Implementation of the EIRA 3 Intervention by Targeting Primary Health Care Practitioners: Effectiveness in Increasing Physical Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.2.1. PHC Centers
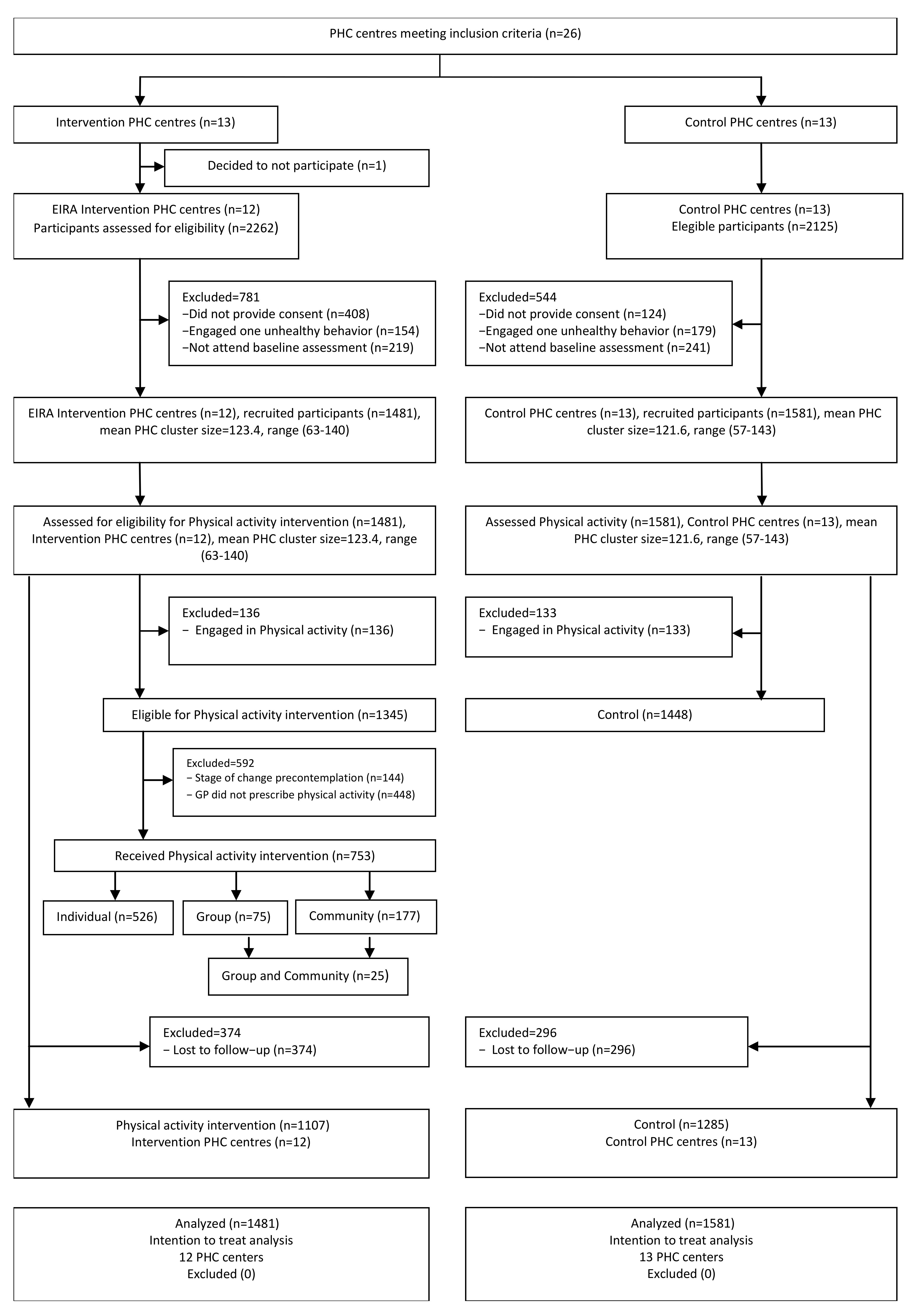
2.2.2. Participants
2.2.3. Assignment of PHC Centers to Different Interventions
2.2.4. Intervention Group
2.2.5. Usual Care Group
2.3. Outcomes
2.3.1. Primary Outcome
2.3.2. Secondary Outcomes
2.4. Sample Size
2.5. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Non-Communicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 November 2020).
