Effects of Fluoride and Calcium Phosphate-Based Varnishes in Children at High Risk of Tooth Decay: A Randomized Clinical Trial
Abstract
:1. Background
2. Materials and Methods
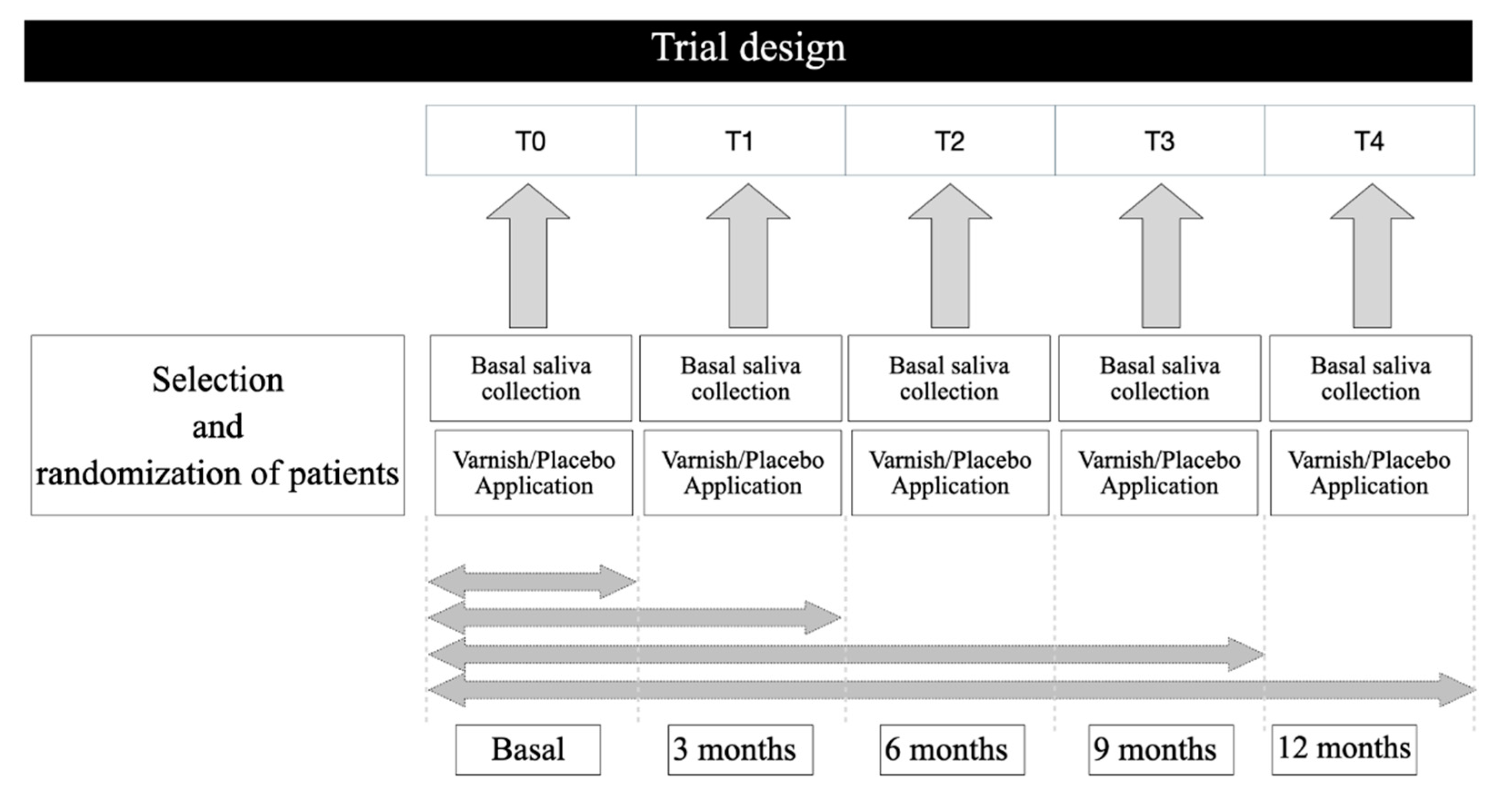
2.1. Trial Design
2.2. Participants
2.3. Patient Selection
2.4. Saliva Samples
2.5. Experimental Groups, Application of Varnishes
2.6. Outcome Measures
2.6.1. Caries Index
2.6.2. Plaque Index
2.6.3. pH and Latic Acid
2.6.4. Fluoride
2.6.5. Chemical Elements
2.7. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Caries Index
3.3. Hygiene Index
3.4. pH
3.5. Lactic Acid
3.6. Fluoride
3.7. Chemical Elements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPP-ACP | calcium phosphate stabilized with casein phosphopeptide (CPP-ACP) |
| M | molar |
| DMFS | decayed, missing, and filled surfaces in permanent teeth |
| dmfs | decayed and filled index by surface in deciduous teeth |
| fTCP | tricalcium phosphate modified by fumaric acid |
| ICDAS | International Caries Detection and Assessment System criteria |
| WHO | World Health Organization |
References
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Caries dental. Nat. Rev. Dis. Prim. 2017, 25, 17030. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.; Enax, J. Early childhood caries: Epidemiology, aetiology, and prevention. Int. J. Dent. 2018, 22, 1415873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, J.; Fontana, M.; Wolff, M. Novel anticaries and remineralization agents: Future research needs. J. Dent. Res. 2018, 97, 125–127. [Google Scholar] [CrossRef]
- Hegde, M.N.; Attavar, S.; Shetty, N.; Hegde, N.D.; Hegde, N.N. Saliva as a biomarker for dental caries: A systematic review. J. Conserv. Dent. 2019, 22, 2–6. [Google Scholar] [CrossRef]
- Kühnisch, J.; Ekstrand, K.R.; Pretty, I.; Twetman, S.; Van Loveren, C.; Gizani, S.; Spyridonos Loizidou, M. Best clinical practice guidance for management of early caries lesions in children and young adults: An EAPD policy document. Eur. Arch. Paediatr. Dent. 2016, 17, 3–12. [Google Scholar] [CrossRef]
- González-Cabezas, C.; Fernandez, C.E. Recent advances in remineralization therapies for caries lesions. Adv. Dent. Res. 2018, 29, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.; Clarkson, J.E. Fluoride varnish for caries prevention: Efficacy and implementation. Caries Res. 2016, 50, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, A. ADA clinical recommendations on topical fluoride for caries prevention. Evid.-Based Dent. 2014, 15, 38–39. