Association of the Reproductive Period with Decreased Estimated Glomerular Filtration Rate in Menopausal Women: A Study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection and Laboratory Measurements
2.3. Diabetes, Hypertension, and Hyperlipidemia in Menopausal Women
2.4. Metabolic Equivalents of Tasks
2.5. Decreased Renal Function
2.6. Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
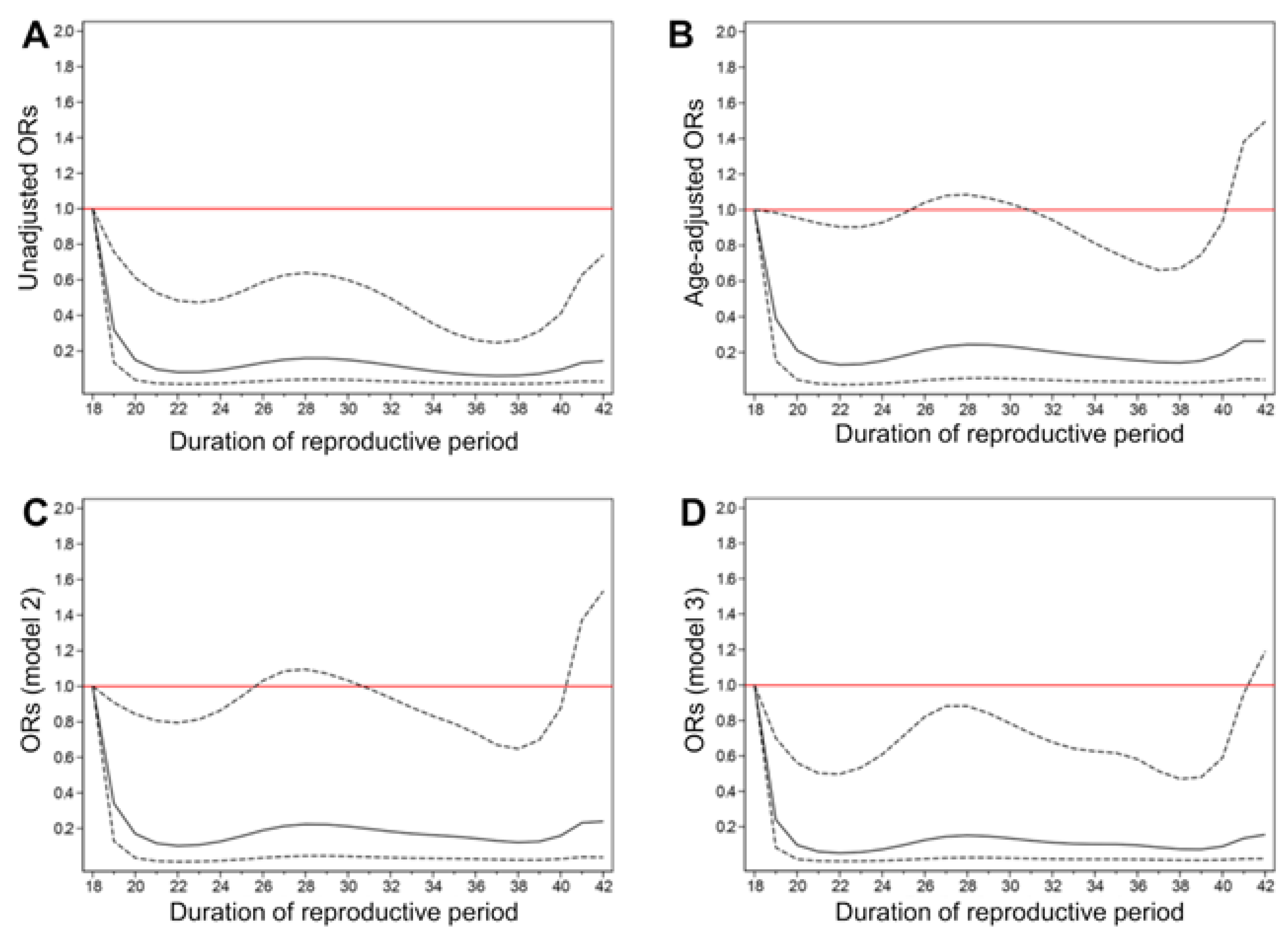
3.2. Association between the Reproductive Period and Decreased Renal Function
3.3. Multivariable Analyses for the Development of Decreased Renal Function
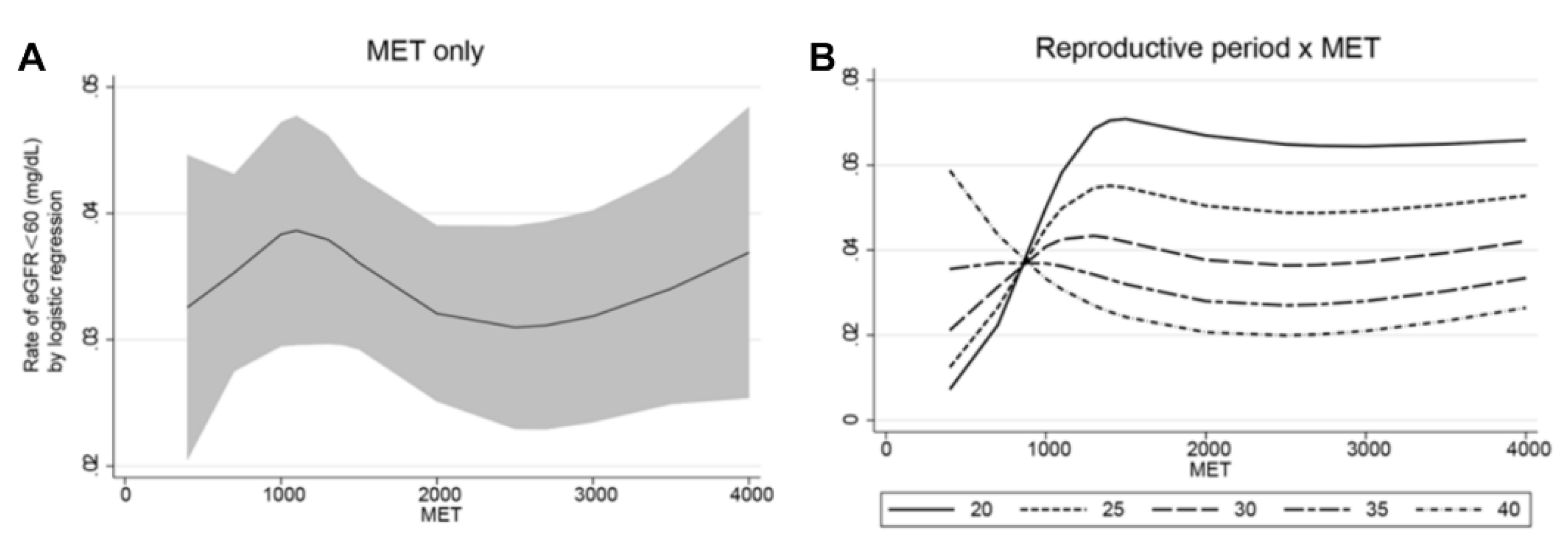
3.4. Interaction between Reproductive Period and Physical Activity on Decreased Renal Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, P.E.; Levin, A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Bikbov, B.; Perico, N.; Remuzzi, G. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018, 139, 313–318. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; CKD Prognosis Consortium. Decline in Estimated Glomerular Filtration Rate and Subsequent Risk of End-Stage Renal Disease and Mortality. JAMA 2014, 311, 2518. [Google Scholar] [CrossRef]
- Hallan, S.I.; Matsushita, K.; Sang, Y.; Mahmoodi, B.K.; Black, C.; Ishani, A.; Kleefstra, N.; Naimark, D.; Roderick, P.; Tonelli, M.; et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012, 308, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kondo, K. Chronic kidney disease in postmenopausal women. Hypertens Res. 2012, 35, 142–147. [Google Scholar] [CrossRef]
- Vellanki, K.; Hou, S. Menopause in CKD. Am. J. Kidney Dis. 2018, 71, 710–719. [Google Scholar] [CrossRef]
- Prince, M.J.; Acosta, D.; Guerra, M.; Huang, Y.; Jimenez-Velazquez, I.Z.; Llibre Rodriguez, J.J.; Valhuerdi, A. Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China; A 10/66 population-based cohort study. PLoS One 2018, 13, e192889. [Google Scholar] [CrossRef]
- Smith, C.A.; McCleary, C.A.; Murdock, G.A.; Wilshire, T.W.; Buckwalter, D.K.; Bretsky, P.; Buckwalter, J.G. Lifelong Estrogen Exposure and Cognitive Performance in Elderly Women. Brain Cogn. 1999, 39, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Park, C.; Yun, Y.D.; Jee, S.H. Duration of ovarian hormone exposure and gynecological cancer risk in Korean women: The Korean Heart Study. Cancer Epidemiol. 2016, 41, 1–7. [Google Scholar] [CrossRef]
