Mood and Changes in Alcohol Consumption in Young Adults during COVID-19 Lockdown: A Model Explaining Associations with Perceived Immune Fitness and Experiencing COVID-19 Symptoms
Abstract
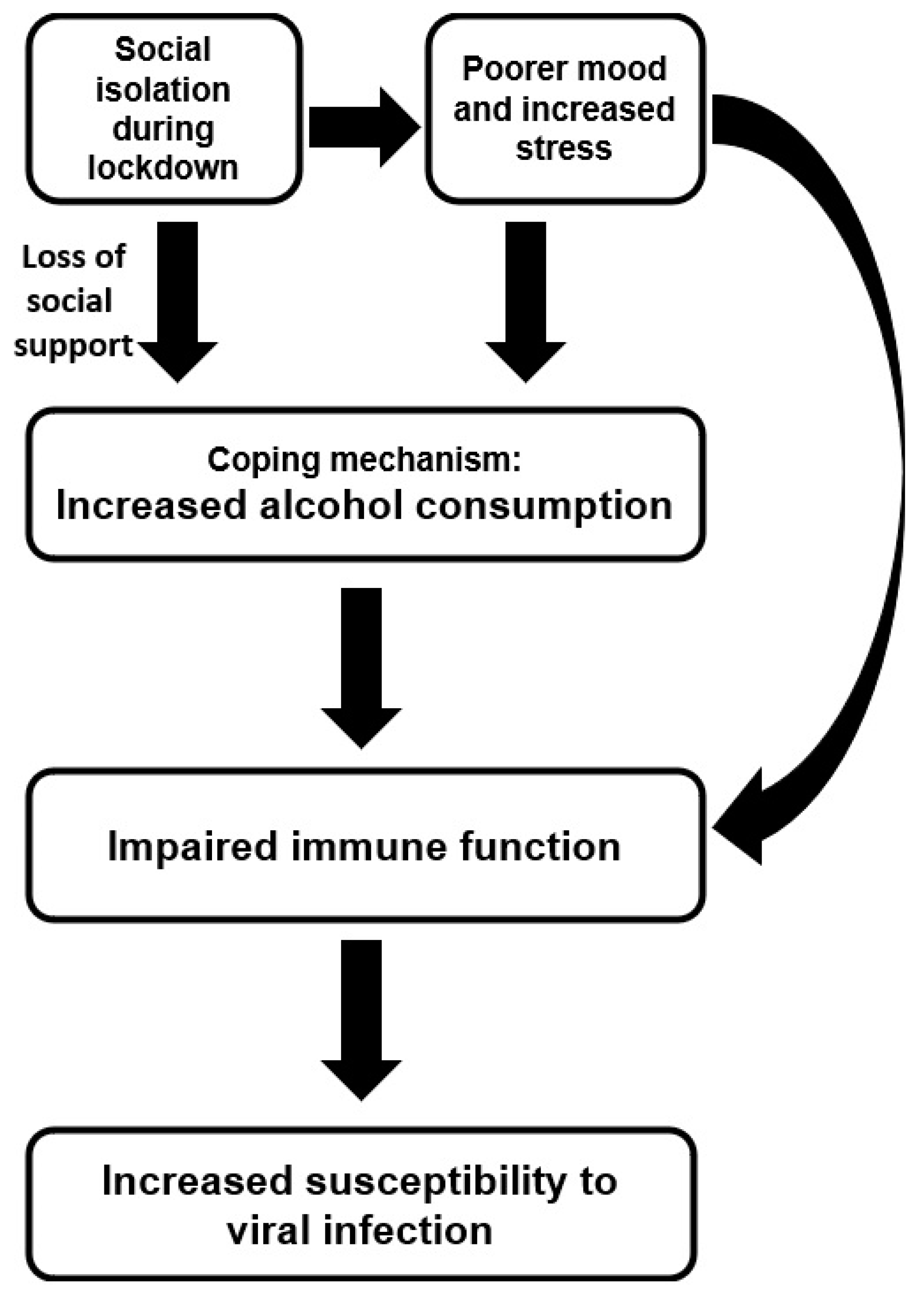
:1. Introduction
2. Materials and Methods
2.1. Survey Content
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Virtual Press Conference on COVID-19. Available online: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2 (accessed on 1 February 2021).
