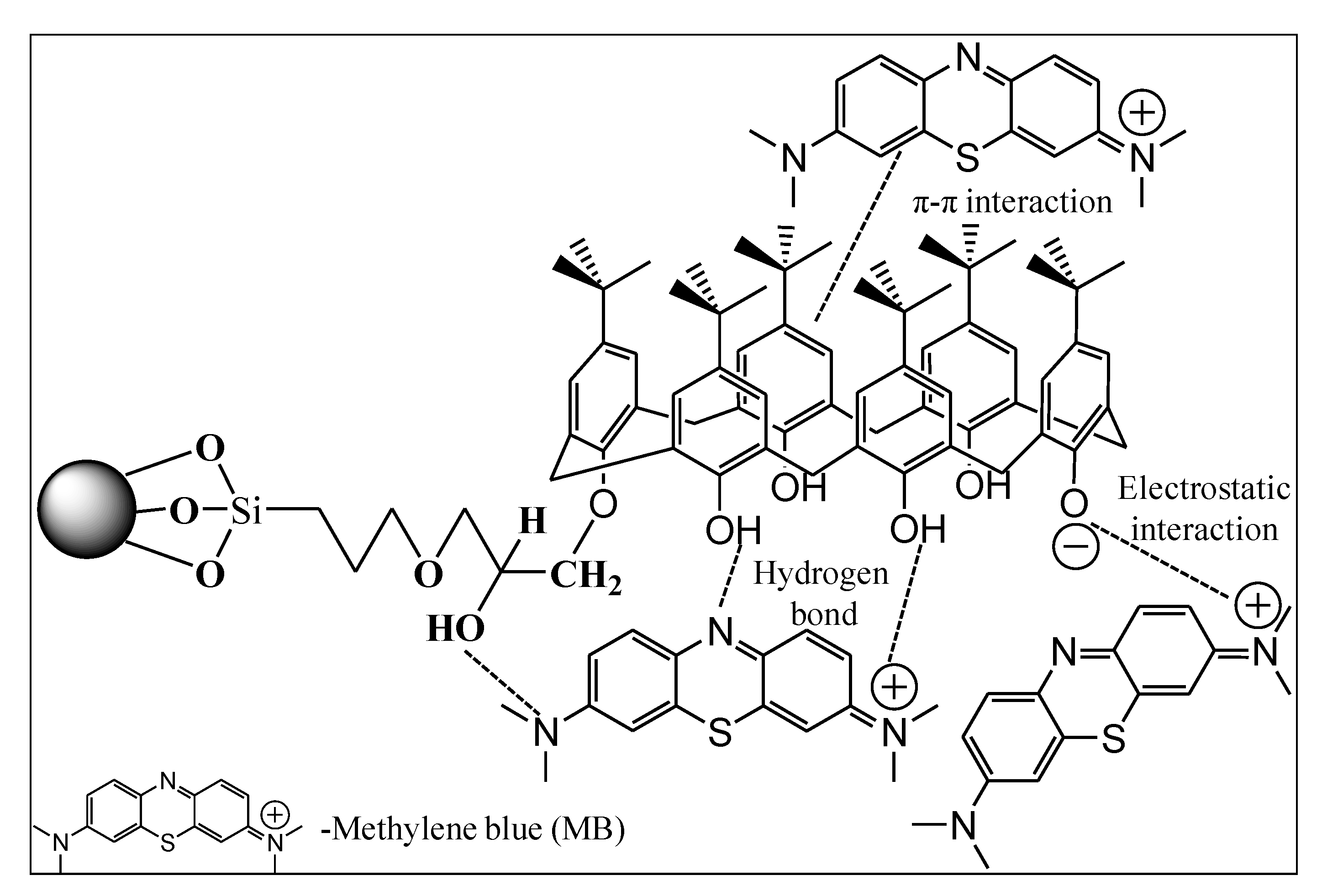
Calix[n]arenes are third-generation host molecules of multifunctionalisable macromolecules after crownethers and cyclodextrins. They are formed from intrinsic pores and synthesised from formaldehyde and para-substituted phenols. They have basket-shaped hollow cavity confirmations with different ring sizes depending on the synthetic condition. Typically, these macromolecules possess a hydrophilic lower rim, mainly due to the presence of phenolic functions. On the other side, aromatic functions and aliphatic side groups that surround a hollow cavity with many dimensions depend on the specific phenolic units incorporated, which form a hydrophobic upper rim [
25]. Calix[n]arene’s unique confirmations and complexation properties lend well in studies of synthetic, natural hosts, and the water treatment industry [
26,
27].