- Rook, A. An investigation into the longevity of Cambridge sportsmen. Br. Med. J. 1954, 1, 773–777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mora, S.; Cook, N.; Buring, J.E.; Ridker, P.M.; Lee, I.M. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation 2007, 116, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Kahn, E.B.; Ramsey, L.T.; Brownson, R.C.; Heath, G.W.; Howze, E.H.; Powell, K.E.; Stone, E.J.; Rajab, M.W.; Corso, P. The effectiveness of interventions to increase physical activity. A systematic review. Am. J. Prev. Med. 2002, 22, 73–107. [Google Scholar] [CrossRef]
- Townsend, N.; Wickramasinghe, K.; Williams, J.; Bhatnagar, P.; Rayner, M. Physical Activity Statistics 2015; British Heart Foundation: London, UK, 2015. [Google Scholar]
- Westerterp, K.R. Daily physical activity and ageing. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Chastin, S.F.M.; Skelton, D.A. Prevalence of sedentary behavior in older adults: A systematic review. Int. J. Environ. Res. Public Health 2013, 10, 6645–6661. [Google Scholar] [CrossRef]
- Cárdenas Fuentes, G.; Bawaked, R.A.; Martínez González, M.Á.; Corella, D.; Subirana Cachinero, I.; Salas-Salvadó, J.; Estruch, R.; Serra-Majem, L.; Ros, E.; Lapetra Peralta, J.; et al. Association of physical activity with body mass index, waist circumference and incidence of obesity in older adults. Eur. J. Public Health 2018, 28, 944–950. [Google Scholar] [CrossRef]
- Zou, Q.; Wang, H.; Su, C.; Du, W.; Ouyang, Y.; Jia, X.; Wang, Z.; Ding, G.; Zhang, B. Longitudinal association between physical activity and blood pressure, risk of hypertension among Chinese adults: China Health and Nutrition Survey 1991–2015. Eur. J. Clin. Nutr. 2021, 75, 274–282. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- WHO/Europe. Physical Activity—Data and Statistics. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/physical-activity/data-and-statistics. (accessed on 20 November 2020).
- Kohl, H.W., 3rd; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. Lancet Physical Activity Series Working Group. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- The European Public Health Alliance (EPHA), the ECDA (ECDA) and the NA. Towards an EU Strategic Framework for the Prevention of Non-communicable Diseases (NCDs). Available online: https://epha.org/joint-paper-i-towards-an-eu-strategic-framework-for-the-prevention-of-ncds/ (accessed on 2 October 2021).
- WHO. Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity. (accessed on 20 November 2020).
- Grandes, G.; Sánchez, A.; Torcal, J.; Sánchez-Pinilla, R.O.; Lizarraga, K.; Serra, J. Targeting physical activity promotion in general practice: Characteristics of inactive patients and willingness to change. BMC Public Health 2008, 8, 172. [Google Scholar] [CrossRef]
- Lewis, B.S.; Lynch, W.D. The effect of physician advice on exercise behavior. Prev. Med. 1993, 22, 110–121. [Google Scholar] [CrossRef]
- Grandes, G.; Sanchez, A.; Sanchez-Pinilla, R.O.; Torcal, J.; Montoya, I.; Lizarraga, K.; Serra, J.; PEPAF Group. Effectiveness of physical activity advice and prescription by physicians in routine primary care a cluster randomized trial. Arch. Intern. Med. 2009, 169, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Hillsdon, M.; Thorogood, M. Interventions for promoting physical activity. Cochrane Database Syst. Rev. 2005, 1, CD003180. [Google Scholar] [CrossRef]
- Reis, R.S.; Salvo, D.; Ogilvie, D.; Lambert, E.V.; Goenka, S.; Brownson, R.C. Lancet Physical Activity Series 2 Executive Committee. Scaling up physical activity interventions worldwide: Stepping up to larger and smarter approaches to get people moving. Lancet 2016, 388, 1337–1348. [Google Scholar] [CrossRef]
- Sanchez, A.; Bully, P.; Martinez, C.; Grandes, G. Effectiveness of physical activity promotion interventions in primary care: A review of reviews. Prev. Med. 2015, 76, S56–S67. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.P.; Blunt, W.; Bartol, C.; Pulford, R.W.; De Cruz, A.; Simmavong, P.K.; Gavarkovs, A.; Newhouse, I.; Pearson, E.; Ostenfeldt, B.; et al. HealtheStepsTM Study Protocol: A pragmatic randomized controlled trial promoting active living and healthy lifestyles in at-risk Canadian adults delivered in primary care and community-based clinics. BMC Public Health 2017, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta-Del-Olmo, E.; Pombo, H.; Pons-Vigués, M.; Casajuana-Closas, M.; Pujol-Ribera, E.; López-Jiménez, T.; Cabezas-Peña, C.; Martín-Borràs, C.; Serrano-Blanco, A.; Rubio-Valera, M.; et al. Complex multiple risk intervention to promote healthy behaviours in people between 45 to 75 years attended in primary health care (EIRA study): Study protocol for a hybrid trial. BMC Public Health 2018, 18, 874. [Google Scholar]
- Marshall, A.L.; Smith, B.J.; Bauman, A.E.; Kaur, S. Reliability and validity of a brief physical activity assessment for use by family doctors. Br. J. Sports Med. 2005, 39, 294–297. [Google Scholar] [CrossRef]