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment—a systematic review. BMC Oral Health 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, N.; Cai, F.; Huq, N.L.; Burrow, M.; Reynolds, E. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Bagheri, R.; Walker, G.; Yuan, Y.; Stanton, D.; Reynolds, C.; Reynolds, E. Effect of calcium phosphate addition to fluoride containing dental varnishes on enamel demineralization. Aust. Dent. J. 2016, 61, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llena, C.; Leyda, A.M.; Forner, L. CPP-ACP and CPP-ACFP versus fluoride varnish in remineralisation of early caries lesions. A prospective study. Eur. J. Paediatr. Dent. 2015, 16, 181–186. [Google Scholar] [PubMed]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Yiu, C.; Ekambaram, M. A review of enamel remineralisation potential of calcium- and phosphate-based remineralisation systems. Oral Health Prev. Dent. 2017, 15, 415–420. [Google Scholar] [CrossRef]
- Philip, N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Casein phosphopeptide-amorphous calcium phosphate: The scientific evidence. Adv. Dent. Res. 2009, 21, 25–29. [Google Scholar] [CrossRef]
- Karlinsey, R.L.; Pfarrer, A.M. Fluoride plus functionalized β-TCP. Adv. Dent. Res. 2012, 24, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Al Dehailan, L.; Lippert, F.; González-Cabezas, C.; Eckert, G.; Martinez-Mier, E. Fluoride concentration in saliva and biofilm fluid following the application of three fluoride varnishes. J. Dent. 2017, 60, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Downey, D.; Dennison, J.; Eckert, G.J.; Flannagan, S.E.; Neiva, G.F.; Yaman, P.; González-Cabezas, C. Fluoride levels in unstimulated whole saliva following clinical application of different 5% NaF varnishes. Caries Res. 2018, 52, 431–438. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, Y.; Yuan, H.; Tao, S.; Cheng, Y.; Li, J.; He, L. Efficacy of fluorides and CPP-ACP vs. fluorides monotherapy on early caries lesions: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0196660. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Manton, D.; Cochrane, N.J.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Reynolds, E. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled In Situ trial. J. Dent. 2011, 39, 518–525. [Google Scholar] [CrossRef]
- Rechmann, P.; Kinsel, R.; Featherstone, J.D.B. Integrating caries management by risk assessment (CAMBRA) and prevention strategies into the contemporary dental practice. Compend. Contin. Educ. Dent. 2018, 39, 226–233. [Google Scholar]
- Pitts, N.B. How the detection, assessment, diagnosis and monitoring of caries integrate with personalized caries management. Monogr. Oral Sci. 2009, 21, 1–14. [Google Scholar] [CrossRef]
