Does Long-Term Training in a Water Immersion Environment Change Interoception?
Abstract
1. Introduction
2. Methods
2.1. Participants
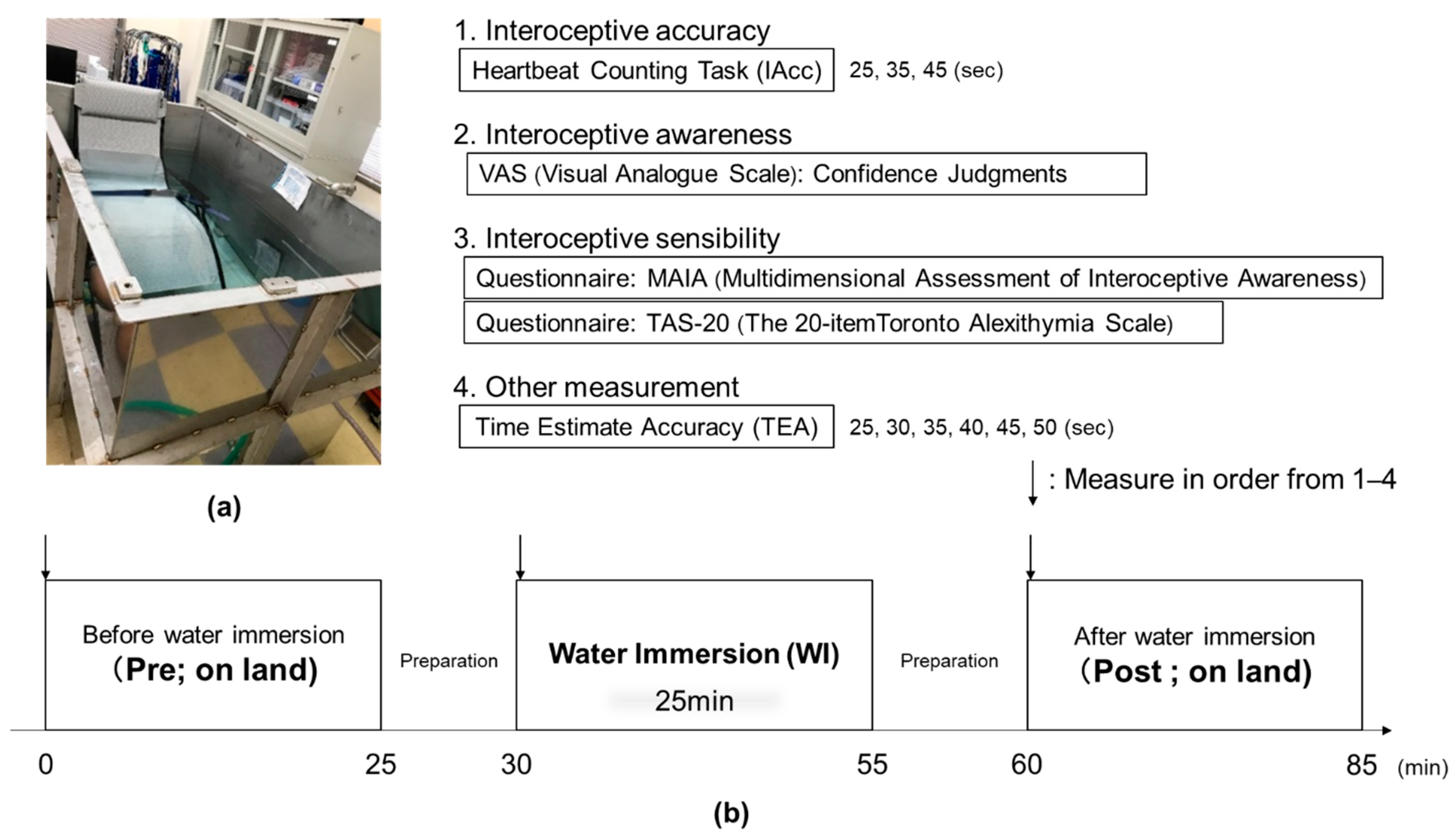
2.2. Experimental Protocol
2.3. Interoceptive Accuracy Data
2.4. Interoceptive Sensibility Data
2.4.1. Multidimensional Assessment of Interoceptive Awareness (MAIA) Questionnaire
2.4.2. Twenty-Item Toronto Alexithymia Scale (TAS-20) Questionnaire
2.5. Interoceptive Awareness Data (Confidence Judgments)
2.6. Time Estimation Accuracy Data
2.7. Statistical Analysis
3. Results
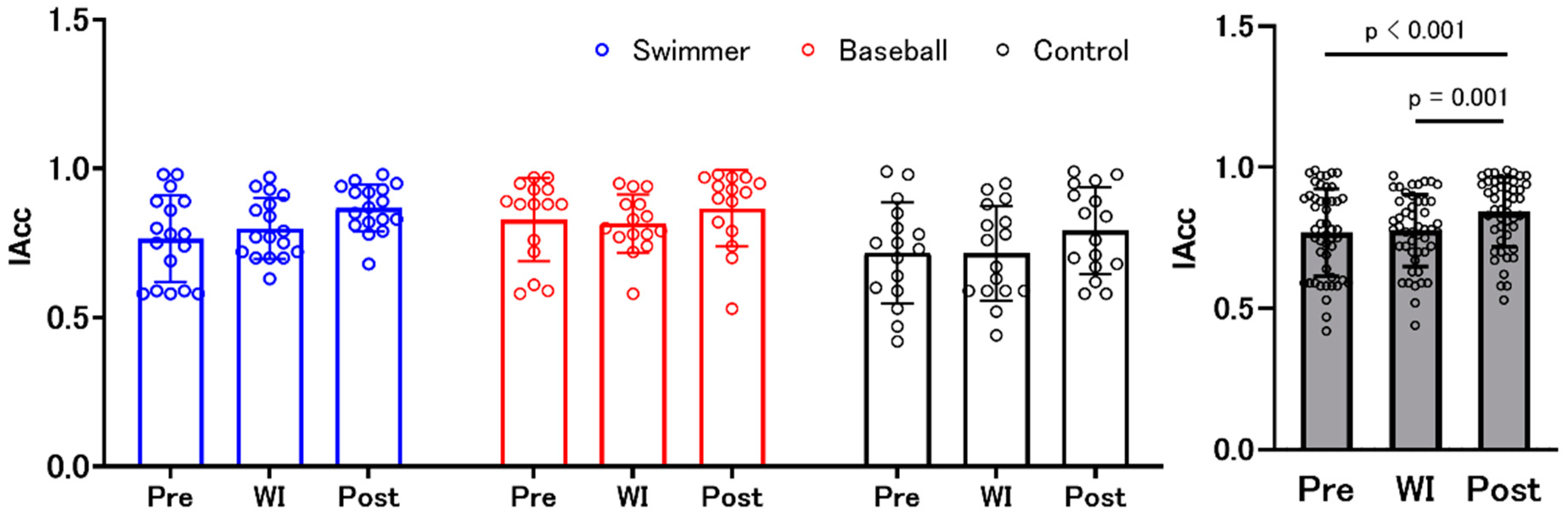
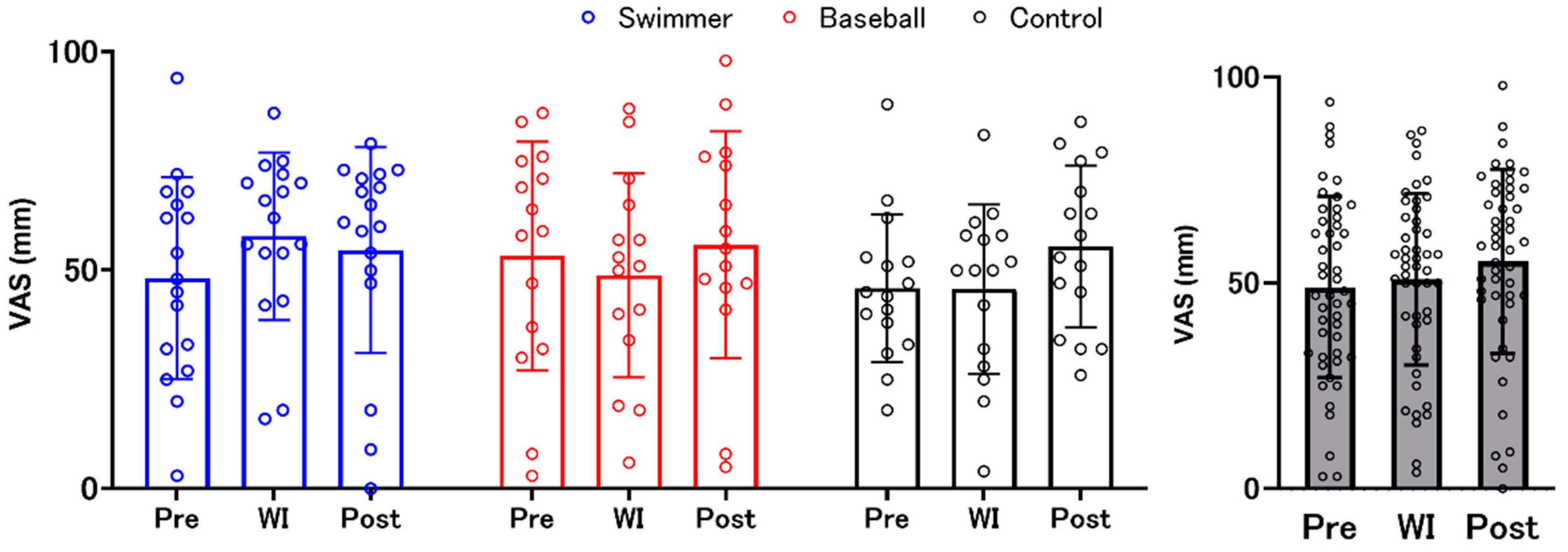
3.1. Interoceptive Accuracy and Interoceptive Awareness (Confidence Judgments)
3.2. MAIA (Interoceptive Sensibility)
3.3. TAS-20
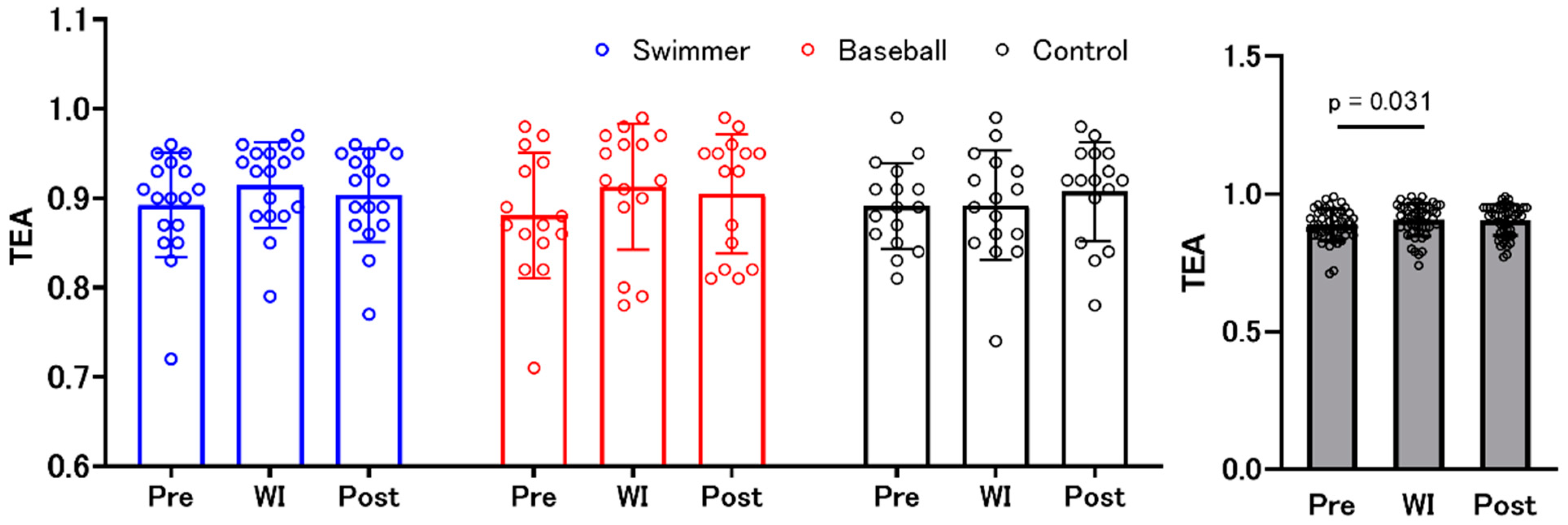
3.4. Time Estimation Accuracy
3.5. Heart Rate
3.6. Correlation between the Change in IAcc Scores and Other Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Tsakiris, M. The multisensory basis of the self: From body to identity to others [Formula: See text]. Q. J. Exp. Psychol. 2017, 70, 597–609. [Google Scholar] [CrossRef]
- Sherrington, C.S. The Integrative Action of the Nervous System; Yale University Press, Constable & Comp., Ltd.: New Haven, CT, USA, 1906. [Google Scholar]
- Domschke, K.; Stevens, S.; Pfleiderer, B.; Gerlach, A.L. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clin. Psychol. Rev. 2010, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cameron, O.G. Interoception: The inside story—A model for psychosomatic processes. Psychosom. Med. 2001, 63, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R. How the brain creates the mind. Sci. Am. 1999, 281, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.D.; Lacasse, K. Bodily influences on emotional feelings: Accumulating evidence and extensions of William James’s theory of emotion. Emot. Rev. 2014, 6, 27–34. [Google Scholar] [CrossRef]
- Scherer, K.R. The dynamic architecture of emotion: Evidence for the component process model. Cogn. Emot. 2009, 23, 1307–1351. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tajadura-Jiménez, A.; Tsakiris, M. Balancing the “inner” and the “outer” self: Interoceptive sensitivity modulates self-other boundaries. J. Exp. Psychol. Gen. 2014, 143, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef]
- Schandry, R. Heart beat perception and emotional experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Drescher, V.M. Perception of gastric contractions and self-control of gastric motility. Psychophysiology 1980, 17, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Drescher, V.M.; Heiman, P.; Blackwell, B. Relation of heart rate control to heartbeat perception. Biofeedback Self-Regul. 1977, 2, 317–392. [Google Scholar] [CrossRef]