- Puig-Ribera, A.; Martín-Cantera, C.; Puigdomenech, E.; Real, J.; Romaguera, M.; Magdalena-Belio, J.F.; Recio-Rodríguez, J.I.; Rodriguez-Martin, B.; Arietaleanizbeaskoa, M.S.; Repiso-Gento, I.; et al. Screening physical activity in family practice: Validity of the Spanish version of a brief physical activity questionnaire. PLoS ONE 2015, 10, e0136870. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Redding, C.; Evers, K. The transtheoretical model and stage of change. In Health Behavior and Health Education: Theory, Research, and Practice; Jossey-Bass: San Francisco, CA, USA, 2002; pp. 99–120. [Google Scholar]
- Bully, P.; Sánchez, Á.; Zabaleta-del-Olmo, E.; Pombo, H.; Grandes, G. Evidence from interventions based on theoretical models for lifestyle modification (physical activity, diet, alcohol and tobacco use) in primary care settings: A systematic review. Prev. Med. 2015, 76, S76–S93. [Google Scholar] [CrossRef]
- Goldstein, M.G.; Whitlock, E.P.; DePue, J. Planning Committee of the Addressing Multiple Behavioral Risk Factors in Primary Care Project. Multiple behavioral risk factor interventions in primary care: Summary of research evidence. Am. J. Prev. Med. 2004, 27, 61–79. [Google Scholar] [CrossRef]
- Bully, P.; Sanchez, A.; Grandes, G.; Pombo, H.; Arietalenizbeaskoa, M.S.; Arce, V.; Martinez, C.; PVS Group. Metric properties of the “prescribe healthy life” screening questionnaire to detect healthy behaviors: A cross-sectional pilot study. BMC Public Health 2016, 16, 1228. [Google Scholar] [CrossRef]
- Zabaleta-del-Olmo, E.; Bolibar, B.; García-Ortíz, L.; García-Campayo, J.; Llobera, J.; Bellón, J.Á.; Ramos, R. Building interventions in primary health care for long-term effectiveness in health promotion and disease prevention. A focus on complex and multi-risk interventions. Prev. Med. 2015, 76, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Pons-Vigués, M.; Berenguera, A.; Coma-Auli, N.; Pombo-Ramos, H.; March, S.; Asensio-Martínez, A.; Moreno-Peral, P.; Mora-Simón, S.; Martínez-Andrés, M.; Pujol-Ribera, E. Health-care users, key community informants and primary health care workers’ views on health, health promotion, health assets and deficits: Qualitative study in seven Spanish regions. Int J. Equity Health 2017, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Berenguera, A.; Pons-Vigués, M.; Moreno-Peral, P.; March, S.; Ripoll, J.; Rubio-Valera, M.; Pombo-Ramos, H.; Asensio-Martínez, A.; Bolaños-Gallardo, E.; Martínez-Carazo, C.; et al. Beyond the consultation room: Proposals to approach health promotion in primary care according to health-care users, key community informants and primary care centre workers. Health Expect. 2017, 20, 896–910. [Google Scholar] [CrossRef]
- Cantera, C.M.; Puigdomènech, E.; Ballvé, J.L.; Arias, O.L.; Clemente, L.; Casas, R.; Roig, L.; Pérez-Tortosa, S.; Díaz-Gete, L.; Granollers, S. Effectiveness of multicomponent interventions in primary healthcare settings to promote continuous smoking cessation in adults: A systematic review. BMJ Open 2015, 5, e008807. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo-Fernandez, J.A.; Recio-Rodríguez, J.I.; Patino-Alonso, M.C.; Pérez-Arechaederra, D.; Rodriguez-Sanchez, E.; Gomez-Marcos, M.A.; García-Ortiz, L. Effectiveness of interventions applicable to primary health care settings to promote Mediterranean diet or healthy eating adherence in adults: A systematic review. Prev. Med. 2015, 76, S39–S55. [Google Scholar] [CrossRef]
- March, S.; Torres, E.; Ramos, M.; Ripoll, J.; García, A.; Bulilete, O.; Medina, D.; Vidal, C.; Cabeza, E.; Llull, M.; et al. Adult community health-promoting interventions in primary health care: A systematic review. Prev. Med. 2015, 76, S94–S104. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Bueno, C.; Cavero-Redondo, I.; Martínez-Andrés, M.; Arias-Palencia, N.; Ramos-Blanes, R.; Salcedo-Aguilar, F. Effectiveness of multifactorial interventions in primary health care settings for primary prevention of cardiovascular disease: A systematic review of systematic reviews. Prev Med. 2015, 76, S68–S75. [Google Scholar] [CrossRef]
- Recio-Rodríguez, J.I.; Martín-Cantera, C.; González-Viejo, N.; Gómez-Arranz, A.; Arietaleanizbeascoa, M.S.; Schmolling-Guinovart, Y.; Maderuelo-Fernandez, J.A.; Pérez-Arechaederra, D.; Rodriguez-Sanchez, E.; Gómez-Marcos, M.A.; et al. Effectiveness of a smartphone application for improving healthy lifestyles, a randomized clinical trial (EVIDENT II): Study protocol. BMC Public Health 2014, 14, 254. [Google Scholar] [CrossRef]
- SemFYC. Actualización del Programa de Actividades Preventivas y de Promoción de la Salud 2018. Available online: https://www.semfyc.es/actualizacion-del-programa-de-actividades-preventivas-y-de-promocion-de-la-salud-2018/ (accessed on 23 November 2020).