- Dawes, C. Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva. J. Physiol. 1975, 244, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, L.M.; Cury, J.A. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpastes. Monogr. Oral Sci. 2013, 23, 108–124. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierichs, R.J.; Stausberg, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Caries-preventive effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF varnishes on sound dentin and artificial dentin caries In Vitro. Caries Res. 2018, 52, 199–211. [Google Scholar] [CrossRef]
- Said, S.N.B.M.; Ekambaram, M.; Yiu, C.K.Y. Effect of different fluoride varnishes on remineralization of artificial enamel carious lesions. Int. J. Paediatr. Dent. 2017, 27, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.; Kousvelari, E.; Vastardis, H. Saliva in the “Omics” era: A promising tool in paediatrics. Oral Dis. 2019, 25, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Pyati, S.A.; Kumar, R.N.; Kumar, V.; Kumar, N.H.P.; Reddy, K.M.P. Salivary flow rate, pH, buffering capacity, total protein, oxidative stress and antioxidant capacity in children with and without dental caries. J. Clin. Pediatr. Dent. 2018, 42, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tanaka, T.; Shigemi, T.; Saeki, K.; Fujita, Y.; Morikawa, K.; Nakashima, H.; Takahashi, S.; Watanabe, S.; Maki, K. Al and Fe levels in mixed saliva of children related to elution behavior from teeth and restorations. J. Trace Elem. Med. Biol. 2011, 25, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Dodawad, R.; Raju, O.; Os, R. Evaluation of flow rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children—an In Vivo study. Int. J. Clin. Pediatr. Dent. 2009, 2, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Tulunoglu, O.; Demirtas, S. Total antioxidant levels of saliva in children related to caries, age, and gender. Int. J. Paediatr. Dent. 2006, 16, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.-Y. Salivary biomarkers for dental caries. Periodontology 2000 2015, 70, 128–141. [Google Scholar] [CrossRef]
- Cury, J.A.; de Oliveira, B.H.; dos Santos, A.P.P.; Tenuta, L.M.A. Are fluoride releasing dental materials clinically effective on caries control? Dent. Mater. 2016, 32, 323–333. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, N.; Wang, Z.; Li, L.; Zhang, J.; Ma, R.; Nie, H.; Li, Z. Influences of pH and iron concentration on the salivary microbiome in individual humans with and without caries. Appl. Environ. Microbiol. 2017, 83, e02412–e02416. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, T.; Freitas-Fernandes, L.B.; Angeli, R.; Muniz, A.M.S.; Gonsalves, E.; Santos, R.; Nadal, J.; Almeida, F.C.L.; Valente, A.P.; Souza, I.P.R. Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics 2012, 9, 657–666. [Google Scholar] [CrossRef]