- Desmedt, O.; Luminet, O.; Corneille, O. The heartbeat counting task largely involves non-interoceptive processes: Evidence from both the original and an adapted counting task. Biol. Psychol. 2018, 138, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Zamariola, G.; Frost, N.; Van Oost, A.; Corneille, O.; Luminet, O. Relationship between interoception and emotion regulation: New evidence from mixed methods. J. Affect. Disord. 2019, 246, 480–485. [Google Scholar] [CrossRef]
- Bagby, R.M.; Taylor, G.J.; Parker, J.D. The twenty-item Toronto alexithymia scale--II. Convergent, discriminant, and concurrent validity. J. Psychosom. Res. 1994, 38, 33–40. [Google Scholar] [CrossRef]
- Mehling, W.E.; Price, C.; Daubenmier, J.J.; Acree, M.; Bartmess, E.; Stewart, A. The multidimensional assessment of interoceptive awareness (MAIA). PLoS ONE 2012, 7, e48230. [Google Scholar] [CrossRef]
- Aitken, R.C. A growing edge of measurement of feelings using visual analogue scales. R. Soc. Med. 1969, 62, 989–993. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef]
- Strigo, I.A.; Craig, A.D. Interoception, homeostatic emotions and sympathovagal balance. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2016, 371, 28080968. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Manassei, M.F.; Hamilton-Fletcher, G.; In den Bosch, Y.; Critchley, H.D.; Engels, M. Interoceptive dimensions across cardiac and respiratory axes. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2016, 371, 28080971. [Google Scholar] [CrossRef]
- Paulus, M.P.; Flagan, T.; Simmons, A.N.; Gillis, K.; Kotturi, S.; Thom, N.; Johnson, D.C.; Van Orden, K.F.; Davenport, P.W.; Swain, J.L. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS ONE 2012, 7, e29394. [Google Scholar] [CrossRef]
- Tsakiris, M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia 2010, 48, 703–712. [Google Scholar] [CrossRef]
- Ainley, V.; Tsakiris, M. Body conscious? Interoceptive awareness, measured by heartbeat perception, is negatively correlated with self-objectification. PLoS ONE 2013, 8, e55568. [Google Scholar] [CrossRef]
- Myers, T.A.; Crowther, J.H. Is self-objectification related to interoceptive awareness? An examination of potential mediating pathways to disordered eating attitudes. Psychol. Women Q. 2008, 32, 172–180. [Google Scholar] [CrossRef]