- Qiu, C.; Chen, H.; Wen, J.; Zhu, P.; Lin, F.; Huang, B.; Chen, G. Associations Between Age at Menarche and Menopause With Cardiovascular Disease, Diabetes, and Osteoporosis in Chinese Women. J. Clin. Endocrinol. Metab. 2013, 98, 1612–1621. [Google Scholar]
- Kim, K.; Ko, G.J.; Moon, J.Y. Reproductive history and incident CKD In middle-aged Women. Nephrol. Dial. Transpl. 2020, 353, 1105. [Google Scholar]
- Kim, C.; Saran, R.; Hood, M.; Karvonen-Gutierrez, C.; Peng, M.Q.; Randolph, J.F., Jr.; Harlow, S.D. Changes in kidney function during the menopausal transition: The Study of Women’s Health Across the Nation (SWAN)—Michigan site. Menopause 2020, 27, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Jhee, J.H.; Joo, Y.S.; Lee, S.M.; Nam, K.H.; Yun, H.R.; Park, J.T. Association of Reproductive Lifespan Duration and Chronic Kidney Disease in Postmenopausal Women. Mayo Clin. Proc. 2020, 95, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Koo, H. Older menarche age and short reproductive period linked to chronic kidney disease risk. Medicine 2019, 98, e15511. [Google Scholar] [CrossRef]
- Shen, L.; Song, L.; Li, H.; Liu, B.; Zheng, X.; Zhang, L.; Wang, Y. Association between earlier age at natural menopause and risk of diabetes in middle-aged and older Chinese women: The Dongfeng–Tongji cohort study. Diabetes Metab. 2017, 43, 345–350. [Google Scholar] [CrossRef]
- Wang, M.; Hu, R.Y.; Wang, H.; Gong, W.W.; Wang, C.M.; Xie, K.X.; Li, L.M. Age at natural menopause and risk of diabetes in adult women: Findings from the China Kadoorie Biobank study in the Zhejiang area. J. Diabetes Investig. 2018, 9, 762–768. [Google Scholar] [CrossRef]
- Eshtiaghi, R.; Esteghamati, A.; Nakhjavani, M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 2010, 65, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Sim, M.Y.; Park, S.B. Association between duration of reproductive lifespan and Framingham risk score in postmenopausal women. Maturitas 2015, 82, 431–435. [Google Scholar] [CrossRef]
