Effect of Tadalafil Administration on Redox Homeostasis and Polyamine Levels in Healthy Men with High Level of Physical Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
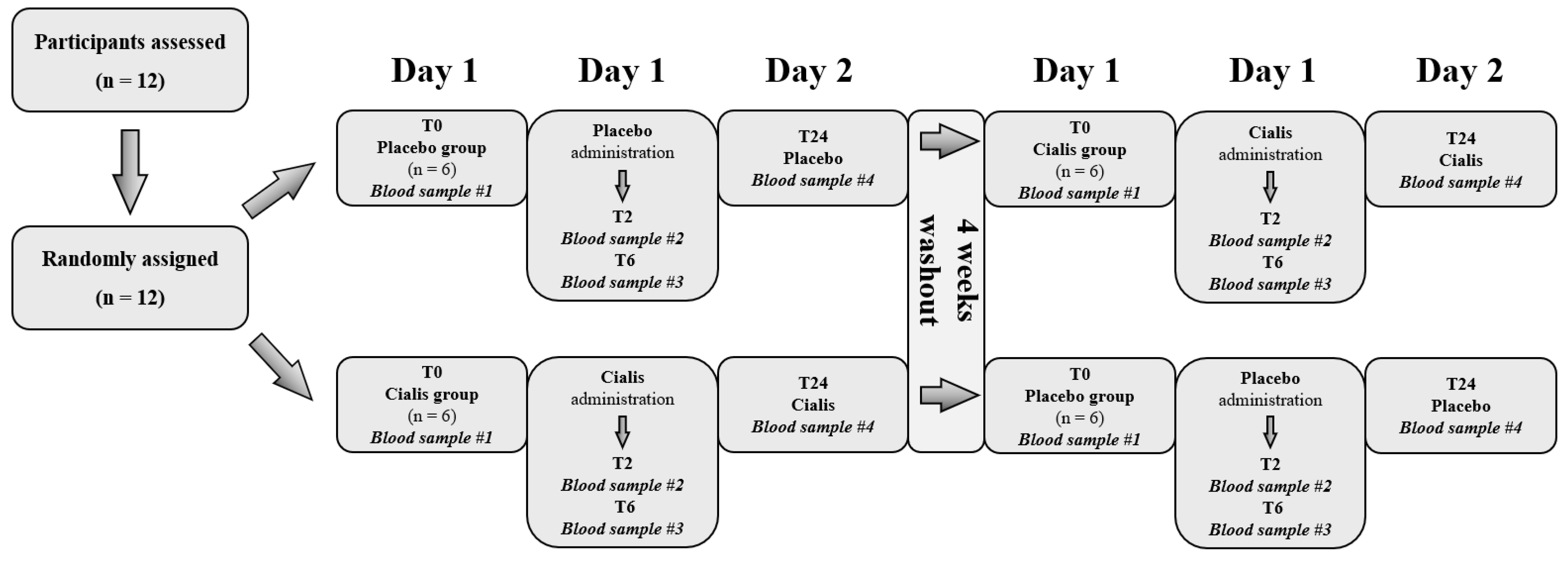
2.2. Experimental Protocol
2.3. Blood Samples Collection
2.4. Biochemical Analysis
2.4.1. Materials
2.4.2. Creatine Kinase and Lactate Dehydrogenase
2.4.3. Glutathione Homeostasis
2.4.4. Total Plasma Free Thiol Determination
2.4.5. Trolox Equivalents Antioxidant Capacity (TAC)
2.4.6. Thiobarbituric Acid Reactive Substances (TBARs)
2.4.7. Protein Carbonyls (PrCar)
2.4.8. Enzymatic Activities
2.4.9. Polyamines Levels
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics of Subjects
3.2. Creatine Kinase and Lactate Dehydrogenase Analysis
3.3. Glutathione Homeostasis
3.4. Total Plasma Free Thiol Determination and Trolox Equivalents Antioxidant Capacity
3.5. Oxidative Damage Analysis
3.6. Enzymatic Activities
3.7. Polyamines Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbin, J.D.; Francis, S.H. Pharmacology of phosphodiesterase-5 inhibitors. Int. J. Clin. Pract. 2002, 56, 453–459. [Google Scholar] [PubMed]
- Wright, P.J. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int. J. Clin. Pract. 2006, 60, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salloum, F.N.; Chau, V.Q.; Hoke, N.N.; Abbate, A.; Varma, A.; Ockaili, R.A.; Toldo, S.; Kukreja, R.C. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 2009, 120, S31–S36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Vachiery, J.L.; Huez, S.; Gillies, H.; Layton, G.; Hayashi, N.; Gao, X.; Naeije, R. Safety, tolerability and pharmacokinetics of an intravenous bolus of sildenafil in patients with pulmonary arterial hypertension. Br. J. Clin. Pharmacol. 2011, 71, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Di Luigi, L.; Sansone, M.; Sansone, A.; Ceci, R.; Duranti, G.; Borrione, P.; Crescioli, C.; Sgrò, P.; Sabatini, S. Phosphodiesterase Type 5 Inhibitors, Sport and Doping. Curr. Sports Med. Rep. 2017, 16, 443–447. [Google Scholar] [CrossRef]
- Georgiadis, G.; Zisis, I.E.; Docea, A.O.; Tsarouhas, K.; Fragkiadoulaki, I.; Mavridis, C.; Karavitakis, M.; Stratakis, S.; Stylianou, K.; Tsitsimpikou, C.; et al. Current Concepts on the Reno-Protective Effects of Phosphodiesterase 5 Inhibitors in Acute Kidney Injury: Systematic Search and Review. J. Clin. Med. 2020, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Iordache, A.M.; Docea, A.O.; Buga, A.M.; Zlatian, O.; Ciurea, M.E.; Rogoveanu, O.C.; Burada, F.; Sosoi, S.; Mitrut, R.; Mamoulakis, C.; et al. Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food Chem. Toxicol. 2020, 135, 111038. [Google Scholar] [CrossRef]
- Morano, S.; Mandosi, E.; Fallarino, M.; Gatti, A.; Tiberti, C.; Sensi, M.; Gandini, L.; Buchetti, B.; Lenti, L.; Jannini, E.A.; et al. Antioxidant treatment associated with sildenafil reduces monocyte activation and markers of endothelial damage in patients with diabetic erectile dysfunction: A double-blind, placebo-controlled study. Eur. Urol. 2007, 52, 1768–1774. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhang, R.; Jiang, X.; Wen, L.; Wu, D.C.; Liu, D.; Yuan, P.; Wang, Y.L.; Jing, Z.C. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc. Res. 2013, 99, 395–403. [Google Scholar] [CrossRef]