- Pereira, J.L.; Duarte, D.; Carneiro, T.J.; Ferreira, S.; Cunha, B.; Soares, D.; Costa, A.L.; Gil, A.M. Saliva NMR metabolomics: Analytical issues in pediatric oral health research. Oral Dis. 2019, 25, 1545–1554. [Google Scholar] [CrossRef]
- Sekhri, P.; Sandhu, M.; Sachdev, V.; Chopra, R. Estimation of trace elements in mixed saliva of caries free and caries active children. J. Clin. Pediatr. Dent. 2018, 42, 135–139. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanaka, T.; Shigemi, T.; Hayashida, Y.; Maki, K. Mn and Cu concentrations in mixed saliva of elementary school children in relation to sex, age, and dental caries. J. Trace Elem. Med. Biol. 2009, 23, 93–99. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.; Sood, P.; Sood, A.; Zaidi, I.; Sinha, A. Saliva as a prediction tool for dental caries: An In Vivo study. J. Oral Biol. Craniofacial Res. 2015, 5, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Tayab, T.; Rai, K.; Kumari, A.V. Evaluating the physicochemical properties and inorganic elements of saliva in caries-free and caries-active children. An In Vivo study. Eur. J. Paediatr. Dent. 2012, 13, 107–112. [Google Scholar]
- Cochrane, N.J.; Shen, P.; Yuan, Y.; Reynolds, E.C. Ion release from calcium and fluoride containing dental varnishes. Aust. Dent. J. 2014, 59, 100–105. [Google Scholar] [CrossRef]
- Rechmann, P.; Bekmezian, S.; Rechmann, B.M.T.; Chaffee, B.W.; Featherstone, J.D.B. MI varnish and MI paste plus in a caries prevention and remineralization study: A randomized controlled trial. Clin. Oral Investig. 2018, 22, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Reyes, S.M.; Martínez-Beneyto, Y.; Serna-Muñoz, C.; Pérez-Silva, A.; Cury, J.A.; Ortiz-Ruiz, A.J. Concentración de flúor y metales pesados en aguas embotelladas: Medidas barrera frente a caries dental y fluorosis. Revista Española Salud Pública 2019, 93, e201912110. [Google Scholar]
- Vogel, G.L. Oral fluoride reservoirs and the prevention of dental caries. Monogr. Oral Sci. 2011, 22, 146–157. [Google Scholar] [CrossRef]
- Brookes, S.J.; Shore, R.C.; Robinson, C.; Wood, S.R.; Kirkham, J. Copper ions inhibit the demineralisation of human enamel. Arch. Oral Biol. 2003, 48, 25–30. [Google Scholar] [CrossRef]