- Mansoor, H.; Elgendy, I.Y.; Segal, R.; Hartzema, A. Duration of Reproductive Years and the Risk of Cardiovascular and Cerebrovascular Events in Older Women: Insights from the National Health and Nutrition Examination Survey. J. Women’s Health 2017, 26, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.M.; Hills, A.P.; Hunter, G.R.; Weinsier, R.L.; Schutz, Y. Metabolic equivalent: One size does not fit all. J. Appl. Physiol. 2005, 99, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Xiao, J.; Shen, C.; Chu, M.J.; Gao, Y.X.; Xu, G.F.; Huang, J.P.; Cai, H. Physical Activity and Sedentary Behavior Associated with Components of Metabolic Syndrome among People in Rural China. PLoS One 2016, 11, e147062. [Google Scholar] [CrossRef]
- Jurj, A.L.; Wen, W.; Gao, Y.T.; Matthews, C.E.; Yang, G.; Li, H.L.; Shu, X.O. Patterns and correlates of physical activity: A cross-sectional study in urban Chinese women. BMC Public Health 2007, 7, 213. [Google Scholar] [CrossRef]
- Kim, K.Z.; Shin, A.; Lee, J.; Myung, S.K.; Kim, J. The Beneficial Effect of Leisure-Time Physical Activity on Bone Mineral Density in Pre- and Postmenopausal Women. Calcif. Tissue Int. 2012, 91, 178–185. [Google Scholar] [CrossRef]
- Dallanezi, G.; Freire, B.F.A.; Nahás, E.A.P.; Nahás-Neto, J.; Corrente, J.E.; Mazeto, G.M.F.D.S. Physical Activity Level of Post-menopausal Women with Low Bone Mineral Density. Revista Brasileira de Ginecologia e Obstetrícia. RBGO Gynecol. Obstet. 2016, 38, 225–230. [Google Scholar]
- Qin, L.; Yang, Z.; Zhang, W.; Gu, H.; Li, X.; Zhu, L.; Su, Q. Syndrome and osteoporotic fracture: A population-based study in China. BMC Endocr. Disord. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Qin, L.; Yang, Z.; Zhang, W.; Gu, H.; Li, X.; Zhu, L.; Su, Q. Physical activity levels and associated cardiovascular disease risk factors among postmenopausal rural women of Bangladesh. Indian. Heart J. 2018, 70, S161–S166. [Google Scholar]
- Janssen, I. Menopause and the Metabolic Syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008, 168, 1568. [Google Scholar] [CrossRef]
- Karvinen, S.; Jergenson, M.J.; Hyvärinen, M.; Aukee, P.; Tammelin, T.; Sipilä, S.; Laakkonen, E.K. Menopausal Status and Physical Activity Are Independently Associated with Cardiovascular Risk Factors of Healthy Middle-Aged Women: Cross-Sectional and Longitudinal Evidence. Front. Endocrinol. 2019, 10, 589. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, B.; Wang, R.; Zhu, M.; Shao, Y.; Wang, N.; Chen, W. Cohort profile: Protocol and baseline survey for the Shanghai Suburban Adult Cohort and Biobank (SSACB) study. BMJ Open 2020, 10, e35430. [Google Scholar] [CrossRef]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 2002, 15, 83–96. [Google Scholar]