- Koka, S.; Das, A.; Salloum, F.N.; Kukreja, R.C. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic. Biol. Med. 2013, 60, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Antinozzi, C.; Sgrò, P.; Marampon, F.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Di Luigi, L. Sildenafil Counteracts the In Vitro Activation of CXCL-9, CXCL-10 and CXCL-11/CXCR3 Axis Induced by Reactive Oxygen Species in Scleroderma Fibroblasts. Biology 2021, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Di Luigi, L.; Duranti, G.; Antonioni, A.; Sgrò, P.; Ceci, R.; Crescioli, C.; Sabatini, S.; Lenzi, A.; Caporossi, D.; Del Galdo, F.; et al. The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species. Antioxidants 2020, 9, 786. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef]
- Perk, H.; Armagan, A.; Naziroğlu, M.; Soyupek, S.; Hoscan, M.B.; Sütcü, R.; Ozorak, A.; Delibas, N. Sildenafil citrate as a phosphodiesterase inhibitor has an antioxidant effect in the blood of men. J. Clin. Pharm. Ther. 2008, 33, 635–640. [Google Scholar] [CrossRef]
- Ozdegirmenci, O.; Kucukozkan, T.; Akdag, E.; Topal, T.; Haberal, A.; Kayir, H.; Oter, S.; Akyol, M.; Uzbay, T. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J. Matern. Fetal Neonatal Med. 2011, 24, 317–323. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Vinciguerra, I.; De Lutiis, M.A.; Grilli, A.; Felaco, M.; Patruno, A. Phosphodiesterase type-5 inhibitor and oxidative stress. Int. J. Immunopathol. Pharmacol. 2008, 21, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Arikan, D.C.; Bakan, V.; Kurutas, E.B.; Sayar, H.; Coskun, A. Protective effect of tadalafil on ischemia/reperfusion injury of rat ovary. J. Pediatr. Surg. 2010, 45, 2203–2209. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.X.; Lin, H.C.; Qiu, X.F.; Gao, J.; Dai, Y.T.; Wang, R. The effects of long-term administration of tadalafil on STZ-induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J. Androl. 2012, 14, 616–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, T.; Rashed, L.; Kotb, K.; Taymour, M. Effect of testosterone and frequent low-dose sildenafil/tadalafil on cavernous tissue oxidative stress of aged diabetic rats. Andrologia 2012, 44, 411–415. [Google Scholar] [CrossRef]
- Koka, S.; Aluri, H.S.; Xi, L.; Lesnefsky, E.J.; Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: Potential role of NO/SIRT1/PGC-1α signaling. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1558–H1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeneye, A.A.; Benebo, A.S. Chemopreventive Effect of Tadalafil in Cisplatin-Induced Nephrotoxicity in Rats. Niger. J. Physiol. Sci. 2016, 31, 1–10. [Google Scholar] [PubMed]
- Sabatini, S.; Sgrò, P.; Duranti, G.; Ceci, R.; Di Luigi, L. Tadalafil alters energy metabolism in C2C12 skeletal muscle cells. Acta Biochim. Pol. 2011, 58, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Duranti, G.; Ceci, R.; Sgrò, P.; Sabatini, S.; Di Luigi, L. Influence of the PDE5 inhibitor tadalafil on redox status and antioxidant defense system in C2C12 skeletal muscle cells. Cell stress and chaperones. Cell Stress Chaperones 2017, 22, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, F.; Tafuri, D.; Vasilescu, M.; Ionescu Anca, M. Pharmacologically active substances and dietary supplements used by athletes - the European and Italian regulation. Med. Sport 2014, 2, 2309–2314. [Google Scholar]