- Dalgleish, T. The emotional brain. Nature Reviews. Neuroscience 2004, 5, 583–589. [Google Scholar] [CrossRef]
- Haase, L.; Thom, N.J.; Shukla, A.; Davenport, P.W.; Simmons, A.N.; Stanley, E.A.; Paulus, M.P.; Johnson, D.C. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc. Cogn. Affect. Neurosci. 2016, 11, 182–190. [Google Scholar] [CrossRef]
- Schaan, L.; Schulz, A.; Nuraydin, S.; Bergert, C.; Hilger, A.; Rach, H.; Hechler, T. Interoceptive accuracy, emotion recognition, and emotion regulation in preschool children. Int. J. Psychophysiol. Int. Organ. Psychophysiol. 2019, 138, 47–56. [Google Scholar] [CrossRef]
- Bringoux, L.; Marin, L.; Nougier, V.; Barraud, P.A.; Raphel, C. Effects of gymnastics expertise on the perception of body orientation in the pitch dimension. J. Vestib. Res. 2000, 10, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Gaigg, S.B.; Calvo-Merino, B. I can feel my heartbeat: Dancers have increased interoceptive accuracy. Psychophysiology 2018, 55, e13008. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.M.; Ulbrich, P.; Schandry, R. Interoceptive sensitivity and physical effort: Implications for the self-control of physical load in everyday life. Psychophysiology 2007, 44, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M. Do we really need a central governor to explain brain regulation of exercise performance? Eur. J. Appl. Physiol. 2008, 104, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.P.; Potterat, E.G.; Taylor, M.K.; Van Orden, K.F.; Bauman, J.; Momen, N.; Padilla, G.A.; Swain, J.L. A neuroscience approach to optimizing brain resources for human performance in extreme environments. Neurosci. Biobehav. Rev. 2009, 33, 1080–1088. [Google Scholar] [CrossRef]
- Hirao, T.; Vogt, T.; Masaki, H. Difference in interoception between long-distance runners and sprinters: An Event-related Potential Study. Med. Sci. Sports Exerc. 2020, 52, 1367–1375. [Google Scholar] [CrossRef]
- Schirmer-Mokwa, K.L.; Fard, P.R.; Zamorano, A.M.; Finkel, S.; Birbaumer, N.; Kleber, B.A. Evidence for enhanced interoceptive accuracy in professional musicians. Front. Behav. Neurosci. 2015, 9, 349. [Google Scholar] [CrossRef]
- Sato, D.; Yamazaki, Y.; Yamashiro, K.; Onishi, H.; Baba, Y.; Ikarashi, K.; Maruyama, A. Elite competitive swimmers exhibit higher motor cortical inhibition and superior sensorimotor skills in a water environment. Behav. Brain Res. 2020, 395, 112835. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Petzschner, F.H.; Weber, L.A.; Wellstein, K.V.; Paolini, G.; Do, C.T.; Stephan, K.E. Focus of attention modulates the heartbeat evoked potential. NeuroImage 2019, 186, 595–606. [Google Scholar] [CrossRef]
