The Thermal Influence of Oral Rehabilitation on the Cranio-Cervico-Mandibular Complex: A Thermographic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Sample
2.3. Thermographic Procedure
2.4. Data Analysis
3. Results
3.1. Thermographic Characterization of the CCMC Structures
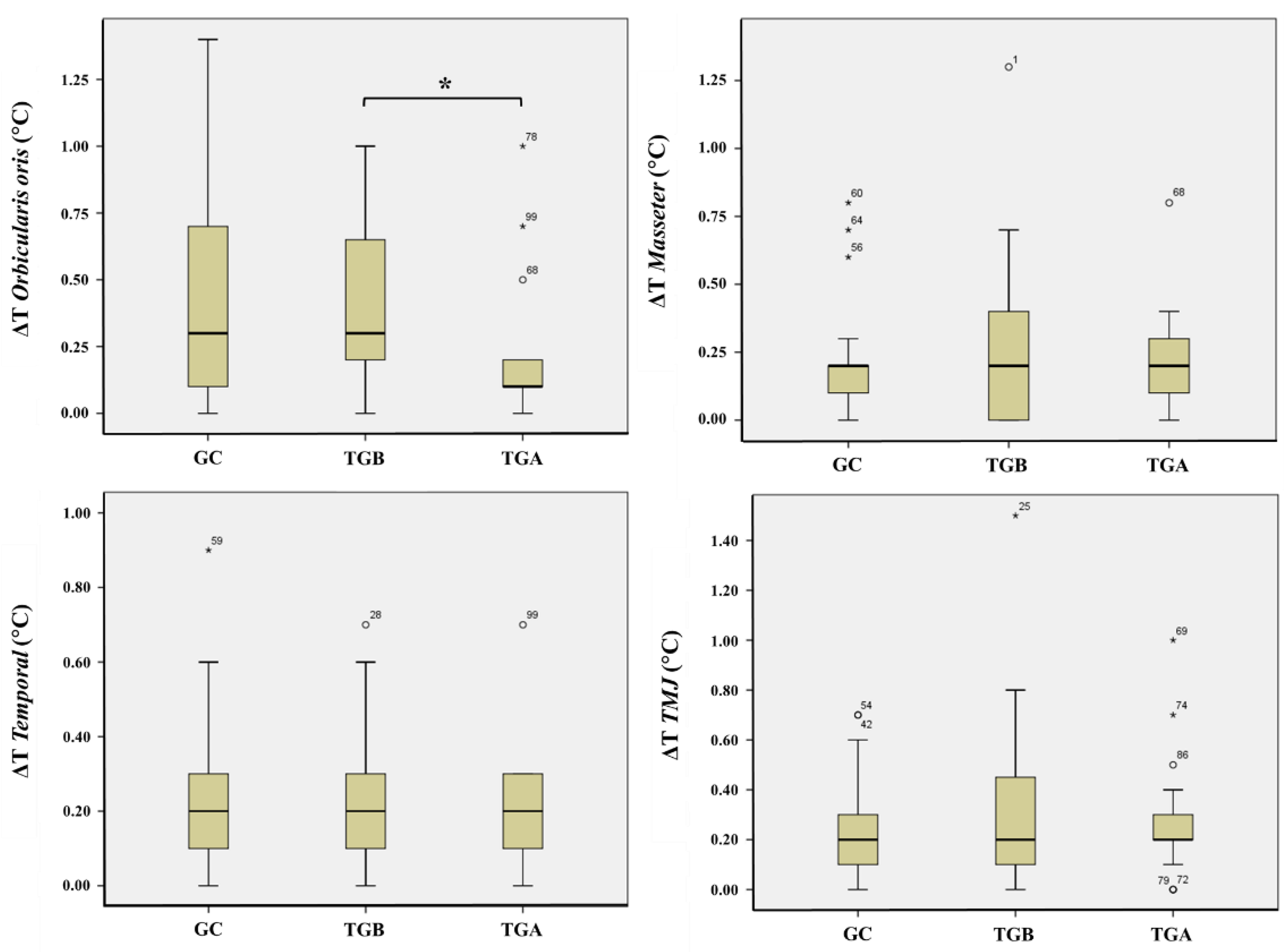
3.2. Comparative Analysis of the Thermal Difference (ΔT) of the CCMC Structures
3.3. Comparative Analysis of the Health Status of the CCMC Structures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, P.W. Dental Functional Morphology—How Teeth Work; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Dawson, P.E. Functional Occlusion: From TMJ to Smile Design; Mosby Elsevier: Maryland Heights, MO, USA, 2007. [Google Scholar]
- Okeson, J.P. Management of Temporomandibular Disorders and Occlusion, 6th ed.; Mosby: Maryland Heights, MO, USA, 1994. [Google Scholar]
- De Souza, F.I.; de Souza Costa, A.; Dos Santos Pereira, R.; Dos Santos, P.H.; de Brito, R.B., Jr.; Rocha, E.P. Assessment of Satisfaction Level of Edentulous Patients Rehabilitated with Implant-Supported Prostheses. Int. J. Oral Maxillofac. Surg. 2016, 31, 884–890. [Google Scholar] [CrossRef]
- Bortoluzzi, M.C.; Traebert, J.; Lasta, R.; Da Rosa, T.N.; Capella, D.L.; Presta, A.A. Tooth loss, chewing ability and quality of life. Contemp. Clin. Dent. 2012, 3, 393–397. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; de Resende, C.M.; Lopes, A.L.; Farias-Neto, A.; Carreiro Ada, F. Association between prosthetic factors and temporomandibular disorders in complete denture wearers. Gerodontology 2014, 31, 308–313. [Google Scholar] [CrossRef]
- Bevilaqua-Grossi, D.; Chaves, T.C.; de Oliveira, A.S. Cervical spine signs and symptoms: Perpetuating rather than predisposing factors for temporomandibular disorders in women. J. Appl. Oral Sci. 2007, 15, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuginski-Barbosa, J.; Macedo, H.R.; Bigal, M.E.; Speciali, J.G. Signs of temporomandibular disorders in migraine patients: A prospective, controlled study. Clin. J. Pain 2010, 26, 418–421. [Google Scholar] [CrossRef]
- Fontijn-Tekampl, E.; Slagter, A.P.; van’t Hof, M.A.; Geertman, M.E.; Kalk, W. Bite Forces with Mandibular Implant-retained Overdentures. J. Dent. Res. 1998, 77, 1832–1839. [Google Scholar] [CrossRef]
- Van Kampen, F.M.; van der Bilt, A.; Cune, M.S.; Bosman, F. The influence of various attachment types in mandibular implant-retained overdentures on maximum bite force and EMG. J. Dent. Res. 2002, 81, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Zanotti, G.; Tartaglia, G.M. Maximal bite forces in healthy young adults as predicted by surface electromyography. J. Dent. 2004, 32, 451–457. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Tartaglia, G.M.; Maglione, M.; Simion, M.; Sforza, C. Neuromuscular coordination of masticatory muscles in subjects with two types of implant-supported prostheses. Clin. Oral Implant. Res. 2004, 15, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Van der Bilt, A.; Tekamp, A.; van der Glas, H.; Abbink, J. Bite force and electromyograpy during maximum unilateral and bilateral clenching. Eur. J. Oral Sci. 2008, 116, 217–222. [Google Scholar] [CrossRef]