- Qiu, Y.; Zhao, Q.; Gu, Y.; Wang, N.; Yu, Y.; Wang, R.; Zhao, G. Association of Metabolic Syndrome and Its Components with Decreased Estimated Glomerular Filtration Rate in Adults. Ann. Nutr. Metab. 2019, 75, 168–178. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Q.; Gu, Y.; Wang, N.; Yu, Y.; Wang, R.; Zhao, G. Association of hypertriglyceridemic waist phenotype with renal function impairment: A cross-sectional study in a population of Chinese adults. Nutr. Metab. 2020, 17, 1–10. [Google Scholar] [CrossRef]
- Aschner, P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes. Res. Clin. Pr. 2017, 132, 169–170. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Oja, P. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Foundation, N.K. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney. Dis. 2012, 60, 850–886. [Google Scholar]
- Feng, Y.; Hong, X.; Wilker, E.; Li, Z.; Zhang, W.; Jin, D.; Xu, X. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 2008, 196, 590–597. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, J.; Yuan, H.; Chen, Z. Duration of reproductive lifespan and age at menarche in relation to metabolic syndrome in postmenopausal Chinese women. J. Obs. Gynaecol. Re. 2016, 42, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; Van Der Schouw, Y.T.; Onland-Moret, N.C.; Sharp, S.J.; Ong, K.K.; Khaw, K.T.; InterAct Consortium. Age at menopause, reproductive life span, and type 2 diabetes risk: Results from the EPIC-InterAct study. Diabetes. Care. 2013, 36, 1012–1019. [Google Scholar] [CrossRef]
- Godsland, I.F. Oestrogens and insulin secretion. Diabetologia 2005, 48, 2213–2220. [Google Scholar] [CrossRef]
- Kummer, S.; von Gersdorff, G.; Kemper, M.J.; Oh, J. The influence of gender and sexual hormones on incidence and outcome of chronic kidney disease. Pediatr. Nephrol. 2012, 27, 1213–1219. [Google Scholar] [CrossRef]
- Gava, A.L.; Freitas, F.P.S.; Meyrelles, S.S.; Silva, I.V.; Graceli, J.B. Gender-dependent effects of aging on the kidney. Braz. J. Med. Biol. Res. 2011, 44, 905–913. [Google Scholar] [CrossRef][Green Version]
