Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists
Abstract
1. Introduction
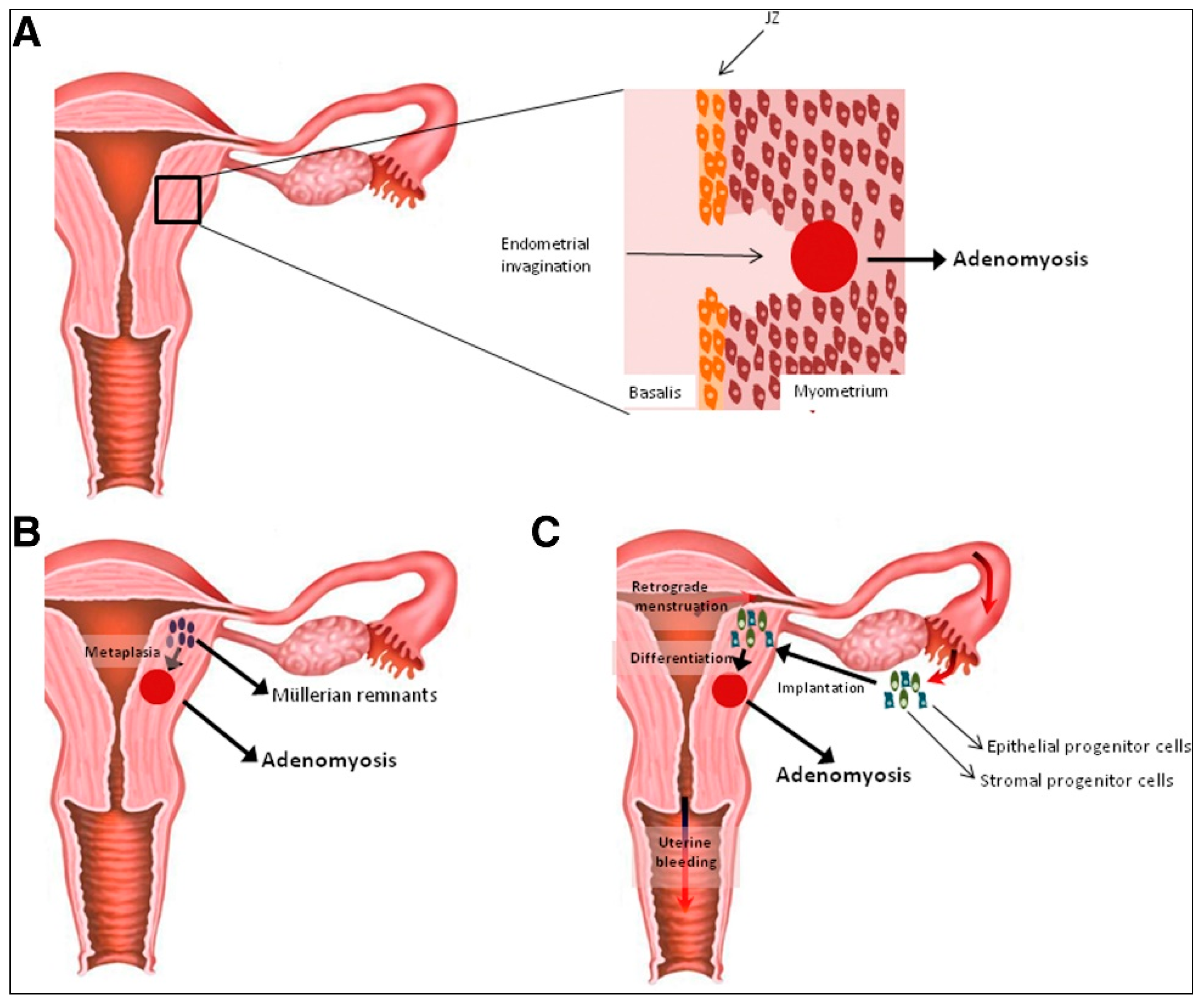
2. Hypotheses on the Origin of Uterine Adenomyosis
2.1. Theory of Endometrial Invasion in the Pathogenesis of Adenomyosis
2.2. Hypothesis of De Novo Generation of Adenomyotic Lesions
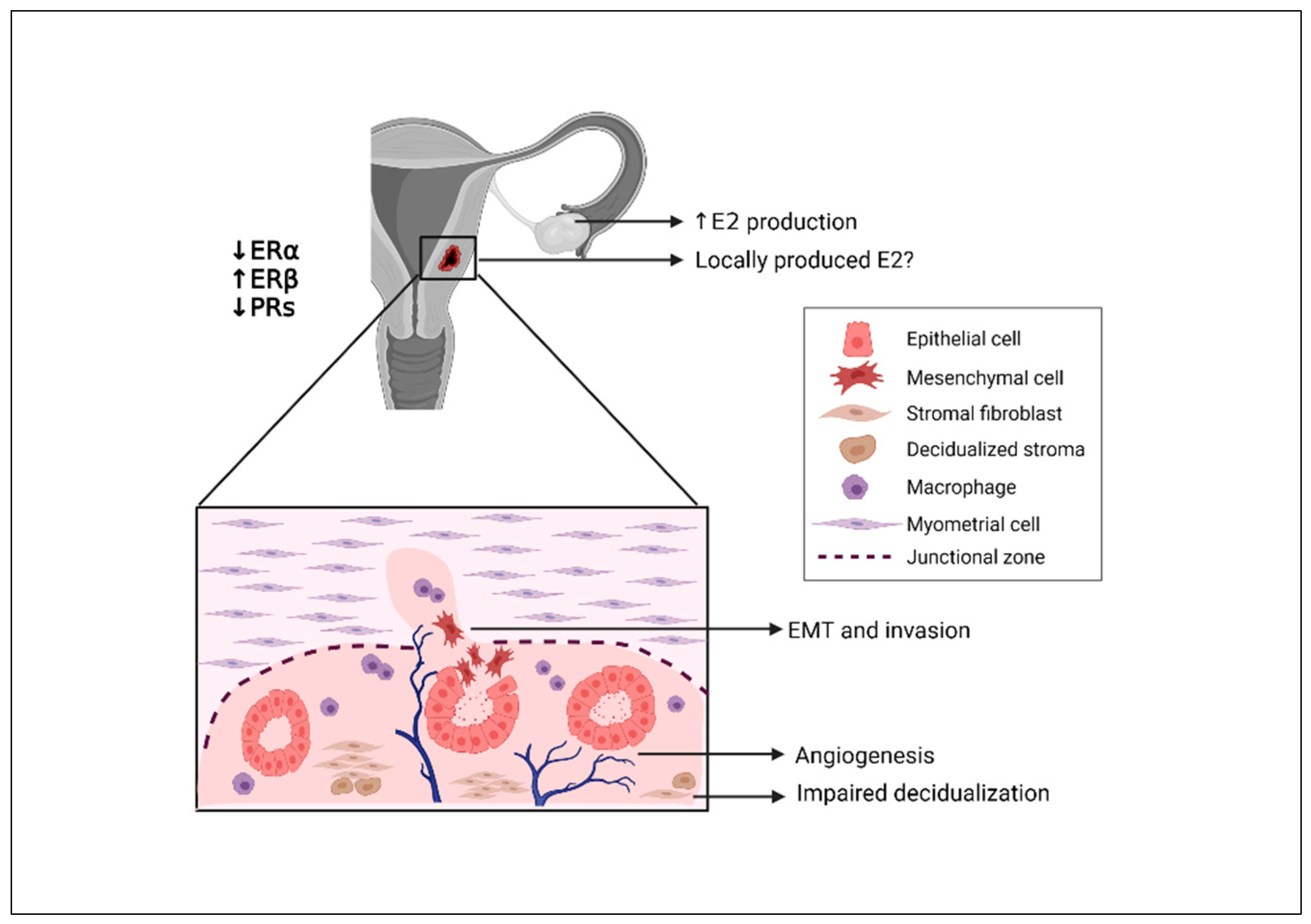
3. Role and Causes of Hyperestrogenism in the Pathogenesis of Adenomyosis
3.1. Impact of Estrogen on Endometrial Cells
3.2. Evidence of Hyperestrogenism in the Myometrium
3.3. Potential Interaction of Estrogen and the Immune Response
3.4. Origin of Aberrant Estrogen Signaling in Adenomyosis
4. Evidence of Endometrial Progesterone Resistance
4.1. Origin of Progesterone Resistance and the Role of ERs
4.2. Is Progesterone Resistance Linked to Infertility?
5. Medical Treatment of Adenomyosis
5.1. Current Medical Therapies for Adenomyosis: The Need for Novel Options
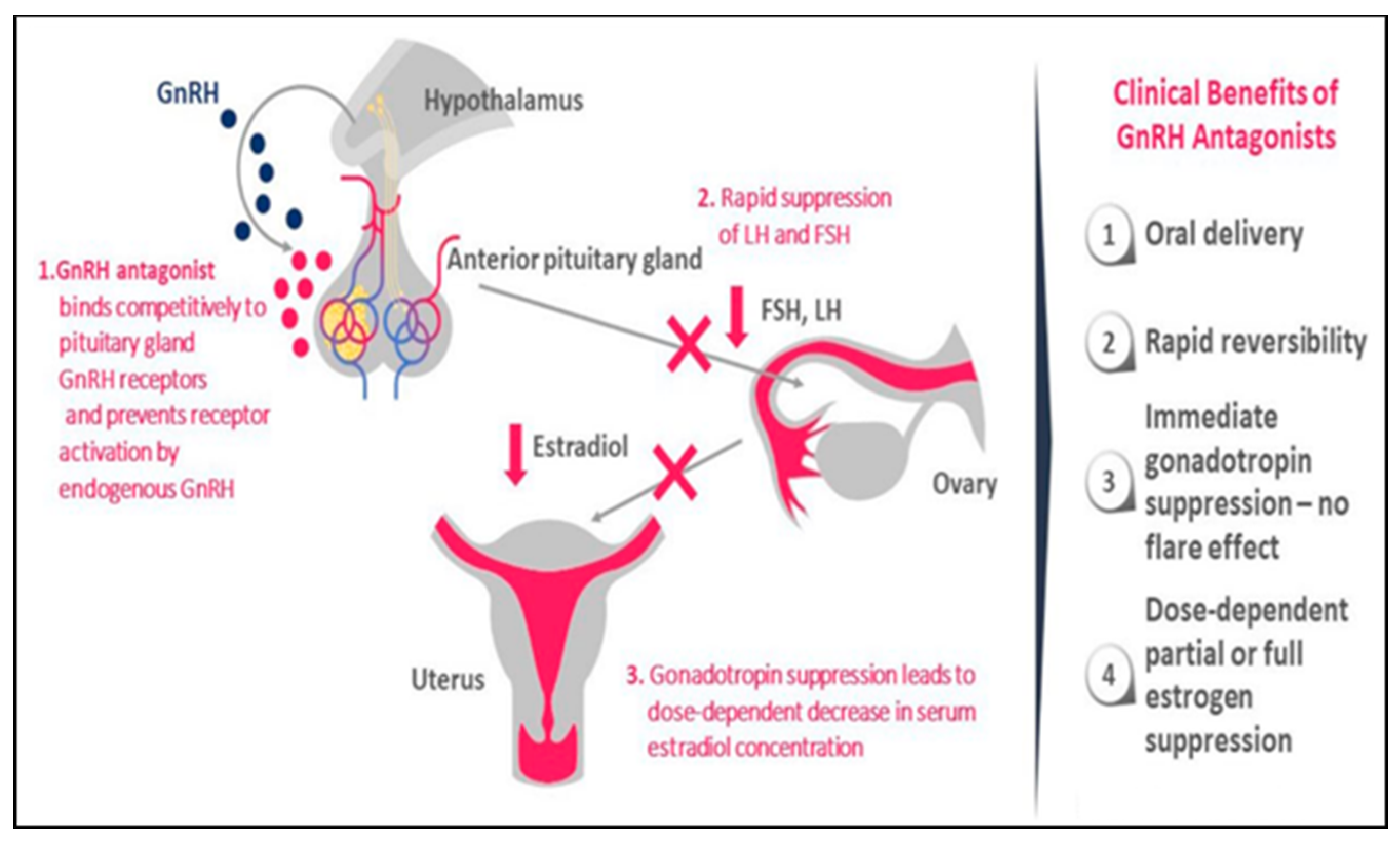
5.2. Treating Adenomyosis Symptoms with GnRH Antagonists: A Promising New Approach
5.3. The Potential Link between Adenomyosis and Endometriosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naftalin, J.; Hoo, W.; Pateman, K.; Mavrelos, D.; Holland, T.; Jurkovic, D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum. Reprod. 2012, 27, 3432–3439. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.-M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021, 28, 2087–2097. [Google Scholar] [CrossRef]
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Bazot, M.; Daraï, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef]
- Brosens, I.; Gordts, S.; Habiba, M.; Benagiano, G. Uterine Cystic Adenomyosis: A Disease of Younger Women. J. Pediatr. Adolesc. Gynecol. 2015, 28, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Gordts, S.; Bazot, M.; Brosens, I.; Benagiano, G. Exploring the challenges for a new classification of adenomyosis. Reprod. Biomed. Online 2020, 40, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis. Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Nirgianakis, K.; Kalaitzopoulos, D.R.; Schwartz, A.S.K.; Spaanderman, M.; Kramer, B.W.; Mueller, M.D.; Mueller, M. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2021, 42, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment. United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef]