- Knoll, J.F.; Hodapp, V. A comparison between two methods for assessing heartbeat perception. Psychophysiology 1992, 29, 218–222. [Google Scholar] [CrossRef]
- Sugawara, A.; Terasawa, Y.; Katsunuma, R.; Sekiguchi, A. Effects of interoceptive training on decision making, anxiety, and somatic symptoms. BioPsychoSoc. Med. 2020, 14, 7. [Google Scholar] [CrossRef]
- Ainley, V.; Tajadura-Jiménez, A.; Fotopoulou, A.; Tsakiris, M. Looking into myself: Changes in interoceptive sensitivity during mirror self-observation. Psychophysiology 2012, 49, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Knapp-Kline, K.; Kline, J.P. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: Slow and steady wins the race. Biol. Psychol. 2005, 69, 387–396. [Google Scholar] [CrossRef]
- Fujino, H. Further validation of the Japanese version of the Multidimensional Assessment of Interoceptive Awareness. BMC Res. Notes 2019, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Bojner Horwitz, E.; Lennartsson, A.K.; Theorell, T.P.G.; Ullén, F. Engagement in dance is associated with emotional competence in interplay with others. Front. Psychol. 2015, 6, 1096. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.J.; Bagby, R.M.; Parker, J.D.A. The 20-item Toronto alexithymia scale. IV. Reliability and factorial validity in different languages and cultures. J. Psychosom. Res. 2003, 55, 277–283. [Google Scholar] [CrossRef]
- Komaki, G.; Maeda, M.; Arimura, T.; Nakata, A.; Shinoda, H.; Ogata, I.; Shimura, M.; Kawamura, N.; Kubo, C. The reliability and factorial validity of the Japanese version of the 20-Item Toronto alexithymia Scale. J. Psychosom. Res. 2003, 55, 143. [Google Scholar] [CrossRef]
- Desmedt, O.; Corneille, O.; Luminet, O.; Murphy, J.; Bird, G.; Maurage, P. Contribution of time estimation and knowledge to heartbeat counting task performance under original and adapted instructions. Biol. Psychol. 2020, 154, 107904. [Google Scholar] [CrossRef]
- Wittmann, M. The inner sense of time: How the brain creates a representation of duration. Nat. Rev. Neurosci. 2013, 14, 217–223. [Google Scholar] [CrossRef]
- Zamariola, G.; Maurage, P.; Luminet, O.; Corneille, O. Interoceptive accuracy scores from the heartbeat counting task are problematic: Evidence from simple bivariate correlations. Biol. Psychol. 2018, 137, 12–17. [Google Scholar] [CrossRef]