- Fontijn-Tekamp, F.A.; Slagter, A.P.; Van Der Bilt, A.; Van, T.H.M.A.; Witter, D.J.; Kalk, W. Biting and chewing in overdentures, full dentures, and natural dentitions. J. Dent. Res. 2000, 79, 1519–1524. [Google Scholar] [CrossRef]
- Feine, J.S.; Lund, J.P. Measuring chewing ability in randomized controlled trials with edentulous populations wearing implant prostheses. J. Oral Rehabil. 2006, 33, 301–308. [Google Scholar] [CrossRef]
- Fueki, K.; Kimoto, K.; Ogawa, T.; Garrett, N.R. Effect of implant-supported or retained dentures on masticatory performance: A systematic review. J. Prosthet. Dent. 2007, 98, 470–477. [Google Scholar] [CrossRef]
- Elsyad, M.A.; Hegazy, S.A.; Hammouda, N.I.; Al-Tonbary, G.Y.; Habib, A.A. Chewing efficiency and electromyographic activity of masseter muscle with three designs of implant-supported mandibular overdentures. A cross-over study. Clin. Oral Implant. Res. 2014, 25, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Von der Gracht, I.; Derks, A.; Haselhuhn, K.; Wolfart, S. EMG correlations of edentulous patients with implant overdentures and fixed dental prostheses compared to conventional complete dentures and dentates: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2017, 28, 765–773. [Google Scholar] [CrossRef]
- Goiato, M.C.; Garcia, A.R.; dos Santos, D.M. Electromyographic Activity of the Mandible Muscles at the Beginning and End of Masticatory Cycles in Patients with Complete Dentures. Gerontology 2008, 54, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Gratt, B.M.; Sickles, E.A. Thermographic characterization of the asymptomatic temporomandibular joint. J. Orofac. Pain 1993, 7, 7–14. [Google Scholar] [PubMed]
- Pogrel, M.A.; McNeill, C.; Kim, J.M. The assessment of trapezius muscle symptoms of patients with temporomandibular disorders by the use of liquid crystal thermography. Oral Surg. Oral Med. Oral Pathol Oral Radiol. Endodontology 1996, 82, 145–151. [Google Scholar] [CrossRef]
- Steed, P.A. The Utilization of Contact Liquid Crystal Thermography in the Evaluation of Temporomandibular Dysfunction. Cranio J. Craniomandib. Sleep Pract. 1991, 9, 120–128. [Google Scholar] [CrossRef]
- Dibai Filho, A.V.; Packer, A.C.; Costa, A.C.; Rodrigues-Bigaton, D. Accuracy of infrared thermography of the masticatory muscles for the diagnosis of myogenous temporomandibular disorder. J. Manip. Physiol. Ther. 2013, 36, 245–252. [Google Scholar] [CrossRef]