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Ingram, J.; Maciejewski, G.; Hand, C.J. Changes in diet, sleep, and physical activity are associated with differences in negative mood during COVID-19 lockdown. Front. Psychol. 2020, 11, 2328. [Google Scholar]
- Di Renzo, L.; Gualtieri, P.; Cinelli, G.; Bigioni, G.; Soldati, L.; Attinà, A.; Bianco, F.F.; Caparello, G.; Camodeca, V.; Carrano, E.; et al. Psychological aspects and eating habits during COVID-19 home confinement: Results of EHLC-COVID-19 Italian online survey. Nutrients 2020, 12, 2152. [Google Scholar] [CrossRef] [PubMed]
- Górnicka, M.; Drywień, M.E.; Zielinska, M.A.; Hamułka, J. Dietary and lifestyle changes during COVID-19 and the subsequent lockdowns among Polish adults: A Cross-sectional online survey PLifeCOVID-19 study. Nutrients 2020, 12, 2324. [Google Scholar] [CrossRef]
- Althobaiti, Y.S.; Alzahrani, M.A.; Alsharif, N.A.; Alrobaie, N.S.; Alsaab, H.O.; Uddin, M.N. The Possible Relationship between the Abuse of Tobacco, Opioid, or Alcohol with COVID-19. Healthcare 2021, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, R. Alcohol consumption and alcohol-related problems during the COVID-19 pandemic: A narrative review. Australas. Psychiatry 2020, 28, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.M.; Litt, D.M.; Stewart, S.H. Drinking to cope with the pandemic: The unique associations of COVID-19-related perceived threat and psychological distress to drinking behaviors in American men and women. Addict. Behav. 2020, 110, 106532. [Google Scholar] [CrossRef]
- Finlay, I.; Gilmore, I. COVID-19 and alcohol—A dangerous cocktail. BMJ 2020, 369, m1987. [Google Scholar] [CrossRef]
- Biddle, N.; Edwards, B.; Gray, M.; Sollis, K. Alcohol Consumption during the COVID-19 Period: May 2020; The Australian National University: Canberra, Australia, 2020; Available online: https://csrm.cass.anu.edu.au/research/publications/alcohol-consumption-during-covid-19-period-may-2020 (accessed on 1 February 2021).
- White, H.R.; Stevens, A.K.; Hayes, K.; Jackson, K.M. Changes in Alcohol Consumption Among College Students Due to COVID-19: Effects of Campus Closure and Residential Change. J. Stud. Alcohol Drugs 2020, 81, 725–730. [Google Scholar] [CrossRef]
- Clay, J.M.; Parker, M.O. Alcohol use and misuse during the COVID-19 pandemic: A potential public health crisis? Lancet Public Health 2020, 5, e259. [Google Scholar] [CrossRef]
- De Wit, H.; Söderpalm, A.H.; Nikolayev, L.; Young, E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol. Clin. Exp. Res. 2003, 27, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Lechner, W.V.; Laurene, K.R.; Patel, S.; Anderson, M.; Grega, C.; Kenne, D.R. Changes in alcohol use as a function of psychological distress and social support following COVID-19 related University closings. Addict. Behav. 2020, 110, 106527. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, S.M.; Jetelina, K.K.; Knell, G. Longer time spent at home during COVID-19 pandemic is associated with binge drinking among US adults. Am. J. Drug Alcohol Abus. 2020, 1–9, epub ahead of print. [Google Scholar] [CrossRef]
- De Goeij, M.C.; Suhrcke, M.; Toffolutti, V.; van de Mheen, D.; Schoenmakers, T.M.; Kunst, A.E. How economic crises affect alcohol consumption and alcohol-related health problems: A realist systematic review. Soc. Sci. Med. 2015, 131, 131–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisely, S.; Warren, N.; McMahon, L.; Dalais, C.; Henry, I.; Siskind, D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: Rapid review and meta-analysis. BMJ 2020, 369, m1642. [Google Scholar] [CrossRef]
- Hogarth, L.; Hardy, L.; Mathew, A.R.; Hitsman, B. Negative mood-induced alcohol-seeking is greater in young adults who report depression symptoms, drinking to cope, and subjective reactivity. Exp. Clin. Psychopharmacol. 2018, 26, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Clay, J.M.; Adams, C.; Archer, P.; English, M.; Hyde, A.; Stafford, L.D.; Parker, M.O. Psychosocial stress increases craving for alcohol in social drinkers: Effects of risk-taking. Drug Alcohol Depend. 2018, 185, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Koob, G.F.; Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001, 24, 97–129. [Google Scholar] [CrossRef]
- The National Institute on Alcohol Abuse and Alcoholism. Alcohol Across the Lifespan: 50 Years of Evidence-Based Diagnosis, Prevention, and Treatment Research. Available online: https://www.niaaa.nih.gov/agenda-niaaa-50th-anniversary-science-symposium (accessed on 1 February 2021).