The efficiency of wastewater contaminant removal is influenced by several process variables. The traditional one-parameter-at-a-time approach is employed to optimise these variables. However, the main drawback of the traditional approach is that only one parameter is varied at a time, while the interactive effects among variables are not studied. Moreover, many experiments must be done using this approach, making it time-consuming and costly. Therefore, the experimental design must account for the maximum adsorption of the water effluents, helping both to reduce the cost as well as reduce the consumption of valuable resources such as energy and materials. Response surface methodology (RSM) can be carried out to optimise the effective variables while keeping a minimum number of experiments and considering the mutual interaction of the factors and their individual effects. Previous studies have demonstrated the use of RSM modelling for the adsorption of water effluents, including heavy metals, dyes, and pharmaceutical waste, with promising results [
28,
29].
According to the authors’ knowledge, there is no comprehensive analysis on lead sulphide (PbS) with the host molecule, calixarene, as an adsorbent for the removal of dye wastewater. Although previous works on metal sulphide adsorbents have effectiveness in the removal of pollutants, none has described the interactive effects between the factors studied [
23,
24]. Indeed, the present work of metal sulphide in terms of analysis and optimisation is a novel and a new approach as compared to previous studies. This research article investigates calix[6]arene-modified PbS as an alternative nanoadsorbent for the removal