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International physical activity questionnaire: Reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Ramos, R.; Solanas, P.; Cordón, F.; Rohlfs, I.; Elosua, R.; Sala, J.; Masiá, R.; Faixedas, M.T.; Marrugat, J. Comparación de la función de Framingham original y la calibrada del REGICOR en la predicción del riesgo coronario poblacional. Med. Clin. 2003, 121, 521–526. [Google Scholar] [CrossRef]
- Marrugat, J.; Solanas, P.; D’Agostino, R.; Sullivan, L.; Ordovas, J.; Cordón, F.; Ramos, R.; Sala, J.; Masià, R.; Rohlfs, I.; et al. Coronary risk estimation in Spain using a calibrated Framingham function. Rev. Esp. Cardil. 2003, 56, 253–261. [Google Scholar] [CrossRef]
- Adams, G.; Gulliford, M.C.; Ukoumunne, O.C.; Eldridge, S.; Chinn, S.; Campbell, M.J. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J. Clin. Epidemiol. 2004, 57, 785–794. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Morgan, F.; Battersby, A.; Weightman, A.L.; Searchfield, L.; Turley, R.; Morgan, H.; Jagroo, J.; Ellis, S. Adherence to exercise referral schemes by participants-what do providers and commissioners need to know? A systematic review of barriers and facilitators. BMC Public Health 2016, 16, 227. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Holmes, M.; Everson-Hock, E.; Davis, S.; Woods, H.B.; Anokye, N.; Tappenden, P.; Kaltenthaler, E. A systematic review and economic evaluation of exercise referral schemes in primary care: A short report. Health Technol. Assess. 2015, 19, 1–110. [Google Scholar] [CrossRef] [PubMed]
- Pavey, T.G.; Taylor, A.H.; Fox, K.R.; Hillsdon, M.; Anokye, N.; Campbell, J.L.; Foster, C.; Green, C.; Moxham, T.; Mutrie, N.; et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: Systematic review and meta-analysis. BMJ 2011, 343, d6462. [Google Scholar] [CrossRef]
- Williams, N.H.; Hendry, M.; France, B.; Lewis, R.; Wilkinson, C. Effectiveness of exercise-referral schemes to promote physical activity in adults: Systematic review. Br. J. GenPract. 2007, 57, 979–986. [Google Scholar] [CrossRef]
- Schutte, B.A.M.; Haveman-Nies, A.; Preller, L. One-year results of the Beweeg-Kuur lifestyle intervention implemented in Dutch primary healthcare settings. Biomed. Res. Int. 2015, 2015, 484823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arija, V.; Villalobos, F.; Pedret, R.; Vinuesa, A.; Timón, M.; Basora, T.; Aguas, D.; Basora, J.; Pas-a-Pas Research Group. Effectiveness of a physical activity program on cardiovascular disease risk in adult primary health-care users: The “Pas-a-Pas” community intervention trial. BMC Public Health 2017, 17, 576. [Google Scholar] [CrossRef]
- Orrow, G.; Kinmonth, A.L.; Sanderson, S.; Sutton, S. Effectiveness of physical activity promotion based in primary care: Systematic review and meta-analysis of randomised controlled trials. BMJ 2012, 344, e1389. [Google Scholar] [CrossRef]
- Martín-Borràs, C.; Giné-Garriga, M.; Puig-Ribera, A.; Martín, C.; Solà, M.; Cuesta-Vargas, A.I.; PPAF Group. A new model of exercise referral scheme in primary care: Is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open 2018, 8, e017211. [Google Scholar] [CrossRef]