- Fung, M.M.; Poddar, S.; Bettencourt, R.; Jassal, S.K.; Barrett-Connor, E. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: The Rancho Bernardo Study. Menopause 2011, 18, 629–637. [Google Scholar] [CrossRef]
- Rossouw, J.E. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Craig, M.C.; Maki, P.M.; Murphy, D.G. The Women’s Health Initiative Memory Study: Findings and implications for treatment. Lancet Neurol. 2005, 4, 190–194. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Henderson, V.W.; Shoupe, D.; Budoff, M.J.; Hwang-Levine, J.; Azen, S.P. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. New Engl. J. Med. 2016, 374, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Harman, S.M.; Black, D.M.; Naftolin, F.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Hodis, H.N. Arterial Imaging Outcomes and Cardiovascular Risk Factors in Recently Menopausal Women: A Randomized Trial. Ann. Intern. Med. 2014, 161, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xiong, L.; Luo, Y.; Huang, Z.; Yi, B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: Evidence from a meta-analysis. BMC Nephrol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Chen, K.; Yan, W.; Wang, A.; Wang, W.; Mu, Y. Association between Duration of Exercise (MET Hours per Week) and the Risk of Decreased eGFR: A Cross-Sectional Study Based on a Large Chinese Population. J. Diabetes. Res. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sárközy, M.; Kovács, Z.Z.; Kovács, M.G.; Gáspár, R.; Szűcs, G.; Dux, L. Mechanisms and Modulation of Oxidative/Nitrative Stress in Type 4 Cardio-Renal Syndrome and Renal Sarcopenia. Front. Physiol. 2018, 9, 1648. [Google Scholar] [CrossRef] [PubMed]
- Barili, A.; da Silva Corralo, V.; Cardoso, A.M.; Mânica, A.; Bonadiman, B.D.S.R.; Bagatini, M.D.; De Sá, C.A. Acute responses of hemodynamic and oxidative stress parameters to aerobic exercise with blood flow restriction in hypertensive elderly women. Mol. Biol. Rep. 2018, 45, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 5503) | Length of Reproductive Period (Years) | p | ||||
|---|---|---|---|---|---|---|---|
| Q1: 18–31 (n = 1103) | Q2: 31–33 (n = 1105) | Q3: 33–35 (n = 1098) | Q4: 35–37 (n = 1102) | Q5: 37–45 (n = 1095) | |||
| Reproductive period (years) | 34.0 (18.0–45.0) | 29.0 (18.0–31.0) | 32.0 (31.0–33.0) | 34.0 (33.0–35.0) | 36.0 (35.0–37.0) | 39.0 (37.0–45.0) | <0.001 |