of methylene blue (MB) dye. A statistical technique, RSM, is introduced to assess the actions of various independent factors on the system response. Independent variables are performed to optimise the MB adsorption to observe the adsorption capacity.
Figure 1 shows the overall procedure for this study.
2. Experimental Works
2.1. Materials and Methods
Lead (II) acetate (Pb(CH3COO)2, purity, 99%), sodium hydroxide (NaOH), potassium hydroxide (KOH), hydrogen chloride (HCl, purity, 37%), toluene (C6H5CH3), triethylamine N(CH2CH3)3, hydrogen nitrate (HNO3, purity, 69%), ethanol (C2H5OH, purity, 95%), sodium bicarbonate (Na2CO3), sodium sulphide (Na2S, purity, 99%), and methylene blue (MB, purity, 99%) were purchased from R & M chemicals (Semenyih, Malaysia). Dithioglycerol (DTG), thioglycerol (TGL), and 3-glyxidoxypropyltrimethoxy silane (C9H20O5Si, purity, 98%) were procured from Sigma-Aldrich (Gillingham, UK). Potassium nitrate (KNO3) and p-tert-butyl-calix[6]arene (C66H84O6, purity, 96%) were obtained from HmbG chemicals (Hamrburg, Germany) and Alfa Aesar (Tewksbury, MA, USA), respectively.
2.2. Synthesis of Calix[6]Arene-Modified Lead Sulphide(Pbs)
The lead sulphide (PbS) nanoparticles were prepared based on a previous procedure, with slight modifications [
30]. The precipitant of PbS was collected by mixing the solution in ethanol at a 3:1 ratio and followed by drying in an oven for 2 h at 80 °C. calix[6]arene-modified PbS was further modified by mixing 3-glycidoxypropyltrimethoxy silane (4.18 mmol),