- Hegde, M.N.; Hegde, N.; Ashok, A.; Shetty, S. Biochemical indicators of dental caries in saliva: An In Vivo study. Caries Res. 2014, 48, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Dame, Z.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Pathak, M.U.; Shetty, V.; Kalra, D. Trace elements and oral health: A systematic review. J. Adv. Oral Res. 2016, 7, 12–20. [Google Scholar] [CrossRef]
- Sejdini, M.; Meqa, K.; Berisha, N.; Çitaku, E.; Aliu, N.; Krasniqi, S.; Salihu, S. The effect of Ca and Mg concentrations and quantity and their correlation with caries intensity in school-age children. Int. J. Dent. 2018, 2018, 2759040. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, K.S.; Zareena, H.S.; Kumar, M.A. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 2015, 6, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Togni, L.; Mascitti, M.; Santarelli, A.; Contaldo, M.; Romano, A.; Serpico, R.; Rubini, C. Unusual conditions impairing saliva secretion: Developmental anomalies of salivary glands. Front. Physiol. 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Product | Manufacturer | Composition |
|---|---|---|
| MI Varnish | GC, Leuven, Belgium | 30–50% polyvinyl acetate, 10–30% hydrogenated MSDS rosin, 20–30% ethanol, 5% sodium fluoride, 1–5% CPP-ACP, 1–5% silicon dioxide |
| Clinpro White Varnish | 3 M ESPE, Saint Paul, MN, US | 30–75% pentaerythritol glycerol ester of colophony resin, 10–15% n-hexane, 1–15% ethyl alcohol, 5% sodium fluoride, 1–5% flavor enhancer, 1–5% thickener, 1–5% food grade flavor, <5% fTCP. |
| Characteristic | Control Group | Clinpro Group | MI Group | p Value |
|---|---|---|---|---|
| Age-years | 6.91 ± 2.59 | 7.6 ± 2.36 | 6.81 ± 2.63 | p = 0.766 (One-way ANOVA) |
| Sex-Age Female Male | 8 (6.12 ± 2.36) 4 (8.50 ± 3.51) p = 0.214 (Mann-Whitney test) | 4 (6.25 ± 1.26) 6 (8.50 ± 2.59) p = 0.171 (Mann-Whitney test) | 4 (6.00 ± 2.16) 7 (7.29 ± 2.93) p = 0.527 (Mann-Whitney test) | p = 0.283 (χ2 test) |
| Baseline (T0) | 12 Months Follow-Up (T4) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| dmfs (mean ± SD) | ds | ms | fs | dmfs mean ± SD | ds | ms | fs | ||
| Control group | 18.8 ± 10.8 | 10.33 | 0.5 | 1.33 | 17.8 ± 21.5 | 0.75 | 1.75 | 9 | p = 0.765 (Wilcoxon test) |
| Clinpro group | 27.6 ± 18.5 | 14.2 | 1.8 | 0.7 | 37.6 ± 21.6 (α) | 0.00 | 5.33 | 12.66 | p = 0.191 (Wilcoxon test) |
| MI group | 20.2 ± 15.2 | 13.45 | 0.27 | 0.82 | 27.2 ± 21.5 | 6.63 | 0.81 | 7.54 | p = 0.387 (Paired t-test) |
| p = 0.353 (One-way ANOVA) | p = 0.032 (Kruskal–Wallis test) | ||||||||
| DMFS mean ± SD | DS | MS | FS | DMFS mean ± SD | DS | MS | FS | ||