- Ceci, R.; Duranti, G.; Sgrò, P.; Sansone, M.; Guidetti, L.; Baldari, C.; Sabatini, S.; Di Luigi, L. Effects of tadalafil administration on plasma markers of exercise-induced muscle damage, IL6 and antioxidant status capacity. Eur. J. Appl. Physiol. 2015, 115, 531–539. [Google Scholar] [CrossRef]
- Di Luigi, L.; Baldari, C.; Pigozzi, F.; Emerenziani, G.P.; Gallotta, M.C.; Iellamo, F.; Ciminelli, E.; Sgrò, P.; Romanelli, F.; Lenzi, A.; et al. The long-acting phosphodiesterase inhibitor tadalafil does not influence athletes’ VO2max, aerobic, and anaerobic thresholds in normoxia. Int. J. Sports Med. 2008, 29, 110–115. [Google Scholar] [CrossRef]
- Duranti, G.; La Rosa, P.; Dimauro, I.; Wannenes, F.; Bonini, S.; Sabatini, S.; Parisi, P.; Caporossi, D. Effects of salmeterol on skeletal muscle cells: Metabolic and proapoptotic features. Med. Sci. Sports Exerc. 2011, 43, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Colamartino, M.; Duranti, G.; Ceci, R.; Sabatini, S.; Testa, A.; Cozzi, R. A multi-biomarker analysis of the antioxidant efficacy of Parkinson’s disease therapy. Toxicol. In Vitro 2017, 47, 1–7. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Podestà, M.A.; Garavaglia, M.L.; Portinaro, N.M.; Milzani, A.; Badalamenti, S.; Dalle-Donne, I. Plasma protein thiolation index (PTI) as a biomarker of thiol-specific oxidative stress in haemodialyzed patients. Free Radic. Biol. Med. 2015, 89, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Beltran Valls, M.R.; Duranti, G.; Dimauro, I.; Quaranta, F.; Pittaluga, M.; Sabatini, S.; Caserotti, P.; Parisi, P.; Parisi, A.; et al. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014, 2, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceci, R.; Duranti, G.; Leonetti, A.; Pietropaoli, S.; Spinozzi, F.; Marcocci, L.; Amendola, R.; Cecconi, F.; Sabatini, S.; Mariottini, P.; et al. Adaptive responses of heart and skeletal muscle to spermine oxidase overexpression: Evaluation of a new transgenic mouse model. Free Radic. Biol. Med. 2017, 103, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Magi, F.; Dimauro, I.; Margheritini, F.; Duranti, G.; Mercatelli, N.; Fantini, C.; Ripani, F.R.; Sabatini, S.; Caporossi, D. Telomere length is independently associated with age, oxidative biomarkers, and sport training in skeletal muscle of healthy adult males. Free Radic. Res. 2018, 52, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Baroli, G.; Fratini, E.; Pietropaoli, S.; Marcoli, M.; Mariottini, P.; Cervelli, M. Epileptic seizures and oxidative stress in a mouse model over-expressing spermine oxidase. Amino Acids 2020, 52, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; Imboden, M.T.; Arena, R.; Myers, J. Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing Using Cycle Ergometry: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin. Proc. 2017, 92, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Bentley, D.J.; Dank, S.; Coupland, R.; Midgley, A.; Spence, I. Acute antioxidant supplementation improves endurance performance in trained athletes. Res. Sports Med. 2012, 20, 1–12. [Google Scholar] [CrossRef]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary strategies to recover from exercise-induced muscle damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox homeostasis in sport: Do athletes really need antioxidant support? Res. Sports Med. 2019, 27, 147–165. [Google Scholar] [CrossRef]