p-tert-butyl-calix[6]arene (3.03 mmol), and 0.2 g lead sulphide (PbS) nanoparticles with 3 drops of the catalyst, triethylamine, in 10-mL dry toluene (dried before using molecular sieves). The solution was stirred at room temperature for 6 h in an inert nitrogen gas atmosphere. The calix[6]arene-modified PbS was centrifuged and washed with toluene, ethanol, and distilled water, in that order. Lastly, the calix[6]arene-modified PbS was collected after drying at 80 °C for 3 h.
2.3. Characterisations
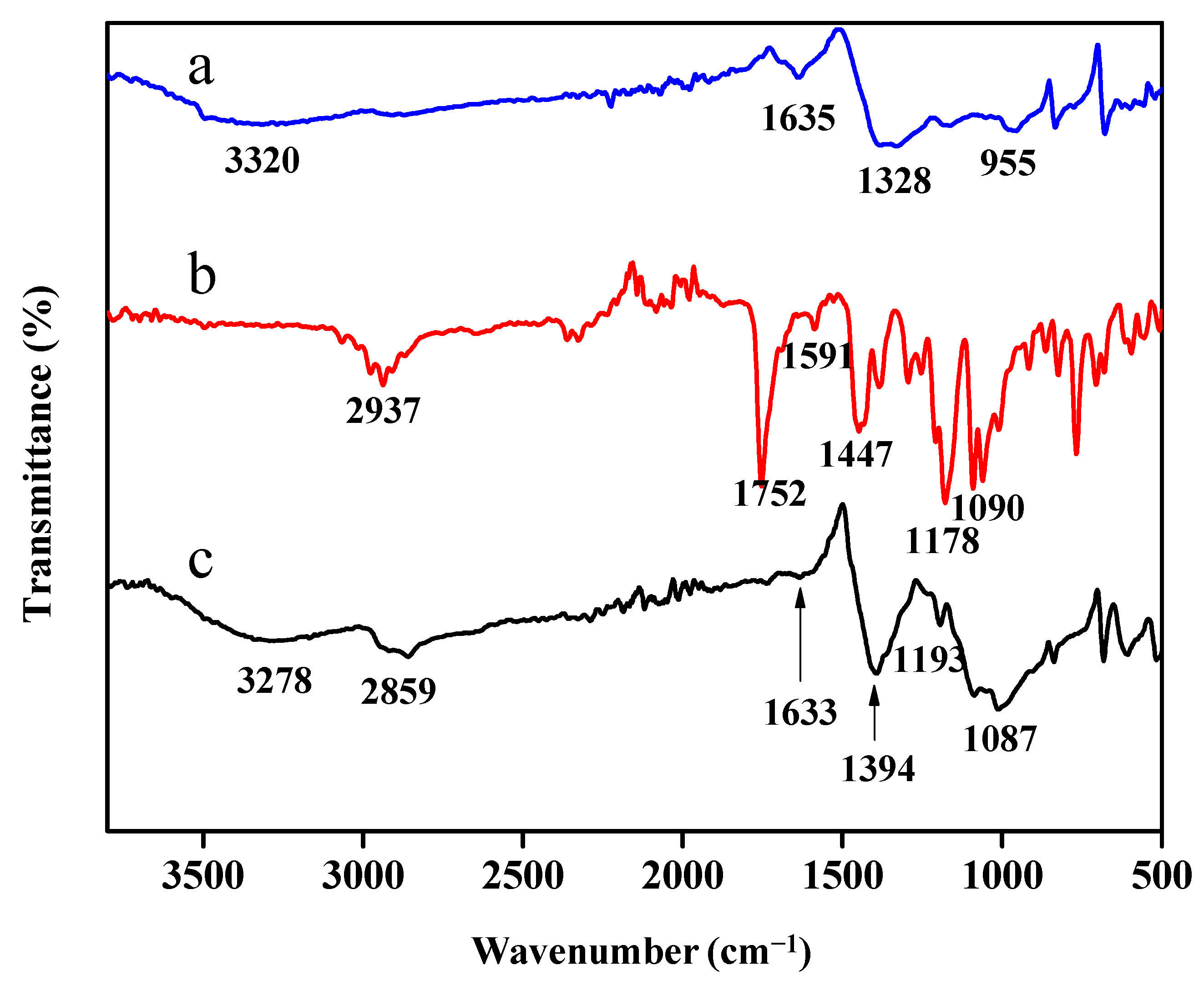
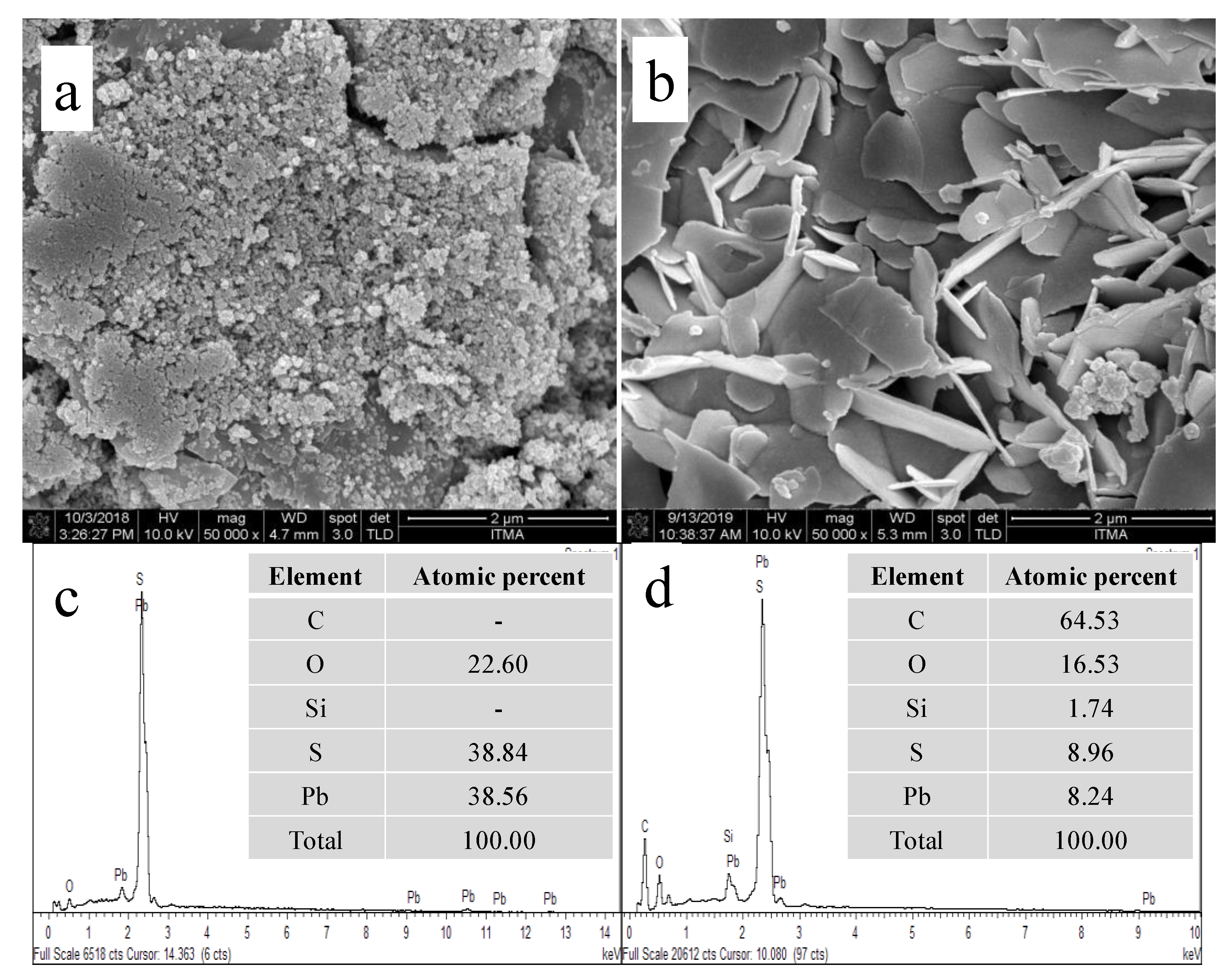
Fourier-transform infrared (FTIR) (Perkin-Elmer, Waltham, MA, USA) was used to examine the chemical functional groups of calix[6]arene-modified PbS. The samples were scanned within the region of 4000–400 cm−1 at room temperature. The surface morphology of the synthesised unmodified PbS and calix[6]arene-modified PbS were evaluated via field emission scanning electron microscopy (FESEM, Quanta 400F) operated in low vacuum. The elements present in calix[6]arene were determined via FESEM coupled with energy-dispersive X-ray (EDX) spectroscopy.
2.4. Determination of the Point of Zero Charge (PZC)
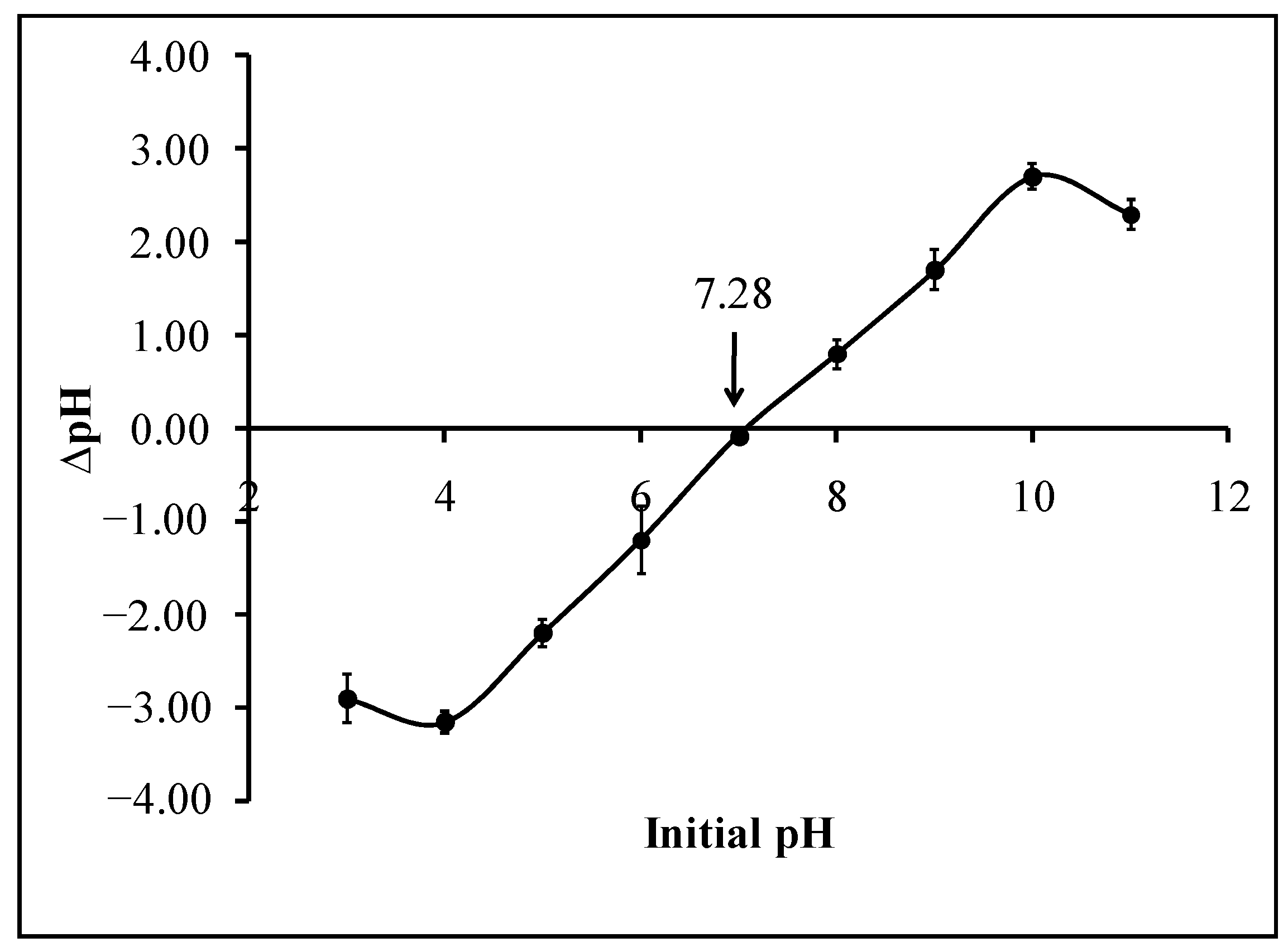
Briefly, 0.10 g of calix[6]arene-modified PbS was added to several conical flasks containing 50.0 mL of 0.01-mol/L KNO3 solution. The initial pH values were adjusted between pH 3 and pH 11. The pH adjustment was made using 0.10-mol/L KOH and 0.10-mol/L HNO3 solutions. All conical flasks were shaken for 48 h under atmospheric conditions. After centrifugation of the samples, the final pH was monitored using a pH meter (Sedolis Sartorius, Goettingen, Germany). The graph of ∆pH versus the initial pH was plotted, and the initial pH value at which ∆pH became zero was considered as the PZC of the calix[6]arene-modified PbS.