- Vannuccini, S.; Luisi, S.; Tosti, C.; Sorbi, F.; Petraglia, F. Role of medical therapy in the management of uterine adenomyosis. Fertil. Steril. 2018, 109, 398–405. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Brosens, J.; Puttemans, P.; Benagiano, G.; Brosens, I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Li, H.-Y.; Huang, C.-H.; Twu, N.-F.; Yen, M.-S.; Wang, P.-H.; Chou, T.-Y.; Liu, Y.-N.; Chao, K.-C.; Yang, M.-H. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J. Pathol. 2010, 222, 261–270. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Maier, V.; Höll, M.; Dietze, R.; Mecha, E.; Omwandho, C.O.; Tinneberg, H.-R.; Meinhold-Heerlein, I.; Konrad, L. Adenomyotic glands are highly related to endometrial glands. Reprod. Biomed. Online 2019, 40, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Z.; Liu, H.; Niu, W.; Wang, X.; Liang, N.; Wang, X.; Wang, Y.; Shi, Y.; Xu, L.; et al. Single-cell transcriptomic analysis of eutopic endometrium and ectopic lesions of adenomyosis. Cell Biosci. 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Li, N.; Yuan, M.; Li, Q.; Zhang, L.; Wang, G.Y. Different macrophages equally induce EMT in endometria of adenomyosis and normal. Reproduction 2017, 154, 79–92. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, Z.; Zhang, C.; Liu, S.; Zhang, Y. ILK-induced epithelial-mesenchymal transition promotes the invasive phenotype in adenomyosis. Biochem. Biophys. Res. Commun. 2018, 497, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Batt, R.E.; Yeh, J. Müllerianosis. four developmental (embryonic) mullerian diseases. Reprod. Sci. 2013, 20, 1030–1037. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; D’Armiento, M.; De Falco, M.; Baldi, A. Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J. Exp. Clin. Cancer Res. 2009, 28, 49. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells. the first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar]
- Kao, A.-P.; Wang, K.-H.; Chang, C.-C.; Lee, J.-N.; Long, C.-Y.; Chen, H.-S.; Tsai, C.-F.; Hsieh, T.-H.; Tsai, E.-M. Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil. Steril. 2011, 95, 1308–1315.e1. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Wu, M.-Y.; Chen, C.-D.; Chen, M.-J.; Yang, Y.-S.; Ho, H.-N. Altered apoptosis and proliferation in endometrial stromal cells of women with adenomyosis. Hum. Reprod. 2007, 22, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Duan, H.; Wang, S.; Quan, Y.-J.; Huang, J.-H.; Guo, Z.