- Loraschi, A.; Galli, N.; Cosentino, M. Dietary supplement and drug use and doping knowledge and attitudes in Italian young elite cyclists. Clin. J. Sport Med. 2014, 24, 238Y44. [Google Scholar] [CrossRef]
- Mazzarino, M.; Cesarei, L.; de la Torre, X.; Fiacco, I.; Robach, P.; Botrè, F. A multi-targeted liquid chromatography-mass spectrometry screening procedure for the detection in human urine of drugs non-prohibited in sport commonly used by the athletes. J. Pharm. Biomed. Anal. 2016, 117, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Frajese, G.V.; Pozzi, F.; Frajese, G. Tadalafil in the treatment of erectile dysfunction; an overview of the clinical evidence. Clin. Interv. Aging 2006, 1, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Choe, H.-S.; Carney, E.W.; Harris, C. Differential antioxidant enzyme activities and glutathione content between rat and rabbit conceptuses. Free Radic. Biol. Med. 2001, 10, 1078–1088. [Google Scholar] [CrossRef]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef]
- Salvi, M.; Toninello, A. Effects of polyamines on mitochondrial Ca(2+) transport. Biochim. Biophys. Acta 2004, 1661, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Cervelli, M.; Amendola, R.; Polticelli, F.; Mariottini, P. Spermine oxidase: Ten years after. Amino Acids 2012, 42, 441–450. [Google Scholar] [CrossRef]
- Cervelli, M.; Angelucci, E.; Germani, F.; Amendola, R.; Mariottini, P. Inflammation, carcinogenesis and neurodegeneration studies in transgenic animal models for polyamine research. Amino Acids 2014, 46, 521–530. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Freeman, B. Free radical chemistry of nitric oxide. Looking at the dark side. Chest 1994, 105, 79S–84S. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Herrera-Martín, G.; Alonso-Iglesias, E. Polyamines are increased in obese children and are related to markers of oxidative/nitrosative stress and angiogenesis. J. Clin. Endocrinol. Metab. 2011, 96, 2821–2825. [Google Scholar] [CrossRef] [Green Version]
- Di Luigi, L.; Baldari, C.; Sgrò, P.; Emerenziani, G.P.; Gallotta, M.C.; Bianchini, S.; Romanelli, F.; Pigozzi, F.; Lenzi, A.; Guidetti, L. The type 5 phosphodiesterase inhibitor tadalafil influences salivary cortisol, testosterone, and dehydroepiandrosterone sulphate responses to maximal exercise in healthy men. J. Clin. Endocrinol. Metab. 2008, 93, 3510–3514. [Google Scholar] [CrossRef] [PubMed]