- Tochikura, I.; Sato, D.; Imoto, D.; Nuruki, A.; Yamashiro, K.; Funada, R.; Maruyama, A. Baseball players’ eye movements and higher coincident-timing task performance. Percept. Mot. Skills 2020, 127, 571–586. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sato, D.; Onishi, H.; Sugawara, K.; Nakazawa, S.; Shimojo, H.; Akatsuka, K.; Nakata, H.; Maruyama, A. Skillspecific changes in somatosensory nogo potentials in baseball players. PLoS ONE 2015, 10, e0142581. [Google Scholar] [CrossRef]
- Critchley, H.D.; Garfinkel, S.N. Interoception and emotion. Curr. Opin. Psychol. 2017, 17, 7–14. [Google Scholar] [CrossRef]
- Ma-Kellams, C. Cross-cultural differences in somatic awareness and interoceptive accuracy: A review of the literature and directions for future research. Front. Psychol. 2014, 5, 1379. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Yamashiro, K.; Onishi, H.; Shimoyama, Y.; Yoshida, T.; Maruyama, A. The effect of water immersion on short-latency somatosensory evoked potentials in human. BMC Neurosci. 2012, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Yamashiro, K.; Yoshida, T.; Onishi, H.; Shimoyama, Y.; Maruyama, A. Effects of water immersion on short- and long-latency afferent inhibition, short-interval intracortical inhibition, and intracortical facilitation. Clin. Neurophysiol. 2013, 124, 1846–1852. [Google Scholar] [CrossRef]
- Patil, M.M.; Linster, C.; Lubenov, E.; Hasselmo, M.E. Cholinergic agonist carbachol enables associative long-term potentiation in piriform cortex slices. J. Neurophysiol. 1998, 80, 2467–2474. [Google Scholar] [CrossRef]
- Rasmusson, D.D.; Dykes, R.W. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Exp. Brain Res. 1988, 70, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Warren, R.A.; Dykes, R.W. Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. II. Cortical neurons excited by somatic stimuli. J. Neurophysiol. 1990, 64, 1212–1222. [Google Scholar] [CrossRef]
- Mourot, L.; Bouhaddi, M.; Gandelin, E.; Cappelle, S.; Dumoulin, G.; Wolf, J.-P.; Rouillon, J.D.; Regnard, J. Cardiovascular autonomic control during short-term thermoneutral and cool headout immersion. Aviat. Space Environ. Med. 2008, 79, 14–20. [Google Scholar] [CrossRef]
- Sato, D.; Yamazaki, Y.; Takahashi, A.; Uetake, Y.; Nakano, S.; Iguchi, K.; Baba, Y.; Nara, R.; Shimoyama, Y. Water immersion decreases sympathetic skin response during color–word Stroop test. PLoS ONE 2017, 12, e0180765. [Google Scholar] [CrossRef]
- Richter, F.; García, A.M.; Arriagada, N.R.; Yoris, A.; Birba, A.; Huepe, D.; Zimmer, H.; Ibáñez, A.; Sedeño, L. Behavioral and neurophysiological signatures of interoceptive enhancements following vagus nerve stimulation. Hum. Brain Mapp. 2021, 42, 1227–1242. [Google Scholar] [CrossRef]