-C. Upregulated Talin1 synergistically boosts β-estradiol-induced proliferation and pro-angiogenesis of eutopic and ectopic endometrial stromal cells in adenomyosis. Reprod. Biol. Endocrinol. 2021, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Peng, G.-Q.; Ban, D.-Y.; Zhang, C.; Zhang, X.-Q.; Li, Y.-P. High-Expression of Neuropilin 1 Correlates to Estrogen-Induced Epithelial-Mesenchymal Transition of Endometrial Cells in Adenomyosis. Reprod. Sci. 2020, 27, 395–403. [Google Scholar] [CrossRef]
- Taylor, A.H.; Kalathy, V.; Habiba, M. Estradiol and tamoxifen enhance invasion of endometrial stromal cells in a three-dimensional coculture model of adenomyosis. Fertil. Steril. 2014, 101, 288–293. [Google Scholar] [CrossRef]
- Huang, T.S.; Chen, Y.J.; Chou, T.Y.; Chen, C.Y.; Li, H.Y.; Huang, B.S.; Tsai, H.W.; Lan, H.Y.; Chang, C.H.; Twu, N.F.; et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J. Cell. Mol. Med. 2014, 18, 1358–1371. [Google Scholar] [CrossRef]
- Barcena de Arellano, M.L.; Oldeweme, J.; Arnold, J.; Schneider, A.; Mechsner, S. Remodeling of estrogen-dependent sympathetic nerve fibers seems to be disturbed in adenomyosis. Fertil. Steril. 2013, 100, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Duan, H.; Wang, S.; Sun, F.-Q.; Gan, L.; Tang, Y.-Q.; Xu, Q.; Li, T.C. Expression Pattern of G-Protein-Coupled Estrogen Receptor in Myometrium of Uteri with and without Adenomyosis. BioMed Res. Int. 2017, 2017, 5974693. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 565–592. [Google Scholar] [CrossRef]
- Bourdon, M.; Santulli, P.; Jeljeli, M.; Vannuccini, S.; Marcellin, L.; Doridot, L.; Petraglia, F.; Batteux, F.; Chapron, C. Immunological changes associated with adenomyosis: A systematic review. Hum. Reprod. Update 2020, 27, 108–129. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.P.; Russell, P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: Adenomyosis and macrophages. J. Reprod. Immunol. 2011, 93, 58–63. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Expression of gamma delta T cells and adhesion molecules in endometriotic tissue in patients with endometriosis and adenomyosis. Am. J. Reprod. Immunol. 1996, 35, 477–482. [Google Scholar] [CrossRef]
- Scheerer, C.; Bauer, P.; Chiantera, V.; Sehouli, J.; Kaufmann, A.; Mechsner, S. Characterization of endometriosis-associated immune cell infiltrates (EMaICI). Arch. Gynecol. Obstet. 2016, 294, 657–664. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Hatazawa, J.; Tanaka, T. Is adenomyosis an immune disease? Hum. Reprod. Update 1998, 4, 360–367. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef]