- Conn, V.S.; Hafdahl, A.R.; Mehr, D.R. Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. Am. J. Public Health 2011, 101, 751–758. [Google Scholar] [CrossRef]
- Leenaars, K.E.F.; Florisson, A.M.E.; Smit, E.; Wagemakers, A.; Molleman, G.R.M.; Koelen, M.A. The connection between the primary care and the physical activity sector: Professionals’ perceptions. BMC Public Health 2016, 16, 1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bull, F.C.L.; Schipper, E.C.C.; Jamrozik, K.; Blanksby, B.A. How can and do Australian doctors promote physical activity? Prev. Med. 1997, 26, 866–873. [Google Scholar] [CrossRef]
- Vuori, I. Role of primary health care in physical activity promotion. Dtsch. Z. Sportmed. 2013, 64, 176–182. [Google Scholar] [CrossRef]
- Din, N.U.; Moore, G.F.; Murphy, S.; Wilkinson, C.; Williams, N.H. Health professionals’ perspectives on exercise referral and physical activity promotion in primary care: Findings from a process evaluation of the National Exercise Referral Scheme in Wales. Health Educ. J. 2015, 74, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Hébert, E.T.; Caughy, M.O.; Shuval, K. Primary care providers’ perceptions of physical activity counselling in a clinical setting: A systematic review. Br. J. Sports Med. 2012, 46, 625–631. [Google Scholar] [CrossRef]
| Basal Characteristics of the Participants | Control (n = 1581) | Intervention (n = 1481) |
|---|---|---|
| Sex, n/N (%) | ||
| Men | 709/1581 (44.8%) | 672/1481 (45.4%) |
| Women | 872/1581 (55.2%) | 809/1481 (54.6%) |
| Age (years), mean ± SD | 58.3 ± 8.3 | 57.7 ± 7.9 |
| (mean 95% CI) | (57.9, 58.7) | (57.3, 58.1) |
| Civil status, n/N (%) | ||
| Married/Co-habitant | 1.055/1575 (67.0%) | 1024/1461 (70.1%) |
| Single/Living apart | 520/1575 (33%) | 437/1461 (29.9%) |
| Work status | ||
| Active | 713/1575 (45.3%) | 661/1456 (45.4%) |
| Inactive | 431/1575 (27.4%) | 424/1456 (29.1%) |
| Retired | 431/1575 (27.4%) | 371/1456 (25.5%) |
| Level of studies | ||
| Higher education, university or similar | 267/1572 (17.0%) | 247/1461 (16.9%) |
| High school | 621/1572(39.5%) | 573/1461 (39.2%) |
| Primary school, not finished primary school, or without studies | 684/1572 (43.5%) | 641/1461 (43.9%) |
| Weight (kg), mean ± SD | 78.77 ± 17.41 | 82.3 ± 17.81 |
| (mean 95% CI) | (77.91, 79.63) | (81.38, 83.21) |
| BMI (kg/m2), mean ± SD | 29.3 ± 5.6 | 30.6 ± 5.9 |
| (mean 95% CI) | (29.0, 29.5) | (30.3, 30.9) |
| BMI, n/N (%) | ||
| Normal | 363/1574 (23.1%) | 228/1454 (15.7%) |
| Overweight | 595/1574 (37.8%) | 491/1454 (33.8%) |
| Obese | 616/1574 (39.1%) | 735/1454 (50.6%) |
| Waist circumference (cm), mean ± SD | 99.9 ± 15.0 | 102.0 ± 14.4 |
| (mean 95% CI) | (99.1, 100.6) | (101.2, 102.7) |
| Physical activity level (MET mins/week) mean ± SD | 1794.2 ± 3018.0 | 1480.0 ± 2382.0 |
| (mean 95% CI) | (1644.2, 19442.2) | (1356.7, 1306.3) |
| Physical activity level, n/N (%) | ||
| Low | 709/1557 (45.5%) | 729/1437 (50.7%) |