2.5. Adsorption Studies
The adsorption potential of calix[6]arene-modified PbS towards MB dye was determined via batch adsorption experiments. In the experiments, a standard MB dye solution of 1000 mg/L was prepared without any purification. The required dye solution concentration was achieved by diluting the stock solution appropriately with deionised water. Then, 0.1-mol/L NaOH or 0.1-mol/L HCl was used to adjust the pH of the solution. The adsorption experiments were executed in a conical flask containing 10.0-mL MB dye solution of specific concentrations. The mixture was agitated in a laboratory shaker at 150 rpm and at a contact time of 60 min. The final concentration of the sample residual dye was determined using a UV–Vis spectrophotometer (Perkin-Elmer Lambda 35, Woburn, MA, USA) at a maximum wavelength of 664 nm. The percentage removal of MB was calculated using the following Equation (1):
where
Ci (mg/L) is the initial concentration of MB solution before adsorption, and
Cf (mg/L) is the final concentration after the MB dye adsorption.
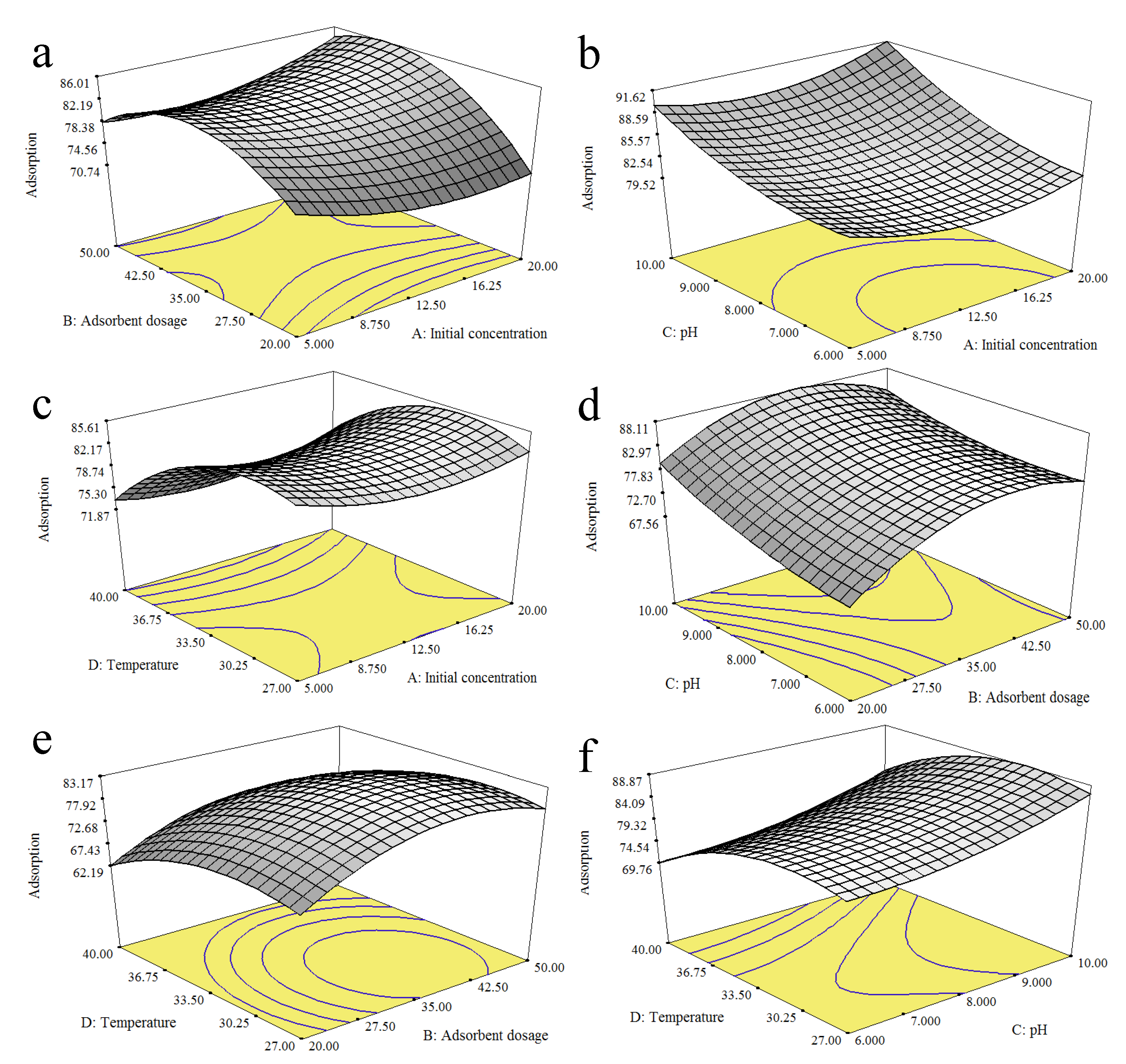
The impacts of various factors, such as the initial concentration (5–20 mg/L), adsorbent dosage (20.00–50.00 mg), initial pH (pH 3–pH 11), and temperature (27–40 °C), were examined. At the end of the experiment, the sample solution was centrifuged for 20 min at 2500 rpm.
2.6. Response Surface Methodology (RSM)