| Age (years) | 61.0 (36.0–74.0) | 63.0 (36.0–74.0) | 62.0 (45.0–74.0) | 59.0 (38.0–74.0) | 60.0 (48.0–74.0) | 60.0 (46.0–74.0) | <0.001 |
| Reason of menopause | <0.001 | ||||||
| Aging | 5183 (94.2%) | 915 (83.0%) | 1060 (95.9%) | 1051 (95.7%) | 1077 (97.7%) | 1080 (98.6%) | |
| Unnatural | 320 (5.8%) | 188 (17.0%) | 45 (4.1%) | 47 (4.3%) | 25 (2.3%) | 15 (1.4%) | |
| Age at menarche (years) | 16.0 (13.0–19.0) | 17.0 (13.0–19.0) | 17.0 (13.0–19.0) | 16.0 (13.0–19.0) | 16.0 (13.0–19.0) | 15.0 (13.0–19.0) | <0.001 |
| Age at menopause (years) | 50.0 (37.0–58.0) | 45.0 (37.0–50.0) | 50.0 (44.0–52.0) | 50.0 (46.0–54.0) | 52.0 (48.0–56.0) | 54.0 (50.0–58.0) | <0.001 |
| Postmenopausal period (years) | 10.0 (0.0–36.0) | 18.0 (0.0–36.0) | 13.0 (0.0–26.0) | 9.0 (0.0–25.0) | 8.0 (0.0–24.0) | 5.0 (0.0–23.0) | <0.001 |
| Pregnancy times | <0.001 | ||||||
| 0 | 31 (0.6%) | 9 (0.8%) | 7 (0.6%) | 6 (0.5%) | 5 (0.5%) | 4 (0.4%) | |
| 1 | 834 (15.2%) | 113 (10.2%) | 135 (12.2%) | 188 (17.1%) | 207 (18.8%) | 191 (17.4%) | |
| 2 | 2304 (41.9%) | 471 (42.7%) | 452 (40.9%) | 453 (41.3%) | 481 (43.6%) | 447 (40.8%) | |
| ≥3 | 2334 (42.4%) | 510 (46.2%) | 511 (46.2%) | 451 (41.1%) | 409 (37.1%) | 453 (41.4%) | |
| BMI (kg/m2) | 24.4 (14.6–43.6) | 24.5 (15.4–40.7) | 24.2 (14.9–42.1) | 24.0 (15.6–43.6) | 24.4 (15.4–40.8) | 24.9 (14.6–39.1) | <0.001 |
| Education level | <0.001 | ||||||
| Low | 3718 (67.6%) | 841 (76.2%) | 823 (74.5%) | 687 (62.6%) | 669 (60.7%) | 698 (63.7%) | |
| Intermediate | 1767 (32.1%) | 258 (23.4%) | 281 (25.4%) | 406 (37.0%) | 429 (38.9%) | 393 (35.9%) | |
| High | 18 (0.3%) | 4 (0.4%) | 1 (0.1%) | 5 (0.5%) | 4 (0.4%) | 4 (0.4%) | |
| Smoking | 0.29 | ||||||
| Never | 5496 (99.9%) | 1100 (99.7%) | 1105 (100.0%) | 1098 (100.0%) | 1101 (99.9%) | 1092 (99.7%) | |
| Former | 1 (<1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | |
| Current | 6 (0.1%) | 3 (0.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 2 (0.2%) | |
| Family history of CKD | 54 (1.0%) | 10 (0.9%) | 12 (1.1%) | 15 (1.4%) | 8 (0.7%) | 9 (0.8%) | 0.58 |
| Alcohol intake | 34 (0.6%) | 5 (0.5%) | 9 (0.8%) | 2 (0.2%) | 9 (0.8%) | 9 (0.8%) | 0.20 |
| T2DM | 1047 (19.0%) | 206 (18.7%) | 213 (19.3%) | 220 (20.0%) | 209 (19.0%) | 199 (18.2%) | 0.85 |
| Hypertension | 3268 (61.2%) | 663 (62.6%) | 656 (61.3%) | 648 (60.0%) | 630 (58.7%) | 671 (63.5%) | 0.15 |