- Capellino, S.; Montagna, P.; Villaggio, B.; Sulli, A.; Soldano, S.; Ferrero, S.; Remorgida, V.; Cutolo, M. Role of Estrogens in Inflammatory Response: Expression of Estrogen Receptors in Peritoneal Fluid Macrophages from Endometriosis. Ann. N. Y. Acad. Sci. 2006, 1069, 263–267. [Google Scholar] [CrossRef]
- Gou, Y.; Li, X.; Li, P.; Zhang, H.; Xu, T.; Wang, H.; Wang, B.; Ma, X.; Jiang, X.; Zhang, Z. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Artymuk, N.; Zotova, O.; Gulyaeva, L. Adenomyosis: Genetics of estrogen metabolism. Horm. Mol. Biol. Clin. Investig. 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Herndon, C.N.; Aghajanova, L.; Balayan, S.; Erikson, D.; Barragan, F.; Goldfien, G.; Vo, K.C.; Hawkins, S.; Giudice, L.C. Global Transcriptome Abnormalities of the Eutopic Endometrium from Women with Adenomyosis. Reprod. Sci. 2016, 23, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef]
- Kitawaki, J.; Noguchi, T.; Amatsu, T.; Maeda, K.; Tsukamoto, K.; Yamamoto, T.; Fushiki, S.; Osawa, Y.; Honjo, H. Expression of Aromatase Cytochrome P450 Protein and Messenger Ribonucleic Acid in Human Endometriotic and Adenomyotic Tissues but not in Normal Endometrium. Biol. Reprod. 1997, 57, 514–519. [Google Scholar] [CrossRef]
- Colette, S.; Lousse, J.C.; Defrere, S.; Curaba, M.; Heilier, J.F.; Van Langendonckt, A.; Mestdagt, M.; Foidart, J.M.; Loumaye, E.; Donnez, J. Absence of aromatase protein and mRNA expression in endometriosis. Hum. Reprod. 2009, 24, 2133–2141. [Google Scholar] [CrossRef][Green Version]
- Colette, S.; Donnez, J. Endometriosis. N. Engl. J. Med. 2009, 360, 1911–1912. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Lam, E.; Brosens, J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 2012, 358, 208–215. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Savaris, R.F.; Groll, J.M.; Young, S.L.; DeMayo, F.J.; Jeong, J.-W.; Hamilton, A.E.; Giudice, L.C.; Lessey, B.A. Progesterone Resistance in PCOS Endometrium: A Microarray Analysis in Clomiphene Citrate-Treated and Artificial Menstrual Cycles. J. Clin. Endocrinol. Metab. 2011, 96, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Cheng, Y.-H.; Yin, P.; Imir, G.; Utsunomiya, H.; Attar, E.; Innes, J.; Kim, J.J. Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol. Cell. Endocrinol. 2006, 248, 94–103. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone Receptor Isoform A But Not B Is Expressed in Endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Lu, Y.; Liu, X.; Guo, S.-W. Immunoreactivity of progesterone receptor isoform B, nuclear factor κB, and IκBα in adenomyosis. Fertil. Steril. 2009, 92, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Mehasseb, M.K.; Panchal, R.; Taylor, A.; Brown, L.; Bell, S.C.; Habiba, M. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil. Steril. 2011, 95, 2228–2235. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Kuroboshi, H.; Mori, T.; Ogi, H.; Itoh, K.; Nakashima, M.; Kitawaki, J. Biological differences between intrinsic and extrinsic adenomyosis with coexisting deep infiltrating endometriosis. Reprod. Biomed. Online 2019, 39, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Einstein, M.; Geissler, W.M.; Chan, H.K.; Elliston, K.O.; Andersson, S. Expression cloning and characterization of human 17 beta-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20 alpha-hydroxysteroid dehydrogenase activity. J. Biol. Chem. 1993, 268, 12964–12969. [Google Scholar] [CrossRef]
- Inoue, S.; Hirota, Y.; Ueno, T.; Fukui, Y.; Yoshida, E.; Hayashi, T.; Kojima, S.; Takeyama, R.; Hashimoto, T.; Kiyono, T.; et al. Uterine adenomyosis is an oligoclonal disorder associated with KRAS mutations. Nat. Commun. 2019, 10, 5785. [Google Scholar] [CrossRef]
- Yoo, J.-Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.-W.; Lessey, B.A. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Adachi, S.; Kase, H.; Motoyama, T.; Inoue, I.; Enomoto, T. Different mutation profiles between epithelium and stroma in endometriosis and normal endometrium. Hum. Reprod. 2019, 34, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Wetendorf, M.; DeMayo, F.J. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int. J. Dev. Biol. 2014, 58, 95–106. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, R.; Cheng, X.; Zhang, Q.; Hu, Y.; Zhang, H.; Cao, Y.; Zhang, M.; Wang, J.; Ding, L.; et al. Decreased expression of NR4A nuclear receptors in adenomyosis impairs endometrial decidualization. Mol. Hum. Reprod. 2016, 22, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jin, Z.; Liu, H.; Xu, C. Impaired decidualization of human endometrial stromal cells from women with adenomyosis †. Biol. Reprod. 2021, 104, 1034–1044. [Google Scholar] [CrossRef]