- Gratt, B.M.; Graff-Radford, S.B.; Shetty, V.; Solberg, W.K.; Sickles, E.A. A 6-year clinical assessment of electronic facial thermography. Dentomaxillofacial Radiol. 1996, 25, 247–255. [Google Scholar] [CrossRef]
- Merla, A.; Romani, G. Biomedical applications of functional infrared imaging. In Proceedings of the 2005 IEEE Conference of Engineering in Medicine and Biology, 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 690–693. [Google Scholar]
- Moreira, A.; Batista, R.; Oliveira, S.; Aguiar Branco, C.; Mendes, J.; Figueiral, M.H. Role of thermography in the assessment of temporomandibular disorders and other musculoskeletal conditions: A systematic review. Proc. Inst. Mech. Eng. H J. Eng. Med. 2021, 235, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Lovgren, A.; Marklund, S.; Visscher, C.M.; Lobbezoo, F.; Haggman-Henrikson, B.; Wanman, A. Outcome of three screening questions for temporomandibular disorders (3Q/TMD) on clinical decision-making. J. Oral Rehabil. 2017, 44, 573–579. [Google Scholar] [CrossRef]
- Lovgren, A.; Parvaneh, H.; Lobbezoo, F.; Haggman-Henrikson, B.; Wanman, A.; Visscher, C.M. Diagnostic accuracy of three screening questions (3Q/TMD) in relation to the DC/TMD in a specialized orofacial pain clinic. Acta Odontol. Scand. 2018, 76, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Ammer, K. The Glamorgan Protocol for recording and evaluation of thermal images of the human body. Thermol. Int. 2008, 18, 125–129. [Google Scholar]
- Fernández Cuevas, I.; Marins, J.; Arnaiz-Lastras, J.; Carmona, P.; Cano, S.; García-Concepción, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 22–58. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef] [PubMed]
- De Caxias, F.P.; Dos Santos, D.M.; Goiato, M.C.; Bitencourt, S.B.; da Silva, E.V.F.; Laurindo, M.C.B., Jr. Effects of mouth rehabilitation with removable complete dentures on stimulus perception and the electromyographic activity of the orbicularis oris muscle. J. Prosthet. Dent. 2018, 119, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.M.; Vitti, M.; Chiarello de Mattos, M.D.G.; Semprini, M.; de Freitas Oliveira Paranhos, H.; Regalo, S.C. Electromyographic analysis of the upper and lower fascicles of the orbicular oris muscle, in edentulous patients, before and after complete denture implantation. Electromyogr. Clin. Neurophysiol. 2003, 43, 315–320. [Google Scholar] [PubMed]
- Grabowski, R.; Kundt, G.; Stahl, F. Interrelation between occlusal findings and orofacial myofunctional status in primary and mixed dentition: Part III: Interrelation between malocclusions and orofacial dysfunctions. J. Orofac. Orthop. 2007, 68, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Saccucci, M.; Tecco, S.; Ierardoa, G.; Luzzi, V.; Festa, F.; Polimeni, A. Effects of interceptive orthodontics on orbicular muscle activity: A surface electromyographic study in children. J. Electromyogr. Kinesiol. 2011, 21, 665–671. [Google Scholar] [CrossRef] [PubMed]