| Past hormone replacement therapy | 787 (15.2%) | 137 (13.2%) | 174 (16.8%) | 169 (16.3%) | 146 (14.1%) | 161 (15.5%) | 0.12 |
| Laboratory parameters | |||||||
| BUN (mg/dL) | 15.1 (3.4–33.9) | 15.1 (5.6–33.9) | 15.1 (6.2–33.9) | 14.9 (3.4–28.6) | 14.9 (7.0–31.1) | 15.1 (6.7–33.9) | 0.080 |
| Cr (mg/dL) | 0.7 (0.1–1.1) | 0.7 (0.4–1.0) | 0.7 (0.3–1.0) | 0.7 (0.4–1.0) | 0.7 (0.1–1.1) | 0.7 (0.1–1.0) | 0.046 |
| HbA1c (%) | 5.8 (3.6–14.7) | 5.8 (3.8–14.7) | 5.8 (3.6–13.0) | 5.8 (3.6–12.4) | 5.8 (4.0–13.2) | 5.8 (3.7–13.0) | 0.601 |
| TC (mg/dL) | 197.7 (73.3–508.9) | 197.7 (88.8–481.4) | 198.1 (95.7–350.4) | 196.5 (73.3–508.9) | 198.1 (87.6–373.6) | 197.6 (85.7–426.7) | 0.610 |
| HDL-C (mg/dL) | 57.0 (14.0–180.2) | 57.0 (15.1–180.2) | 57.0 (20.2–155.0) | 57.8 (20.2–118.2) | 56.6 (17.4–113.6) | 57.4 (14.0–109.7) | 0.562 |
| TG (mg/dL) | 127.4 (29.2–1347.0) | 129.2 (41.6–1347.0) | 125.7 (30.1–1342.0) | 124.8 (29.2–994.7) | 128.3 (38.1–1110.0) | 127.4 (45.1–1296.0) | 0.570 |
| LDL-C (mg/dL) | 111.6 (0.0–327.0) | 110.4 (0.0–327.0) | 113.5 (15.4–257.1) | 111.2 (3.9–320.8) | 111.6 (10.8–279.9) | 111.6 (4.2–317.8) | 0.630 |
| ALB (g/dL) | 5.0 (3.3–6.1) | 4.9 (3.3–6.1) | 5.0 (4.0–5.8) | 5.0 (3.9–6.0) | 5.0 (4.0–5.8) | 4.9 (3.9–5.8) | 0.808 |
| Hb (g/dL) | 13.5 (5.8–18.0) | 13.4 (5.8–16.2) | 13.4 (9.7–16.5) | 13.5 (9.9–18.0) | 13.5 (11.0–16.2) | 13.5 (8.4–15.9) | 0.189 |
| UA (mg/dL) | 4.6 (1.8–10.8) | 4.6 (2.1–10.0) | 4.5 (1.8–9.8) | 4.5 (1.8–10.8) | 4.6 (2.0–9.3) | 4.6 (2.3–9.8) | 0.209 |
| eGFR (mg/dL) | 92.2 (60.1–194.5) | 90.7 (60.7–117.3) | 91.2 (60.8–130.4) | 92.9 (60.3–116.4) | 93.2 (60.1–177.0) | 92.6 (60.3–194.5) | <0.001 |
| MET | 1386 (160–6678) | 1386 (160–6678) | 1386 (160–6678) | 1386 (160–6678) | 1440 (160–6678) | 1386 (160–6678) | 0.164 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Reproductive period | ||||||
| Q1: 18–31 | 1.00 | - | 1.00 | - | 1.00 | - |
| Q2: 31–33 | 0.83 [0.56,1.25] | 0.375 | 0.73 [0.47,1.16] | 0.187 | 0.68 [0.43,1.09] | 0.109 |
| Q3: 33–35 | 0.73 [0.46,1.16] | 0.185 | 0.58 [0.35,0.96] | 0.035 | 0.56 [0.34,0.94] | 0.027 |
| Q4: 35–37 | 0.66 [0.41,1.05] | 0.082 | 0.68 [0.41,1.13] | 0.139 | 0.66 [0.40,1.10] | 0.113 |
| Q5: 37–45 | 0.53 [0.30,0.96] | 0.036 | 0.65 [0.39,1.07] | 0.091 | 0.59 [0.35,1.00] | 0.049 |