| Control group | 0.37 ± 1.32 | 0.46 | 0 | 0.1 | 0.89 ± 1.79 | 0.00 | 0 | 1.77 | p = 1.00 (Wilcoxon test) |
| Clinpro group | 3.70 ± 3.38 (α) | 2.2 | 0 | 0 | 2.02 ± 2.41 | 0.14 | 0 | 0.91 | p = 0.151 (Paired t-test) |
| MI group | 1.94 ± 2.89 | 1.55 | 0 | 0.3 | 1.34 ± 2.05 | 0 | 0 | 1.37 | p = 0.541 (Paired t-test) |
| p = 0.039 (Kruskal–Wallis test) | p = 0.766 (Kruskal–Wallis test) | ||||||||
| Baseline (T0) | 3 Months (T1) | 6 Months (T2) | 9 Months (T3) | 12 Months (T4) | p Value | |
|---|---|---|---|---|---|---|
| Control group | 1.99 ± 0.54 | 1.64 ± 0.65 | 1.15 ± 0.41 a,b | 1.30 ± 0.60 a | 1.42 ± 0.71 a | p = 0.002 |
| Clinpro group | 2.76 ± 0.89 | 1.74 ± 0.55 | 1.77 ± 045 | 1.46 ± 0.66 a | 1.28 ± 0.66 a | p < 0.001 |
| MI group | 2.23 ± 0.74 | 1.74 ± 0.65 a | 1.43 ± 0.62 a | 1.27 ± 0.55 a,b | 1.18 ± 0.27 a,b | p < 0.001 |
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | Baseline | 3 Months | 6 Months | 9 Months | 12 Months | Baseline | 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 7.691 ± 0.277 | 7.892 ± 0.312 | 7.767 ± 0.246 | 7.858 ± 0.345 | 7.850 ± 0.390 | 7.689 ± 0.293 | 7.650 ± 0.295 | 7.470 ± 0.683 | 7.610 ± 0.396 | 7.710 ± 0.325 | 7.627 ± 0.329 | 7.682 ± 0.402 | 7.718 ± 0.309 | 7.945 ± 0.370 | 7.800 ± 0.286 |
| Lactic acid | 19.289 ± 15.332 | 13.978 ± 9.415 | 21.036 ± 17.990 | 22.630 ± 16.428 | 8.438 ± 5.903 | 19.275 ± 10.584 | 26.825 ± 16.797 | 32.712 ± 20.395 | 21.114 ± 17.635 | 18.417 ± 17.425 | 24.362 ± 16.195 | 23.137 ± 18.911 | 27.200 ± 16.339 | 14.930 ± 11.224 | 22.089 ± 12.714 |
| 23Na | 248.194 ± 139.716 | 201.595 ± 66.288 | 218.480 ± 170.891 | 173.850 ± 85.649 | 240.168 ± 135.968 | 225.429 ± 147.144 | 215.098 ± 171.809 | 224.836 ± 124.393 | 220.662 ± 180.556 | 212.320 ± 111.239 | 160.551 ± 70.881 | 181.645 ± 79.014 | 204.946 ± 100.158 | 169.32 ± 82.909 | 162.175 ± 74.268 |
| 27Al | 333.208 ± 183.751 | 234.432 ± 132.795 | 191.094 ± 123.028 | 180.855 ± 111.019 | 293.447 ± 345.301 | 186.702 ± 88.419 | 211.754 ± 142.646 | 513.260 ± 782.415 | 180.783 ± 89.185 | 214.838 ± 209.433 | 352.480 ± 333.027 | 238.117 ± 162.809 | 327.611 ± 273.735 | 227.77 ± 189.359 | 230.263 ± 106.472 |
| 39K | 1025.741 ± 276.149 | 993.496 ± 180.808 | 1002.781 ± 221.995 | 920.151 ± 153.085 | 957.936 ± 228.272 | 936.678 ± 184.584 | 1057.216 ± 289.940 | 1055.634 ± 290.046 | 1105.300 ± 262.103 | 1008.175 ± 213.882 | 936.756 ±192.816 | 981.274 ± 206.305 | 964.686 ± 215.792 | 929.09 ± 254.098 | 937.495 ± 132.456 |