- Ring, C.; Brener, J.; Knapp, K.; Mailloux, J. Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: A cautionary tale about interoceptive awareness. Biol. Psychol. 2015, 104, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Brener, J. Visceral Perception. Biofeedback Behav. 1977, 2, 235–259. [Google Scholar]
- Ring, C.; Brener, J. Influence of beliefs about heart rate and actual heart rate on heartbeat counting. Psychophysiology 1996, 33, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Iodice, P.; Porciello, G.; Bufalari, I.; Barca, L.; Pezzulo, G. An interoceptive illusion of effort induced by false heart-rate feedback. Proc. Natl. Acad. Sci. USA 2019, 116, 13897–13902. [Google Scholar] [CrossRef] [PubMed]
- Keatinge, W.R.; Evans, M. The respiratory and cardiovascular response to immersion in cold and warm water. Q. J. Exp. Physiol. Cogn. Med. Sci. 1961, 46, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, D.R.; Lundgren, C.E.G. The underwater environment: Cardiopulmonary, thermal, and energetic demands. J. Appl. Physiol. 2009, 106, 276–283. [Google Scholar] [CrossRef]
- Bird, F.; House, J.; Massey, H.; Tipton, M. The physiological and subjective responses to repeated cold water immersion in a group of 10–12 year olds. Int. J. Aquat. Res. Educ. 2015, 9, 162–174. [Google Scholar] [CrossRef][Green Version]
- Tipton, M.J.; Mekjavic, I.B.; Eglin, C.M. Permanence of the habituation of the initial responses to cold-water immersion in humans. Eur. J. Appl. Physiol. 2000, 83, 17–21. [Google Scholar] [CrossRef]
- Uchida, Y.; Hitokoto, H.; Kaino, K. Do you always choose what you like? Subtle social cues increase Preference-Choice consistency among Japanese but not among Americans. Front. Psychol. 2017, 8, 169. [Google Scholar] [CrossRef]
- Ainley, V.; Tsakiris, M.; Pollatos, O.; Schulz, A.; Herbert, B.M. Comment on “Zamariola et al. (2018), Interoceptive Accuracy Scores are Problematic: Evidence from Simple Bivariate Correlations”—The empirical data base, the conceptual reasoning and the analysis behind this statement are misconceived and do not support the authors’ conclusions. Biol. Psychol. 2020, 152, 107870. [Google Scholar] [CrossRef]
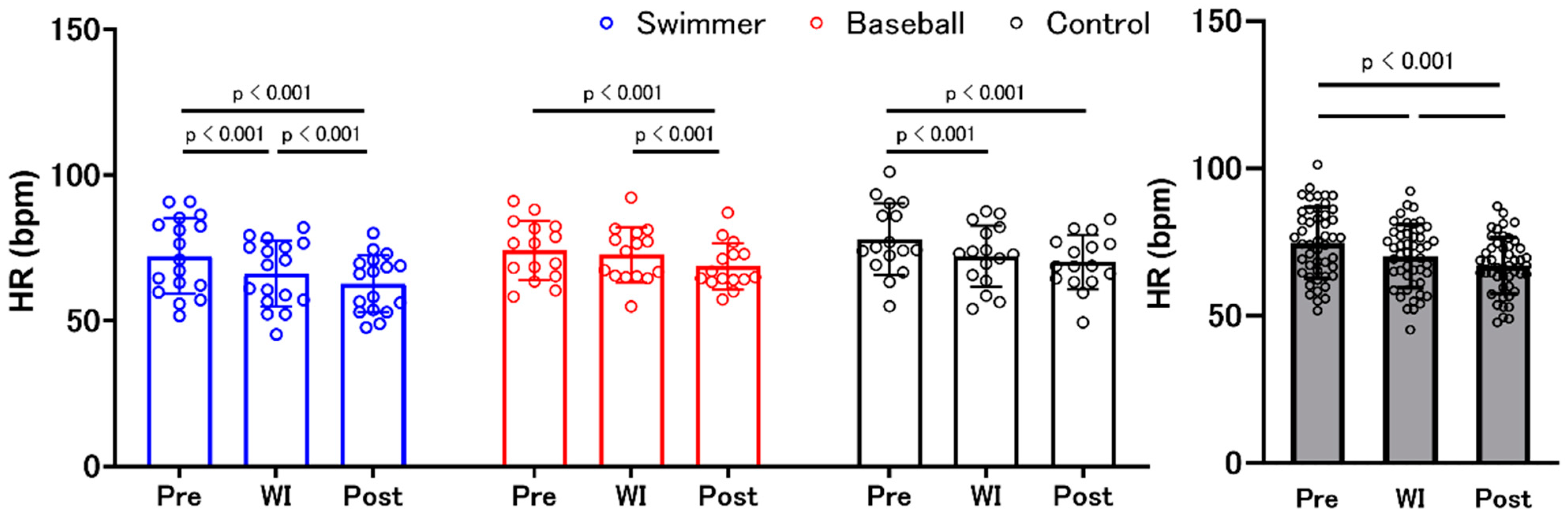
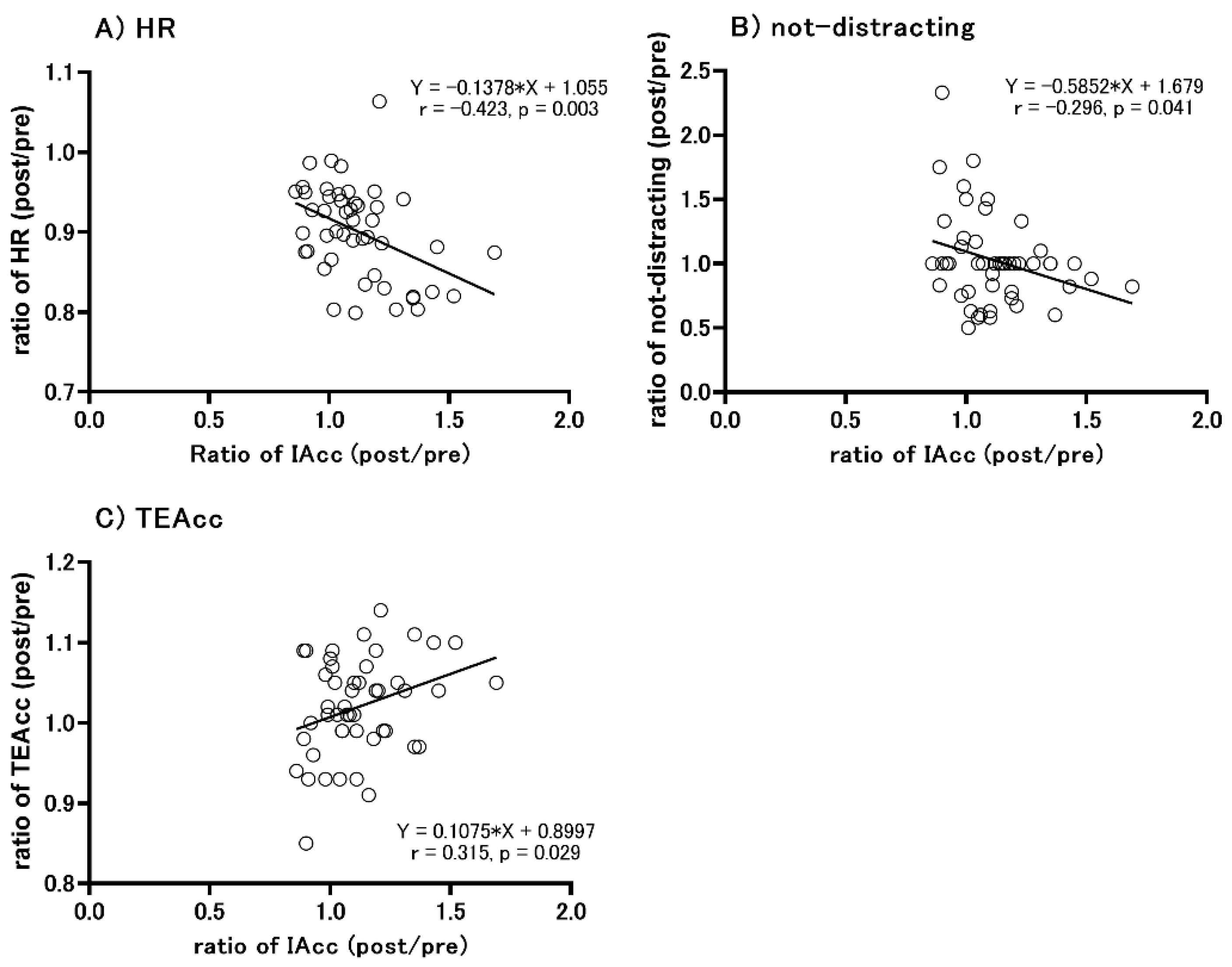
| Swimmer | Baseball | Control | ANOVA (Environment, Group, and Interaction) | Post Hoc Test | |||||
|---|---|---|---|---|---|---|---|---|---|
| MAIA | Noticing (pre) | 3.16 | ±1.01 | 3.18 | ±1.32 | 2.95 | ±0.86 | F (1.428, 64.26) = 0.435, p = 0.649, ηp2 = 0.010 | |