| Per 1-year increase | 0.96 [0.93,1.00] | 0.030 | 0.97 [0.94,1.01] | 0.144 | 0.97 [0.93,1.00] | 0.071 |
| OR (95% CI) | p | |
|---|---|---|
| Reproductive period | ||
| Q1: 18–31 | 1.00 [1.00,1.00] | . |
| Q2: 31–33 | 0.74 [0.46,1.20] | 0.219 |
| Q3: 33–35 | 0.51 [0.29,0.92] | 0.024 |
| Q4: 35–37 | 0.74 [0.44,1.27] | 0.276 |
| Q5: 37–45 | 0.61 [0.34,1.08] | 0.091 |
| MET | 1.10 [0.73,1.65] | 0.656 |
| Reproductive period × MET | ||
| Q1 × MET | 1.00 [1.00,1.00] | . |
| Q2 × MET | 1.22 [0.61,2.42] | 0.573 |
| Q3 × MET | 1.98 [0.91,4.29] | 0.083 |
| Q4 × MET | 0.87 [0.42,1.80] | 0.712 |
| Q5 × MET | 0.43 [0.23,0.81] | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhao, Q.; Jiang, Y.; Wang, N.; Liu, X.; Qiu, Y.; Zhu, J.; Tong, X.; Cui, S.; Zaid, M.; et al. Association of the Reproductive Period with Decreased Estimated Glomerular Filtration Rate in Menopausal Women: A Study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020). Int. J. Environ. Res. Public Health 2021, 18, 10451. https://doi.org/10.3390/ijerph181910451
Yu Y, Zhao Q, Jiang Y, Wang N, Liu X, Qiu Y, Zhu J, Tong X, Cui S, Zaid M, et al. Association of the Reproductive Period with Decreased Estimated Glomerular Filtration Rate in Menopausal Women: A Study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020). International Journal of Environmental Research and Public Health. 2021; 18(19):10451. https://doi.org/10.3390/ijerph181910451
Chicago/Turabian StyleYu, Yuting, Qi Zhao, Yonggen Jiang, Na Wang, Xing Liu, Yun Qiu, Junjie Zhu, Xin Tong, Shuheng Cui, Maryam Zaid, and et al. 2021. "Association of the Reproductive Period with Decreased Estimated Glomerular Filtration Rate in Menopausal Women: A Study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020)" International Journal of Environmental Research and Public Health 18, no. 19: 10451. https://doi.org/10.3390/ijerph181910451
APA StyleYu, Y., Zhao, Q., Jiang, Y., Wang, N., Liu, X., Qiu, Y., Zhu, J., Tong, X., Cui, S., Zaid, M., Li, J., Yu, J., & Zhao, G. (2021). Association of the Reproductive Period with Decreased Estimated Glomerular Filtration Rate in Menopausal Women: A Study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020). International Journal of Environmental Research and Public Health, 18(19), 10451. https://doi.org/10.3390/ijerph181910451