- Di Luigi, L.; Sgrò, P.; Baldari, C.; Gallotta, M.C.; Emerenziani, G.P.; Crescioli, C.; Bianchini, S.; Romanelli, F.; Lenzi, A.; Guidetti, L. The phosphodiesterases type 5 inhibitor tadalafil reduces the activation of the hypothalamus-pituitary-adrenal axis in men during cycle ergometric exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E972–E978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidetti, L.; Emerenziani, G.P.; Gallotta, M.C.; Pigozzi, F.; Di Luigi, L.; Baldari, C. Effect of tadalafil on anaerobic performance indices in healthy athletes. Br. J. Sports Med. 2008, 42, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Van Osta, A.; Moraine, J.J.; Mélot, C.; Mairbäurl, H.; Maggiorini, M.; Naeije, R. Effects of high altitude exposure on cerebral hemodynamics in normal subjects. Stroke 2005, 36, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.C.; Yu, Z.X.; Shen, J.Y.; Wu, B.X.; Xu, K.F.; Zhu, X.Y.; Pan, L.; Zhang, Z.L.; Liu, X.Q.; Zhang, Y.S.; et al. Vardenafil in pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 2011, 183, 1723–1729. [Google Scholar] [CrossRef]
- Leshem, E.; Caine, Y.; Rosenberg, E.; Maaravi, Y.; Hermesh, H.; Schwartz, E. Tadalafil and acetazolamide versus acetazolamide for the prevention of severe high-altitude illness. J. Travel. Med. 2012, 19, 308–310. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, G.S.; von Wulffen, W.; Huppmann, P.; Meis, T.; Ihle, F.; Geiseler, J.; Leuchte, H.H.; Tufman, A.; Behr, J.; Neurohr, C. Haemodynamic changes in pulmonary hypertension in patients with interstitial lung disease treated with PDE-5 inhibitors. Respirology 2014, 19, 700–706. [Google Scholar] [CrossRef]
- Sabri, M.; Beheshtian, E. Comparison of the therapeutic and side effects of tadalafil and sildenafil in children and adolescents with pulmonary arterial hypertension. Pediatr. Cardiol. 2014, 35, 699–704. [Google Scholar] [CrossRef]
- Victor, R.G.; Sweeney, H.L.; Finkel, R.; McDonald, C.M.; Byrne, B.; Eagle, M.; Goemans, N.; Vandenborne, K.; Dubrovsky, A.L.; Topaloglu, H.; et al. Tadalafil DMD Study Group. A phase 3 randomized placebo-controlled trial of tadalafil for Duchenne muscular dystrophy. Neurology 2017, 89, 1811–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, C.M.; Sajeev, G.; Yao, Z.; McDonnell, E.; Elfring, G.; Souza, M.; Peltz, S.W.; Darras, B.T.; Shieh, P.B.; Cox, D.A.; et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: A meta-analysis of disease progression rates in recent multicenter clinical trials. Muscle Nerve 2020, 61, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedky, A.A.; Magdy, Y. Tadalafil versus linaclotide in gastrointestinal dysfunction and depressive behavior in constipation-predominant irritable bowel syndrome. Life Sci. 2020, 256, 117960. [Google Scholar] [CrossRef]
- Pittaluga, M.; Sgadari, A.; Dimauro, I.; Tavazzi, B.; Parisi, P.; Caporossi, D. Physical exercise and redox balance in type 2 diabetics: Effects of moderate training on biomarkers of oxidative stress and DNA damage evaluated through comet assay. Oxid. Med. Cell. Longev. 2015, 2015, 981242. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Duranti, G.; Di Filippo, E.S.; Bondi, D.; Verratti, V.; Doria, C.; Caporossi, C.; Sabatini, S.; Dimauro, I.; Pietrangelo, T. Endurance Training Improves Plasma Superoxide Dismutase Activity in Healthy Elderly. Mech. Ageing Dev. 2019, 185, 111190. [Google Scholar] [CrossRef]
| Subjects’ Characteristics (n = 12) | |
|---|---|
| Age (years) | 24.4 ± 3.4; (19–31) |
| Height (cm) | 172.2 ± 6.7; (157.0–184.0) |
| Weight (kg) | 73.8 ± 5.2; (65.0–84.0) |
| BMI (kg m−2) | 24.9 ± 1.8; (22.0–28.4) |
| VO2max (mL min−1 kg−1) | 47.7 ± 4.9; (40.1–56.0) |