Response surface methodology (RSM) uses experimental designs to optimise the influence of interactive effects on the dependent variable (response). Central composite design (CCD) (Design-Expert Software version 6.0.6) is an appropriate RSM design for this research, because it recognises the effects of some independent variables on the MB adsorption of calix[6]arene-modified PbS. The MB adsorption response data from the suggested experiments was generated via the second-order polynomial equations represented by Equation (2):
where
Y is an objective to optimise the response,
is the constant coefficient,
is the linear coefficient,
is the quadratic coefficient, and
is the interaction coefficient, while
and
represent the coded values of the independent factors.
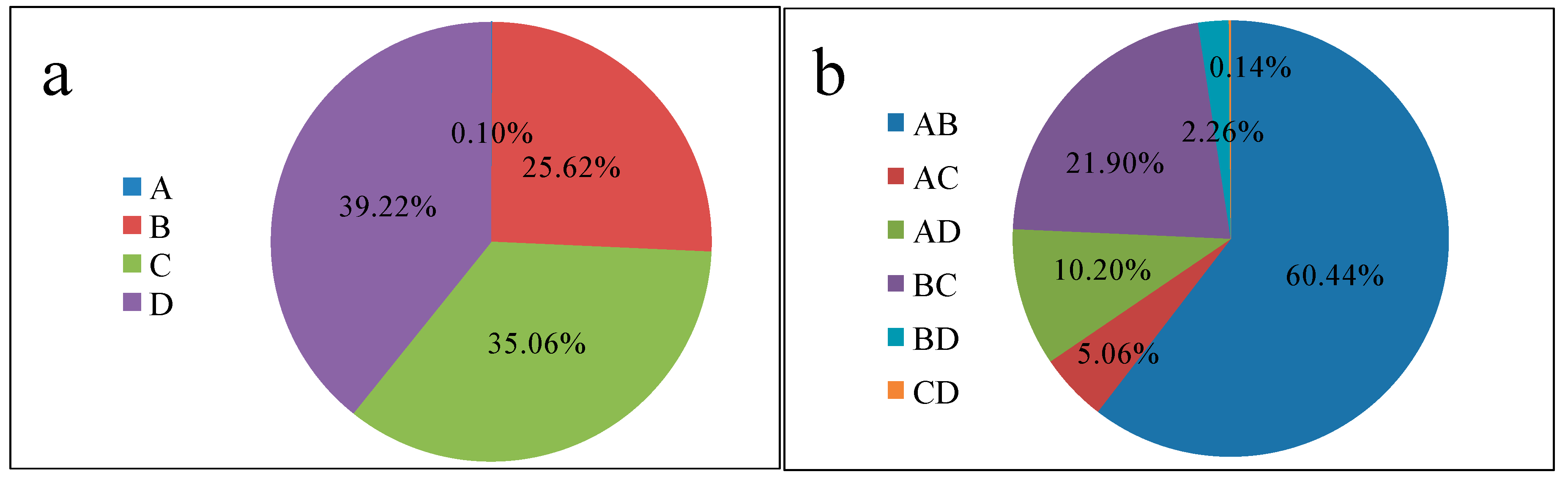
For the purpose of selecting important variables, the parameters used for referable optimum variables were subjected to the initial concentration, adsorbent dosage, pH, and temperature and identified as A, B, C, and D, respectively. The design evaluates the individual factors and their corresponding interactions and identifies the optimum response in the minimum number of runs. The experimental error and reproducibility of the data can be gathered from the centre points. All experiments were demonstrated at a fixed contact time (60 min). The RSM/CCD suggested a total of 30 experimental runs, as presented in
Table 1, including 16 factorial points, 8 axial points, and 6 centre points.