| Moderate | 648/1557 (41.6%) | 570/1437 (39.7%) |
| High | 200/1557 (12.8%) | 138/1437 (9.6%) |
| Follow WHO recommendations (Yes), n/N (%) | 252/1580 (15.9%) | 205/1476 (13.9%) |
| Moderate-intensity physical activity (min/day) mean ± SD | 20.1 ± 56.4 | 16.4 ± 51.5 |
| (mean 95% CI) | (17.2, 22.9) | (13.7, 19.1) |
| High-intensity physical activity (min/day) mean ± SD | 9.2 ± 45.9 | 7.6 ± 36.1 |
| (mean 95% CI) | (6.9, 11.6) | (5.7, 9.5) |
| Time spent sitting (min/day) mean ± SD | 328.7 ± 188.7 | 321.2 ± 176.9 |
| (mean 95% CI) | (319.2, 338.1) | (311.9, 330.4) |
| Stages of change: physical activity, n/N (%) | ||
| Precontemplation | 643/1548 (41.5%) | 152/1270 (12.0%) |
| Contemplation | 389/1548 (25.1%) | 229/1270 (18.0%) |
| Preparation | 204/1548 (13.2%) | 516/1270 (40.6%) |
| Action | 137/1548 (8.9%) | 266/1270 (20.9%) |
| Maintenance/Termination | 175/1548 (11.3%) | 107/1270 (8.4%) |
| Diet, n/N (%) | ||
| Inadequate | 1261/1576 (80.0%) | 1240/1480 (83.8%) |
| Adequate | 315/1576 (20.0%) | 240/1480 (16.2%) |
| Social support DUKE-UNC-11 mean ± SD | 45.5 ± 8.4 | 45.4 ± 8.9 |
| (mean 95% CI) | (45.0, 45.9) | (44.9, 45.9) |
| Health status EQ5D5L mean ± SD | 67.6 ± 20.0 | 65.0 ± 21.0 |
| (mean 95% CI) | (66.6, 68.6) | (63.9, 66.1) |
| Depression (Yes) n/N (%) | 211/1196 (17.6%) | 219/1140 (19.2%) |
| Tobacco use, n/N (%) | ||
| Non-smoker | 444/1581 (28.1%) | 432/1481 (29.2%) |
| Ex-smoker | 440/1581 (27.8%) | 408/1481 (27.5%) |
| Smoker | 697/1581 (44.1%) | 641/1481 (43.3%) |
| Diabetes (Yes), n/N (%) | 323/1579 (20.5%) | 277/1469 (18.9%) |
| Systolic blood pressure (mmHg) mean ± SD | 130.8 ± 17.5 | 132.85 ± 16.5 |
| (mean 95% CI) | (129.8, 131.8) | (131.8, 133.9) |
| Diastolic blood pressure (mmHg) mean ± SD | 79.88 ± 10.31 | 82.27 ± 10.08 |
| (mean 95% CI) | (79.3, 80.5) | (81.6, 82.9) |
| Hypertension (Yes), n/N (%) | 610/1577 (38.7%) | 587/1459 (40.2%) |
| LDL mean ± SD | 108.3 ± 33.9 | 108.0 ± 39.3 |
| (mean 95% CI) | (104.3, 112.3) | (103.2, 112.8) |
| HDL mean ± SD | 45.9 ± 12.2 | 46.7 ± 12.8 |
| (mean 95% CI) | (44.5, 47.4) | (45.14, 48.28) |
| TG mean ± SD | 131.4 ± 81.7 | 135.1 ± 80.1 |
| (mean 95% CI) | (126.2, 136.7) | (129.6, 140.5) |
| Blood sugar mean ± SD | 102.1 ± 29.6 | 104.3 ± 31.8 |
| (mean 95% CI) | (100.2, 103.9) | (102.2, 106.5) |
| Glycated hemoglobin mean ± SD | 7.19 ± 1.37 | 7.12 ± 1.36 |
| (mean 95% CI) | (7.03, 7.35) | (6.95, 7.29) |
| Use of statin (Yes), n/N (%) | 420/1569 (26.8%) | 449/1462 (30.7%) |
| Cardiovascular disease (Yes), n/N (%) | 87/1568 (5.5%) | 87/1452 (6%) |
| REGICOR Cardiovascular risk mean ± SD | 5.4 ± 0.1 | 5.6 ± 0.1 |
| (mean 95% CI) | (5.2, 5.6) | (5.4, 5.7) |
| Baseline | At 12 Months | Per-Protocol | Intention to Treat | |||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 1581) | Intervention (n = 1481) | Control (n = 1285) | Intervention (n = 1107) | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | |
| MET mins/week mean ± SD | 1794.2 ± 3018.0 | 1480.0 ± 2382.0 | 1508.1 ± 2135.3 | 1507.1 ± 1996.0 | 52.37 | 0.534 | 284.09 | 0.335 |