| (WI) | 3.13 | ±0.67 | 3.23 | ±0.99 | 3.03 | ±0.65 | F (2, 45) = 0.162, p = 0.851, ηp2 = 0.007 | ||
| (post) | 3.16 | ±0.66 | 3.22 | ±1.00 | 3.14 | ±0.78 | F (4, 90) = 0.308, p = 0.872, ηp2 = 0.014 | ||
| Not-distracting (pre) | 2.75 | ±0.91 | 2.36 | ±0.91 | 2.50 | ±0.74 | F (2, 90) = 0.323, p = 0.725, ηp2 = 0.007 | ||
| (WI) | 2.61 | ±0.73 | 2.24 | ±0.73 | 2.60 | ±0.96 | F (2, 45) = 1.288, p = 0.286, ηp2 = 0.054 | ||
| (post) | 2.49 | ±0.82 | 2.16 | ±0.58 | 2.73 | ±0.88 | F (4, 90) = 1.314, p = 0.271, ηp2 = 0.055 | ||
| Not-worrying (pre) | 2.18 | ±0.69 | 2.49 | ±0.80 | 2.19 | ±0.60 | F (2, 90) = 0.228, p = 0.797, ηp2 = 0.005 | ||
| (WI) | 1.92 | ±0.60 | 2.56 | ±0.75 | 2.25 | ±0.59 | F (2, 45) = 2.273, p = 0.115, ηp2 = 0.092 | ||
| (post) | 2.16 | ±0.65 | 2.53 | ±0.75 | 2.15 | ±0.49 | F (4, 90) = 1.702, p = 0.159, ηp2 = 0.070 | ||
| Attention regulation (pre) | 3.02 | ±0.64 | 3.12 | ±0.80 | 2.58 | ±0.54 | F (2, 90) = 4.0590, p = 0.021, ηp2 = 0.083 | * Pre vs. WI (p = 0.025) | |
| (WI) | 2.99 | ±0.68 | 3.37 | ±0.51 | 2.78 | ±0.65 | F (2, 45) = 3.511, p = 0.038, ηp2 = 0.135 | * Baseball vs. control (p = 0.034) | |
| (post) | 2.92 | ±0.72 | 3.27 | ±0.71 | 2.62 | ±0.64 | F (4, 90) = 1.571, p = 0.189, ηp2 = 0.065 | ||
| Emotional awareness (pre) | 2.98 | ±0.73 | 3.57 | ±0.70 | 3.26 | ±0.94 | F (2, 90) = 0.055, p = 0.946, ηp2 = 0.001 | ||
| (WI) | 3.11 | ±0.60 | 3.41 | ±0.61 | 3.35 | ±0.87 | F (2, 45) = 1.804, p = 0.176, ηp2 = 0.074 | ||
| (post) | 2.99 | ±0.63 | 3.40 | ±0.79 | 3.49 | ±0.80 | F (4, 90) = 1.798, p = 0.136, ηp2 = 0.074 | ||
| Self-regulation (pre) | 3.09 | ±0.68 | 3.40 | ±0.67 | 2.70 | ±0.73 | F (1.451, 65.286) = 0.220, p = 0.730, ηp2 = 0.005 | ||
| (WI) | 3.10 | ±0.46 | 3.57 | ±0.50 | 2.67 | ±0.56 | F (2, 45) = 9.738, p < 0.001, ηp2 = 0.302 | * Baseball vs. control (p < 0.001) | |
| (post) | 3.15 | ±0.52 | 3.48 | ±0.59 | 2.69 | ±0.63 | F (4, 90) = 0.293, p = 0.882, ηp2 = 0.013 | ||
| Body listening (pre) | 2.69 | ±0.86 | 3.07 | ±0.68 | 2.08 | ±0.86 | F (2, 90) = 1.012, p = 0.367, ηp2 = 0.022 | ||
| (WI) | 2.86 | ±0.69 | 3.07 | ±0.84 | 2.23 | ±0.96 | F (2, 45) = 4.937, p = 0.011, ηp2 = 0.181 | * Baseball vs. control (p = 0.010) | |
| (post) | 2.75 | ±0.89 | 3.07 | ±0.76 | 2.38 | ±0.93 | F (4, 90) = 0.670, p = 0.615, ηp2 = 0.029 | ||
| Trusting (pre) | 3.33 | ±0.76 | 3.78 | ±0.65 | 3.06 | ±1.01 | F (2, 90) = 1.774, p = 0.176, ηp2 = 0.038 | ||
| (WI) | 3.20 | ±0.85 | 3.69 | ±0.65 | 2.94 | ±0.97 | F (2, 45) = 3.380, p = 0.043, ηp2 = 0.131 | * Baseball vs. control (p = 0.048) | |