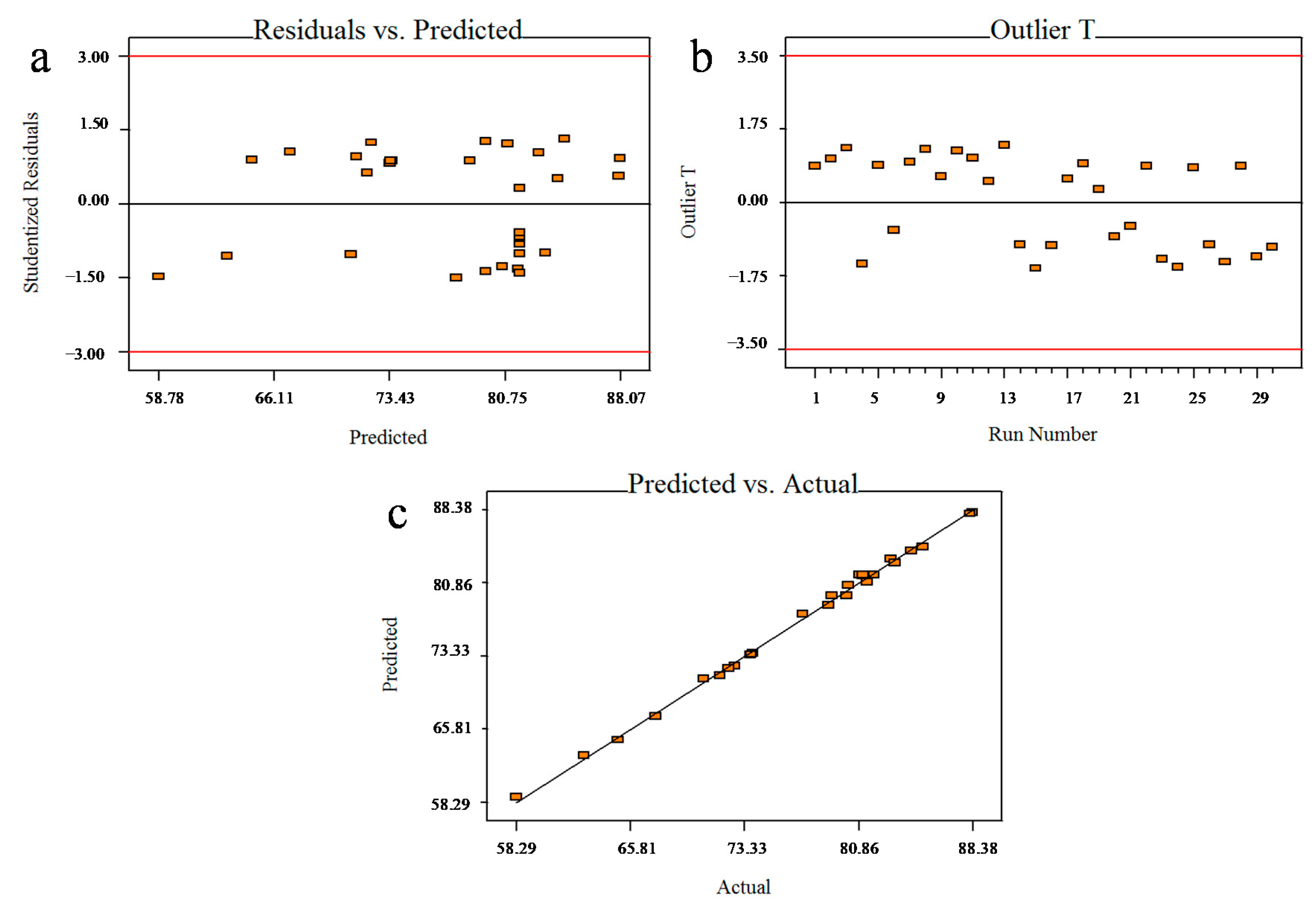
The importance of the model and the identification of the factors’ individual effects and interaction effects were evaluated using analysis of variance (ANOVA). The adequacy of the generated model was evaluated based on the (R2) coefficient, the adjusted coefficient of determination (adjusted R2), and the lack-of-fit test. Based on the p-values and F-values, the optimum conditions were predicted, and three-dimensional response–surface graphs were plotted.