| (mean 95% CI) | (1644.2–19442.2) | (1356.7–1306.3) | (1387.7, 1628.4) | (1385.0, 1629.2) | (−112.61, 217.34) | (−298.24, 866.42) | ||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||||
| Physical activity (IPAQ7) | 0.800 | 0.822 | ||||||
| Low | 709/1557 (45.5%) | 729/1437 (50.7%) | 505/1220 (41.4%) | 473/1082 (43.7%) | Ref | Ref | ||
| Moderate-High | 848/1557 (55.5%) | 708/1437 (49.3%) | 715/1220 (58.6%) | 609/1082 (56.3%) | 0.98 (0.82, 1.16) | 1.02 (0.85, 1.23) | ||
| Follow WHO recommendations | 0.020 | |||||||
| No | 1328/1580 (84.1%) | 1271/1476 (86,1%) | 1059/1231 (86.0%) | 906/1086 (83.4%) | ref | ref | ||
| Yes | 252/1580 (15.9%) | 205/1476 (13.9%) | 172/1231 (14.0%) | 180/1086 (16.6%) | 1.33 (1.05, 1.70) | 0.019 | 1.29 (1.04, 1.60) | |
| Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | |||||
| BMI (kg/m2) mean ± SD | 29.3 ± 5.6 | 30.6 ± 5.9 | 28.97 ± 5.50 | 30.29 ± 5.65 | −0.11 | 0.134 | 0.03 | 0.961 |
| (mean 95% CI) | (29.0–29.5) | (30.3–30.9) | (28.66, 29.28) | (29.94, 30.63) | (−0.25, 0.03) | (−1.22, 1.28) | ||
| Weight (kg) mean ± SD | 78.77 ± 17.41 | 82.3 ± 17.81 | 78.25 ± 17.31 | 81.36 ± 17.34 | −0.30 | 0.097 | −0.04 | 0.982 |
| (mean 95% CI) | (77.91–79.63) | (81.38–83.21) | (77.28, 79.22) | (80.31, 82.41) | (−0.66, 0.05) | (−3.30, 3.22) | ||
| Waist circumference (cm) mean ± SD | 99.9 ± 15.0 | 102.0 ± 14.4 | 98.67 ± 14.80 | 101.43 ± 14.20 | 0.60 | 0.043 | 1.10 | 0.454 |
| (mean 95% CI) | (99.1–100.6) | (101.2–102.7) | (97.82, 99.52) | (100.55, 102.31) | (0.02, 1.18) | (−1.81, 4.00) | ||
| Systolic BP (mmHg) mean ± SD | 130.8 ± 17.5 | 132.85 ± 16.5 | 131.81 ± 17.14 | 131.00 ± 16.42 | −1.96 | 0.001 | −1.13 | 0.497 |
| (mean 95% CI) | (129.8–131.8) | (131.8–133.9) | (130.84, 132.78) | (130.00, 132.01) | (−3.13, −0.79) | (−4.41, 2.16) | ||
| Diastolic BP (mmHg) mean ± SD | 79.88 ± 10.31 | 82.27 ± 10.08 | 80.07 ± 10.26 | 81.07 ± 9.94 | −0.13 | 0.737 | 0.30 | 0.779 |
| (mean 95% CI) | (79.3–80.5) | (81.6–82.9) | (79.49, 80.65) | (80.46, 81.68) | (−0.89, 0.63) | (−1.80, 2.41) | ||
| LDL(mg/dL) mean ± SD | 108.3 ± 33.9 | 108.0 ± 39.3 | 105.75 ± 32.51 | 104.63 ± 32.09 | −1.92 | 0.165 | −2.49 | 0.530 |
| (mean 95% CI) | (104.3–112.3) | (103.2–112.8) | (100.98–110.51) | (99.80–109.46) | (−4.63, 0.79) | (−10.38, 5.39) | ||
| HDL(mg/dL) mean ± SD | 45.9 ± 12.2 | 46.7 ± 12.8 | 47.76 ± 12.85 | 47.28 ± 13.23 | −0.08 | 0.828 | −0.28 | 0.843 |
| (mean 95% CI) | (44.5–47.4) | (45.14–48.28) | (45.87, 49.64) | (45.29, 49.27) | (−0.85, 0.68) | (−3.08, 2.52) | ||
| TG (mg/dL) mean ± SD | 131.4 ± 81.7 | 135.1 ± 80.1 | 130.9 ± 81.7 | 138.5 ± 81.6 | 5.41 | 0.051 | 7.99 | 0.487 |
| (mean 95% CI) | (126.2–136.7) | (129.6–140.5) | (126.5, 135.3) | (132.9, 144.0) | (−0.02, 10.84) | (−14.76, 30.73) | ||
| Blood sugar (mg/dL) mean ± SD | 102.1 ± 29.6 | 104.3 ± 31.8 | 135.94 ± 46.84 | 135.36 ± 38.75 | −0.72 | 0.491 | 0.88 | 0.763 |
| (mean 95% CI) | (100.2–103.9) | (102.2–106.5) | (129.07–142.81) | (129.52–141.19) | (−2.78, 1.34) | (−4.91, 6.68) | ||