| Variables | T0 | T2 | T6 | T24 |
|---|---|---|---|---|
| CK a | 28.31 ± 7.08 | 29.76 ± 6.67 | 33.57 ± 6.43 | 39.70 ± 6.80 # |
| LDH a | 40.90 ± 6.90 | 42.22 ± 7.02 | 45.41 ± 6.85 | 49.53 ± 8.43 * |
| Variables | T0 | T2 | T6 | T24 |
|---|---|---|---|---|
| Total GSH a | 100.48 ± 32.50 | 99.23 ± 33.24 | 102.37 ± 30.32 | 104.46 ± 31.38 |
| GSSG a | 5.64 ± 3.08 | 6.43 ± 3.39 | 7.13 ± 4.16 * | 7.07 ± 3.67 ** |
| GSH/GSSG | 21.52 ± 15.12 | 16.65 ± 5.86 | 17.54 ± 12.34 | 20.02 ± 20.41 |
| FTH b | 3.28 ± 0.39 | 3.20 ± 0.31 | 3.16 ± 0.34 | 3.26 ± 0.43 |
| TAC c | 0.77 ± 0.11 | 0.74 ± 0.11 | 0.75 ± 0.11 | 0.75 ± 0.13 |
| Variables | T0 | T2 | T6 | T24 |
|---|---|---|---|---|
| TBARs a | 0.72 ± 0.08 | 0.75 ± 0.04 | 0.67 ± 0.05 | 0.68 ± 0.07 |
| PrCAR b | 1.63 ± 0.08 | 1.65 ± 0.10 | 1.67 ± 0.08 | 1.56 ± 0.08 |
| Variables | T0 | T2 | T6 | T24 |
|---|---|---|---|---|
| CAT a | 19.13 ± 1.81 | 20.12 ± 1.35 | 20.87 ± 1.62 | 20.02 ± 2.09 |
| SOD a | 4.72 ± 0.70 | 5.05 ± 0.91 | 5.09 ± 1.38 | 5.04 ± 1.00 |
| GPx a | 9.62 ± 1.38 | 9.89 ± 1.81 | 9.93 ± 1.67 | 10.19 ± 2.16 |
| SOD/CAT | 0.25 ± 0.04 | 0.25 ± 0.05 | 0.24 ± 0.07 | 0.25 ± 0.05 |
| SOD/GPx | 0.50 ± 0.11 | 0.53 ± 0.13 | 0.52 ± 0.14 | 0.51 ± 0.13 |
| Variables | T0 | T2 | T6 | T24 |
|---|---|---|---|---|
| Spd a | 22.36 ± 12.47 | 18.06 ± 6.39 | 23.13 ± 11.16 | 20.38 ± 8.16 |
| Spm a | 17.04 ± 7.36 | 15.24 ± 5.09 | 19.55 ± 7.48 | 15.99 ± 4.78 |
| Spd/Spm | 1.29 ± 0.39 | 1.26 ± 0.43 | 1.20 ± 0.40 | 1.31 ± 0.49 |
| Variables and Groups | 1 µM | 2 µM | 10 µM | 100 µM | |
|---|---|---|---|---|---|
| TAC (Trolox® equivalents mM) | TAD | 0.079 ± 0.008 | 0.082 ± 0.012 | 0.109 ± 0.011 | 0.385 ± 0.106 |
| AA | 1.251 ± 0.079 | 1.796 ± 0.091 | 2.165 ± 0.012 | 2.816 ± 0.016 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duranti, G.; Ceci, R.; Di Luigi, L.; Antinozzi, C.; Dimauro, I.; Sabatini, S.; Cervelli, M.; Sgrò, P. Effect of Tadalafil Administration on Redox Homeostasis and Polyamine Levels in Healthy Men with High Level of Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 9962. https://doi.org/10.3390/ijerph18199962
Duranti G, Ceci R, Di Luigi L, Antinozzi C, Dimauro I, Sabatini S, Cervelli M, Sgrò P. Effect of Tadalafil Administration on Redox Homeostasis and Polyamine Levels in Healthy Men with High Level of Physical Activity. International Journal of Environmental Research and Public Health. 2021; 18(19):9962. https://doi.org/10.3390/ijerph18199962
Chicago/Turabian StyleDuranti, Guglielmo, Roberta Ceci, Luigi Di Luigi, Cristina Antinozzi, Ivan Dimauro, Stefania Sabatini, Manuela Cervelli, and Paolo Sgrò. 2021. "Effect of Tadalafil Administration on Redox Homeostasis and Polyamine Levels in Healthy Men with High Level of Physical Activity" International Journal of Environmental Research and Public Health 18, no. 19: 9962. https://doi.org/10.3390/ijerph18199962
APA StyleDuranti, G., Ceci, R., Di Luigi, L., Antinozzi, C., Dimauro, I., Sabatini, S., Cervelli, M., & Sgrò, P. (2021). Effect of Tadalafil Administration on Redox Homeostasis and Polyamine Levels in Healthy Men with High Level of Physical Activity. International Journal of Environmental Research and Public Health, 18(19), 9962. https://doi.org/10.3390/ijerph18199962