| 44Ca | 94.786 ± 35.781 | 88.603 ± 26.733 | 92.572 ± 35.049 | 61.407 ± 21.515 | 70.536 ± 24.694 | 88.898 ± 43.968 | 73.684 ± 30.781 | 66.012 ± 31.476 | 58.324 ± 17.708 | 59.406 ± 24.819 | 73.989 ± 36.378 | 77.978 ± 20.440 | 88.883 ± 68.376 | 84.379 ± 41.876 | 78.395 ± 24.987 |
| 52Cr | 4.013 ± 6.785 | 2.295 ± 1.404 | 1.435 ± 1.072 | 1.559 ± 1.616 | 2.458 ± 3.103 | 1.218 ± 1.473 | 2.254 ± 2.320 | 3.445 ± 4.317 | 4.537 ± 6.220 | 1.871 ± 3.653 | 2.082 ± 1.100 | 2.750 ± 2.905 | 2.933 ± 2.385 | 6.546 ± 13.866 | 2.787 ± 3.289 |
| 55Mn | 55.852 ± 32.229 | 49.281 ± 30.059 | 60.706 ± 21.520 | 48.331 ± 26.383 | 53.574 ±31.954 | 47.842 ± 32.057 | 50.339 ± 42.555 | 40.063 ± 28.127 | 41.016 ± 25.129 | 39.694 ± 28.462 | 42.251 ± 31.591 | 50.519 ±20.276 | 52.462 ± 41.599 | 53.124 ± 27.774 | 51.673 ± 17.636 |
| 57Fe | 147.952 ± 90.313 | 147.144 ± 88.435 | 164.172 ± 88.824 | 140.449 ± 117.831 | 186.385 ± 169.798 | 103.283 ± 49.763 | 151.232 ± 105.901 | 166.435 ± 185.257 | 105.924 ± 73.330 | 122.435 ± 92.716 | 142.072 ± 93.081 | 150.262 ± 125.766 | 220.809 ± 188.328 | 155.36 ± 116.249 | 164.515 ± 94.194 |
| 59Co | 1.646 ± 0.889 | 2.112 ± 1.621 | 1.994 ± 1.072 | 1.899 ± 0.999 | 1.903 ± 1.514 | 1.421 ± 1.170 | 1.300 ± 1.026 | 1.336 ± 0.916 | 1.439 ± 0.920 | 1.329 ± 1.040 | 1.157 ± 0.858 | 1.289 ± 1.163 | 1.359 ± 1.209 | 1.127 ± 1.241 | 1.187 ± 0.947 |
| 63Cu | 70.412 ± 59.314 | 70.833 ± 46.034 | 50.914 ± 25.602 | 63.243 ± 61.103 | 52.858 ± 59.830 | 32.533 ± 24.889 | 68.003 ± 135.975 | 61.914 ± 42.271 | 44.274 ± 38.882 | 39.072 ± 56.660 | 34.676 ± 19.508 | 54.865 ± 34.184 | 96.771 ± 84.260 | 44.290 ± 45.844 | 118.943 ± 283.883 |
| 75As | 2.009 ± 1.452 | 1.923 ± 1.396 | 2.090 ± 1.631 | 2.396 ± 1.397 | 2.691 ± 1.534 | 2.566 ± 1.339 | 2.491 ± 1.233 | 2.466 ± 1.053 | 2.549 ± 1.253 | 2.281 ± 1.246 | 1.843 ± 1.543 | 1.759 ± 1.512 | 1.889 ± 1.496 | 1.966 ± 1.568 | 2.131 ± 1.613 |
| 111Cd | 0.698 ± 0.677 | 0.853 ± 1.405 | 0.437 ± 0.360 | 0.669 ± 0.522 | 0.698 ± 0.677 | 0.359 ± 0.543 | 0.612 ± 0.735 | 1.503 ± 3.908 | 0.483 ± 0.422 | 0.347 ± 0.421 | 0.337 ± 0.283 | 0.384 ± 0.276 | 0.605 ± 0.792 | 0.313 ± 0.181 | 0.816 ± 0.784 |
| 137Ba | 21.002 ± 14.780 | 19.456 ± 11.772 | 12.753 ± 6.548 | 17.328 ± 26.994 | 21.246± 30.018 | 8.790 ± 5.546 | 12.096 ± 9.927 | 11.900 ± 15.873 | 12.707 ± 9.965 | 10.016 ± 8.097 | 19.181 ± 11.538 | 13.971 ± 7.363 | 21.576 ± 19.071 | 19.095 ± 17.679 | 17.238 ± 15.998 |
| 208Pb | 17.077 ± 11.782 | 6.140 ± 9.077 | 3.054 ± 5.685 | 2.423 ± 4.086 | 6.356 ± 9.687 | 4.252 ± 8.489 | 12.496 ± 29.752 | 3.499 ± 8.701 | 1.114 ± 1.427 | 3.801 ± 7.776 | 5.943 ± 9.236 | 2.688 ± 2.487 | 6.397 ± 7.296 | 2.739 ± 3.024 | 4.361 ± 9.152 |