| Group ROI | (Mean Values of Tm; Mean ± SD, °C) | ||
|---|---|---|---|
| CG (n = 33) | TGB (n = 33) | TGA (n = 19) | |
| Superior Oo | 32.86 ± 2.032 | 32.93 ± 0.946 | 32.87 ± 1.164 |
| Inferior Oo | 32.97 ± 2.017 | 33.92 ± 0.961 | 32.94 ± 1.271 |
| Right Masseter | 32.89 ± 1.854 | 32.79 ± 1.078 | 32.87 ± 1.218 |
| Left Masseter | 32.91 ± 1.862 | 32.84 ± 0.908 | 32.96 ± 1.209 |
| Right Temporal | 34.45 ± 1.562 | 34.03 ± 0.554 | 34.05 ± 0.927 |
| Left Temporal | 34.43 ± 1.418 | 34.00 ± 0.576 | 34.08 ± 0.938 |
| Right TMJ | 33.24 ± 1.863 | 33.12 ± 0.825 | 32.96 ± 1.266 |
| Left TMJ | 33.27 ± 1.832 | 33.08 ± 0.729 | 33.11 ± 1.154 |
| Variable Tested | TGB × TGA (Wilcoxon) | CG × TGB (Mann–Whitney U) | CG × TGA (Mann–Whitney U) |
|---|---|---|---|
| ΔT Oo | 0.020 | 0.301 | 0.085 |
| ΔT Masseter | 0.270 | 0.724 | 0.233 |
| ΔT Temporal | 0.063 | 0.611 | 0.975 |
| ΔT TMJ | 0.204 | 0.823 | 0.173 |
| Variable Tested | Health Status of the ROI | ||
|---|---|---|---|
| CG (Healthy/Abnormal) | TGB (Healthy/Abnormal) | TGA (Healthy/Abnormal) | |
| Oo | 20/12 | 15/18 | 15/2 |
| Masseter | 30/3 | 23/10 | 14/5 |
| Temporal | 30/3 | 29/4 | 19/0 |
| TMJ | 27/5 | 24/9 | 14/8 |
| Variable Tested | TGB × TGA (Chi-Square Test) | CG × TGB (Chi-Square Test) | CG × TGA (Chi-Square Test) |
|---|---|---|---|
| ΔT Oo | 0.003 | 0.168 | 0.058 |
| ΔT Masseter | 0.760 | 0.030 | 0.097 |
| ΔT Temporal | 0.114 | 0.689 | 0.176 |
| ΔT TMJ | 0.940 | 0.253 | 0.353 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.; Batista, R.; Oliveira, S.; Mendes, J.; Sampaio-Fernandes, M.; Figueiral, M.H. The Thermal Influence of Oral Rehabilitation on the Cranio-Cervico-Mandibular Complex: A Thermographic Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10441. https://doi.org/10.3390/ijerph181910441
Moreira A, Batista R, Oliveira S, Mendes J, Sampaio-Fernandes M, Figueiral MH. The Thermal Influence of Oral Rehabilitation on the Cranio-Cervico-Mandibular Complex: A Thermographic Analysis. International Journal of Environmental Research and Public Health. 2021; 18(19):10441. https://doi.org/10.3390/ijerph181910441
Chicago/Turabian StyleMoreira, André, Ricardo Batista, Susana Oliveira, Joaquim Mendes, Margarida Sampaio-Fernandes, and Maria Helena Figueiral. 2021. "The Thermal Influence of Oral Rehabilitation on the Cranio-Cervico-Mandibular Complex: A Thermographic Analysis" International Journal of Environmental Research and Public Health 18, no. 19: 10441. https://doi.org/10.3390/ijerph181910441
APA StyleMoreira, A., Batista, R., Oliveira, S., Mendes, J., Sampaio-Fernandes, M., & Figueiral, M. H. (2021). The Thermal Influence of Oral Rehabilitation on the Cranio-Cervico-Mandibular Complex: A Thermographic Analysis. International Journal of Environmental Research and Public Health, 18(19), 10441. https://doi.org/10.3390/ijerph181910441