| (post) | 3.06 | ±0.81 | 3.73 | ±0.75 | 3.06 | ±0.99 | F (4, 90) = 0.985, p = 0.420, ηp2 = 0.042 | ||
| TAS-20 | Total (pre) | 47.24 | ±8.31 | 46.93 | ±7.99 | 46.69 | ±8.76 | F (2, 90) = 3.354, p = 0.039, ηp2 = 0.069 | |
| (WI) | 48.35 | ±7.84 | 47.40 | ±8.96 | 47.50 | ±9.79 | F (2, 45) = 0.170, p = 0.844, ηp2 = 0.007 | ||
| (post) | 50.76 | ±9.81 | 47.00 | ±8.69 | 48.50 | ±9.74 | F (4, 90) = 1.160, p = 0.334, ηp2 = 0.049 | ||
| DIF (pre) | 15.53 | ±5.29 | 13.47 | ±5.08 | 13.63 | ±4.06 | F (2, 90) = 3.564, p = 0.032, ηp2 = 0.073 | * Pre vs. post (p = 0.049) | |
| (WI) | 16.24 | ±5.25 | 13.80 | ±5.45 | 14.88 | ±4.24 | F (2, 45) = 1.227, p = 0.303, ηp2 = 0.052 | ||
| (post) | 16.76 | ±5.45 | 13.80 | ±4.71 | 15.50 | ±3.86 | F (4, 90) = 0.520, p = 0.721, ηp2 = 0.023 | ||
| DDF (pre) | 12.12 | ±2.21 | 12.67 | ±3.79 | 12.94 | ±3.53 | F (2, 90) = 2.635, p = 0.077, ηp2 = 0.055 | ||
| (WI) | 12.71 | ±2.11 | 13.53 | ±4.58 | 12.94 | ±4.22 | F (2, 45) = 0.107, p = 0.889, ηp2 = 0.005 | ||
| (post) | 13.18 | ±2.88 | 13.20 | ±4.26 | 13.56 | ±4.44 | F (4, 90) = 0.549, p = 0.700, ηp2 = 0.024 | ||
| EOT (pre) | 19.59 | ±2.83 | 20.80 | ±4.16 | 20.13 | ±4.40 | F (1.640, 73.815) = 1.154, p = 0.320, ηp2 = 0.025 | ||
| (WI) | 19.41 | ±2.62 | 20.07 | ±4.23 | 19.69 | ±4.39 | F (2, 45) = 0.080, p = 0.923, ηp2 = 0.004 | ||
| (post) | 20.82 | ±3.64 | 20.00 | ±4.49 | 19.44 | ±4.86 | F (4, 90) = 2.570, p = 0.043, ηp2 = 0.103 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, Y.; Sato, D.; Otsuru, N.; Ikarashi, K.; Fujimoto, T.; Yamashiro, K. Does Long-Term Training in a Water Immersion Environment Change Interoception? Int. J. Environ. Res. Public Health 2021, 18, 10259. https://doi.org/10.3390/ijerph181910259
Baba Y, Sato D, Otsuru N, Ikarashi K, Fujimoto T, Yamashiro K. Does Long-Term Training in a Water Immersion Environment Change Interoception? International Journal of Environmental Research and Public Health. 2021; 18(19):10259. https://doi.org/10.3390/ijerph181910259
Chicago/Turabian StyleBaba, Yasuhiro, Daisuke Sato, Naofumi Otsuru, Koyuki Ikarashi, Tomomi Fujimoto, and Koya Yamashiro. 2021. "Does Long-Term Training in a Water Immersion Environment Change Interoception?" International Journal of Environmental Research and Public Health 18, no. 19: 10259. https://doi.org/10.3390/ijerph181910259
APA StyleBaba, Y., Sato, D., Otsuru, N., Ikarashi, K., Fujimoto, T., & Yamashiro, K. (2021). Does Long-Term Training in a Water Immersion Environment Change Interoception? International Journal of Environmental Research and Public Health, 18(19), 10259. https://doi.org/10.3390/ijerph181910259