- Cope, A.G.; Ainsworth, A.J.; Stewart, E.A. Current and Future Medical Therapies for Adenomyosis. Semin. Reprod. Med. 2020, 38, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Silber, S.; Kakinuma, T.; Nagaishi, M.; Kato, K.; Kato, O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod. Biomed. Online 2011, 22, 94–99. [Google Scholar] [CrossRef] [PubMed]
- A Flores, V.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Osuga, Y.; Hayashi, K.; Kanda, S. Long-term use of dienogest for the treatment of primary and secondary dysmenorrhea. J. Obstet. Gynaecol. Res. 2020, 46, 606–617. [Google Scholar] [CrossRef]
- Vannuccini, S.; Petraglia, F. Recent advances in understanding and managing adenomyosis. F1000Research 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Donnez, J. Gonadotropin-releasing hormone antagonist (linzagolix): A new therapy for uterine adenomyosis. Fertil. Steril. 2020, 114, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Conway, F.; Morosetti, G.; Camilli, S.; Martire, F.G.; Sorrenti, G.; Piccione, E.; Zupi, E.; Exacoustos, C. Ulipristal acetate therapy increases ultrasound features of adenomyosis: A good treatment given in an erroneous diagnosis of uterine fibroids. Gynecol. Endocrinol. 2019, 35, 207–210. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Nakashima, M.; Ishimaru, T.; Masuzaki, H. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum. Reprod. 2010, 25, 2878–2890. [Google Scholar] [CrossRef] [PubMed]
- Grow, D.R.; Filer, R.B. Treatment of adenomyosis with long-term GnRH analogues: A case report. Obstet. Gynecol. 1991, 78, 538–539. [Google Scholar]
- Matsushima, T.; Akira, S.; Fukami, T.; Yoneyama, K.; Takeshita, T. Efficacy of Hormonal Therapies for Decreasing Uterine Volume in Patients with Adenomyosis. Gynecol. Minim. Invasive Ther. 2018, 7, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, T.; Akira, S.; Yoneyama, K.; Takeshita, T. Recurrence of uterine adenomyosis after administration of gonadotropin-releasing hormone agonist and the efficacy of dienogest. Gynecol. Endocrinol. 2020, 36, 521–524. [Google Scholar] [CrossRef]
- Park, C.W.; Choi, M.H.; Yang, K.M.; Song, I.O. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin. Exp. Reprod. Med. 2016, 43, 169–173. [Google Scholar] [CrossRef]
- Barbieri, R.L. Endometriosis and the estrogen threshold theory. Relation to surgical and medical treatment. J. Reprod. Med. 1998, 43, 287–292. [Google Scholar]