2.7. Desorption and Reusability Tests
The reusability test of calix[6]arene-modified PbS containing MB solution was examined via a chemical desorption method. This desorption study was performed to investigate the efficiency of the adsorbent. Four different eluents (dH2O, HNO3, (CH3)2CO, and C2H5OH) were used to select the best eluent to elute the MB solutions. The adsorbent was first equilibrated with 0.025-g/L MB solution in a shaker under ambient temperature conditions. At equilibration, the adsorbent was washed with distilled water to remove unadsorbed traces of MB and then dried in an oven for 2 h at 80 °C. The various concentrations of the selected eluent were studied and tested with MB. All residual concentrations of MB were measured using UV absorbance.
2.8. Leaching Tests
The MB solution in each cycle was analysed to examine the amount of lead (Pb) and total suspended solids (TSS) of calix[6]arene-modified PbS. The Standard Methods (2017) 2540D were referred to determine the acceptable value for the Pb and TSS. Briefly, the supernatants were collected after the MB solution was filtered through a preweighed filter. Inductively coupled plasma-mass spectrometry (ICP-MS, Perkin Elmer, DRC-e) was used to analyse the Pb element in the MB supernatant. Meanwhile, the residue retained on the filter was heated at 104 °C until a constant weight was obtained. The concentration of suspended calix[6]arene-modified PbS was calculated using the following Equation (3):
4. Conclusions
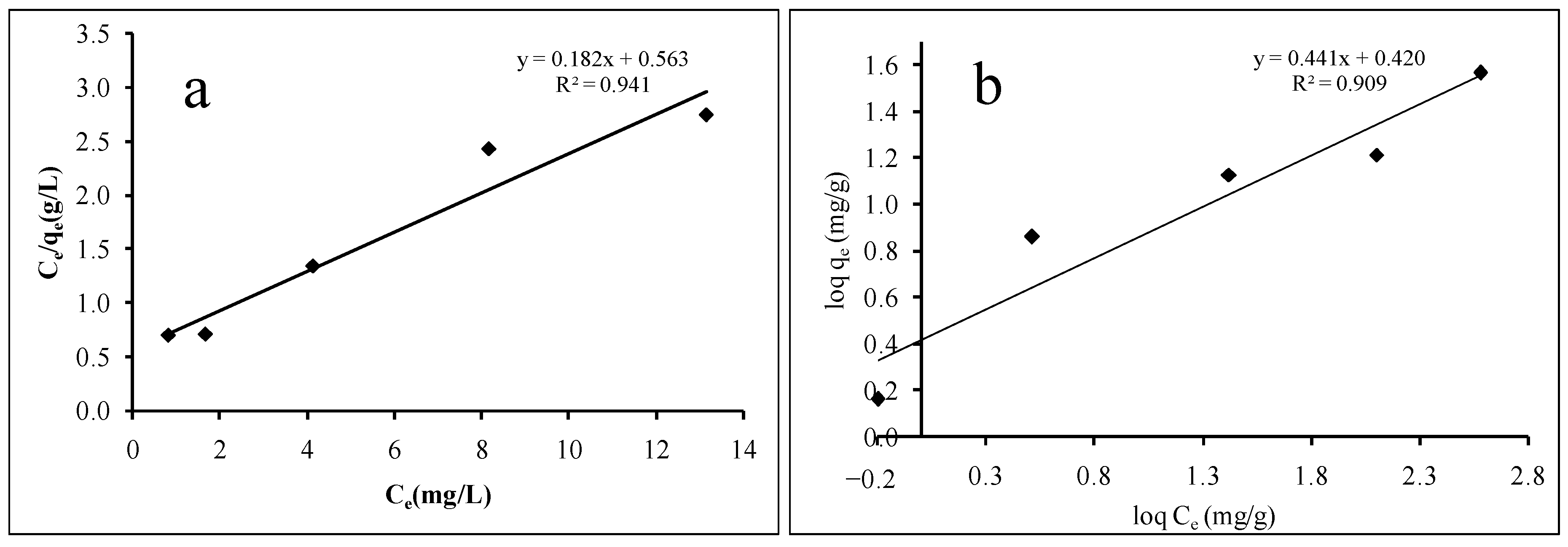
The current paper demonstrated the synthesis of calix[6]arene-modified PbS and its efficient cationic dye adsorption. The effects of the initial concentration, adsorbent dosage, pH, and temperature on the adsorption were studied using the response surface methodology (RSM) called central composite design (CCD). Equilibrium isotherm and kinetic studies were carried out under optimised conditions. The optimised initial concentration, adsorbent dosage, pH, and temperature were 20.00 mg/L of MB dye, 44.00 mg of calix[4]arene-modified PbS, pH 6, and 31.00 °C temperature. The adsorption of the MB dye was in-line with the Langmuir isotherm attributed to the homogenous monolayer adsorption that occurred on the surface of calix[6]arene-modified PbS. The maximum adsorption capacity of calix[6]arene-modified PbS was found to be 5.495 mg/g at the optimal conditions. The kinetic data closely followed the pseudo-second-order kinetic model, indicating chemisorptions as the mechanism of adsorption.