| Glycated hemoglobin (%) mean ± SD | 7.19 ± 1.37 | 7.12 ± 1.36 | 6.97 ± 1.15 | 6.81 ± 1.05 | −0.10 | 0.273 | −0.08 | 0.719 |
| (mean 95% CI) | (7.03–7.35) | (6.95–7.29) | (6.80–7.14) | (6.65–6.97) | (−0.28, 0.08) | (−0.51, 0.35) | ||
| REGICOR mean ± SD | 5.4 ± 0.1 | 5.6 ± 0.1 | 5.33 ± 3.36 | 5.34 ± 3.60 | −0.26 | 0.030 | −0.15 | 0.464 |
| (mean 95% CI) | (5.2–5.6) | (5.4–5.7) | (5.10, 5.55) | (5.08, 5.59) | (−0.50, −0.03) | (−0.56, 0.26) | ||
| Moderate-intensity physical activity (IPAQ7) | 0.038 | 0.100 | ||||||
| (min/day) mean ± SD | 20.1 ± 56.4 | 16.4 ± 51.5 | 16.15 ± 48.73 | 18.54 ± 52.23 | 0.25 | 0.21 | ||
| (mean 95% CI) | (17.2–22.9) | (13.7–19.1) | (13.41, 18.88) | (15.40, 21.68) | (0.01, 0.49) | (−0.04, 0.45) | ||
| High-intensity physical activity (IPAQ7) | 0.491 | 0.402 | ||||||
| (min/day) mean ± SD | 9.2 ± 45.9 | 7.6 ± 36.1 | 6.60 ± 36.61 | 7.08 ± 35.13 | 0.16 | 0.15 | ||
| (mean 95% CI) | (6.9–11.6) | (5.7–9.5) | (4.55, 8.66) | (4.97, 9.19) | (−0.29, 0.60) | (−0.20, 0.49) | ||
| Mins/day spent sitting (IPAQ7) | 0.764 | 0.920 | ||||||
| mean ± SD | 328.7 ± 188.7 | 321.2 ± 176.9 | 314.66 ± 160.94 | 309.48 ± 165.54 | 0.01 | −0.003 | ||
| (mean 95% CI) | (319.2–338.1) | (311.9–330.4) | (305.62, 323.69) | (299.54, 319.43) | (−0.03, 0.04) | (−0.07, 0.06) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Martos, S.; Leiva, A.; Sanchez, Á.; Motrico, E.; Bellón, J.; Aldecoa Landesa, S.; Magallón-Botaya, R.; Casajuana-Closas, M.; Zabaleta-del-Olmo, E.; Bolíbar, B.; et al. Implementation of the EIRA 3 Intervention by Targeting Primary Health Care Practitioners: Effectiveness in Increasing Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 10537. https://doi.org/10.3390/ijerph181910537
Contreras-Martos S, Leiva A, Sanchez Á, Motrico E, Bellón J, Aldecoa Landesa S, Magallón-Botaya R, Casajuana-Closas M, Zabaleta-del-Olmo E, Bolíbar B, et al. Implementation of the EIRA 3 Intervention by Targeting Primary Health Care Practitioners: Effectiveness in Increasing Physical Activity. International Journal of Environmental Research and Public Health. 2021; 18(19):10537. https://doi.org/10.3390/ijerph181910537
Chicago/Turabian StyleContreras-Martos, Sara, Alfonso Leiva, Álvaro Sanchez, Emma Motrico, Juan Bellón, Susana Aldecoa Landesa, Rosa Magallón-Botaya, Marc Casajuana-Closas, Edurne Zabaleta-del-Olmo, Bonaventura Bolíbar, and et al. 2021. "Implementation of the EIRA 3 Intervention by Targeting Primary Health Care Practitioners: Effectiveness in Increasing Physical Activity" International Journal of Environmental Research and Public Health 18, no. 19: 10537. https://doi.org/10.3390/ijerph181910537
APA StyleContreras-Martos, S., Leiva, A., Sanchez, Á., Motrico, E., Bellón, J., Aldecoa Landesa, S., Magallón-Botaya, R., Casajuana-Closas, M., Zabaleta-del-Olmo, E., Bolíbar, B., Maderuelo, J.-Á., & Llobera, J. (2021). Implementation of the EIRA 3 Intervention by Targeting Primary Health Care Practitioners: Effectiveness in Increasing Physical Activity. International Journal of Environmental Research and Public Health, 18(19), 10537. https://doi.org/10.3390/ijerph181910537