- Donnez, J.; Taylor, R.N.; Taylor, H.S. Partial suppression of estradiol. a new strategy in endometriosis management? Fertil. Steril. 2017, 107, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Donnez, O.; Donnez, J. P–299 Efficacy and safety of linzagolix for the treatment of severe adenomyosis. Initial results from a pilot study. Hum. Reprod. 2021, 36 (Suppl. 1), deab130.298. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Fellah, L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019, 111, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Tosti, C.; Marcellin, L.; Bourdon, M.; Lafay-Pillet, M.-C.; Millischer, A.-E.; Streuli, I.; Borghese, B.; Petraglia, F.; Santulli, P. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum. Reprod. 2017, 32, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Busard, M.; Mijatovic, V.; Lüchinger, A.; Bleeker, M.; Pieters-van den Bos, I.C.; Schats, R.; Van Kuijk, C.; Hompes, P.; Van Waesberghe, J. MR imaging of bladder endometriosis and its relationship with the anterior uterine wall: Experience in a tertiary referral centre. Eur. J. Radiol. 2012, 81, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Del Forno, S.; Orsini, B.; Iodice, R.; Degli Esposti, E.; Aru, A.C.; Manzara, F.; Lenzi, J.; Raimondo, D.; Seracchioli, R. Ureteral endometriosis, the hidden enemy: Multivariable fractional polynomial approach for evaluation of preoperative risk factors in the absence of ureteral dilation. Fertil. Steril. 2021, 116, 470–477. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnez, J.; Stratopoulou, C.A.; Dolmans, M.-M. Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists. Int. J. Environ. Res. Public Health 2021, 18, 9941. https://doi.org/10.3390/ijerph18199941
Donnez J, Stratopoulou CA, Dolmans M-M. Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists. International Journal of Environmental Research and Public Health. 2021; 18(19):9941. https://doi.org/10.3390/ijerph18199941
Chicago/Turabian StyleDonnez, Jacques, Christina Anna Stratopoulou, and Marie-Madeleine Dolmans. 2021. "Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists" International Journal of Environmental Research and Public Health 18, no. 19: 9941. https://doi.org/10.3390/ijerph18199941
APA StyleDonnez, J., Stratopoulou, C. A., & Dolmans, M.-M. (2021). Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists. International Journal of Environmental Research and Public Health, 18(19), 9941. https://doi.org/10.3390/ijerph18199941