| 9F | 0.0467 ± 0.0186 | - | - | - | 0.0617 ± 0.0286 | 0.0560 ± 0.0434 | - | - | - | 0.0660 ± 0.0261 | 0.0540 ± 0.0152 | - | - | - | 0.0920 ± 0.0409 |
| CONTROL | CLINPRO | MI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | Baseline | 3 Months | 6 Months | 9 Months | 12 Months | Baseline | 3 Months | 6 Months | 9 Months | 12 Months | |
| 24Mg | 5.964 ± 2.530 b | 6.033 ± 1.878 b,c | 5.679 ± 1.550 b | 4.197 ± 1.875 | 4.763 ± 1.868 | 5.176 ± 1.727 | 5.490 ± 3.819 | 5.955 ± 2.363 | 4.691 ± 2.231 | 5.349 ± 2.612 | 5.099 ± 1.644 | 5.620 ± 2.271 | 6.039 ± 3.399 | 5.177 ± 2.625 | 4.834 ± 1.969 |
| 31P | 205.273 ± 78.814 | 198.102 ± 59.206 a | 198.390 ± 65.518 a | 165.450 ± 52.551 a | 172.776 ± 67.742 a | 178.866 ± 45.689 | 202.141 ± 52.828 | 201.097 ± 62.726 | 198.657 ± 55.295 | 180.379 ± 52.449 | 175.243 ± 88.092 | 189.651 ± 56.666 | 185.310 ± 68.068 | 199.371 ± 147.554 | 172.743 ± 51.287 |
| 66Zn | 697.063 ± 431.615 | 511.339 ± 292.248 | 428.145 ± 166.827 | 377.632 ± 255.334 | 358.914 ± 309.153 b | 360.907 ± 100.713 | 497.673 ± 527.331 | 283.983 ± 138.173 | 436.796 ± 493.820 | 246.976 ± 155.103 | 451.570 ± 261.448 | 297.659 ± 135.544 | 617.446 ± 759.010 | 359.778 ± 281.762 | 306.541 ± 183.673 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poza-Pascual, A.; Serna-Muñoz, C.; Pérez-Silva, A.; Martínez-Beneyto, Y.; Cabello, I.; Ortiz-Ruiz, A.J. Effects of Fluoride and Calcium Phosphate-Based Varnishes in Children at High Risk of Tooth Decay: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 10049. https://doi.org/10.3390/ijerph181910049
Poza-Pascual A, Serna-Muñoz C, Pérez-Silva A, Martínez-Beneyto Y, Cabello I, Ortiz-Ruiz AJ. Effects of Fluoride and Calcium Phosphate-Based Varnishes in Children at High Risk of Tooth Decay: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2021; 18(19):10049. https://doi.org/10.3390/ijerph181910049
Chicago/Turabian StylePoza-Pascual, Andrea, Clara Serna-Muñoz, Amparo Pérez-Silva, Yolanda Martínez-Beneyto, Inmaculada Cabello, and Antonio José Ortiz-Ruiz. 2021. "Effects of Fluoride and Calcium Phosphate-Based Varnishes in Children at High Risk of Tooth Decay: A Randomized Clinical Trial" International Journal of Environmental Research and Public Health 18, no. 19: 10049. https://doi.org/10.3390/ijerph181910049
APA StylePoza-Pascual, A., Serna-Muñoz, C., Pérez-Silva, A., Martínez-Beneyto, Y., Cabello, I., & Ortiz-Ruiz, A. J. (2021). Effects of Fluoride and Calcium Phosphate-Based Varnishes in Children at High Risk of Tooth Decay: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 18(19), 10049. https://doi.org/10.3390/ijerph181910049