- Te Velde, A.A.; Bezema, T.; Van Kampen, A.H.; Kraneveld, A.D.; Hart, B.A.; Van Middendorp, H.; Hack, E.C.; Van Montfrans, J.M.; Belzer, C.; Jans-Beken, L.; et al. Embracing complexity beyond systems medicine: A new approach to chronic immune disorders. Front. Immunol. 2016, 7, 587. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [Green Version]
- Simou, E.; Leonardi-Bee, J.; Britton, J. The effect of alcohol consumption on the risk of ARDS: A systematic review and meta-analysis. Chest 2018, 154, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Fernie, G.; Cole, J.C.; Goudie, A.J.; Field, M. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug Alcohol Depend. 2010, 112, 54–61. [Google Scholar] [CrossRef]
- Clay, J.M.; Parker, M.O. The role of stress-reactivity, stress-recovery and risky decision-making in psychosocial stress-induced alcohol consumption in social drinkers. Psychopharmacology 2018, 235, 3243–3257. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Yeligar, S.M.; Brown, L.A.S. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. Sci. World J. 2012, 2012, 740308. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.J.; Guidot, D.M. Alcohol and the lung. Alcohol Res. 2017, 38, 243. [Google Scholar] [PubMed]
- Blendon, R.J.; Benson, J.M.; DesRoches, C.M.; Raleigh, E.; Taylor-Clark, K. The public’s response to severe acute respiratory syndrome in Toronto and the United States. Clin. Infect. Dis. 2004, 38, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Liu, X.; Fang, Y.; Fan, B.; Fuller, C.J.; Guan, Z.; Yao, Z.; Kong, J.; Lu, J.; Litvak, I.J. Alcohol abuse/dependence symptoms among hospital employees exposed to a SARS outbreak. Alcohol Alcohol. 2008, 43, 706–712. [Google Scholar] [CrossRef]
- Kiani, P.; Merlo, A.; Saeed, H.M.; Benson, S.; Bruce, G.; Hoorn, R.; Kraneveld, A.D.; van de Loo, A.J.; Severeijns, N.R.; Sips, A.S.; et al. Immune Fitness and the Psychosocial and Health Consequences of the COVID-19 Pandemic Lockdown in The Netherlands: Methodology and Design of the CLOFIT Study. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 199–218. [Google Scholar] [CrossRef]
- Verster, J.C.; Sandalova, E.; Garssen, J.; Bruce, G. The use of single-item ratings versus traditional multiple-item questionnaires to assess mood and health. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 183–198. [Google Scholar] [CrossRef]
- Wilson, D.M.C.; Nielsen, E.; Ciliska, D. Lifestyle assessment: Testing the FANTASTIC Instrument. Can. Fam. Physician 1984, 30, 1863–1866. [Google Scholar]
- Sharratt, J.K.; Sharratt, M.T.; Smith, D.M.; Howell, N.J.; Davenport, L. FANTASTIC Lifestyle survey of University of Waterloo employees. Can. Fam. Physician 1984, 30, 1869–1872. [Google Scholar] [PubMed]
- Wilod Versprille, L.J.F.; van de Loo, A.J.A.E.; Mackus, M.; Arnoldy, L.; Sulzer, T.A.L.; Vermeulen, S.A.; Abdulahad, S.; Huls, H.; Baars, T.; Kraneveld, A.D.; et al. Development and validation of the Immune Status Questionnaire (ISQ). Int. J. Environ. Res. Publ. Health 2019, 16, 4743. [Google Scholar] [CrossRef] [Green Version]
- Van Schrojenstein Lantman, M.; Otten, L.S.; Mackus, M.; de Kruijff, D.; van de Loo, A.J.A.E.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Mental resilience, perceived immune functioning, and health. J. Multidiscip. Healthc. 2017, 10, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, J.P.; Shrader, C.H.; Algarin, A.B.; Lee, J.Y.; Fish, J.N. Changes in alcohol use since the onset of COVID-19 are associated with psychological distress among sexual and gender minority university students in the US. Drug Alcohol Depend. 2021, 221, 10859. [Google Scholar] [CrossRef]
- Oldham, M.; Garnett, C.; Brown, J.; Kale, D.; Shahab, L.; Herbec, A. Characterising the patterns of and factors associated with increased alcohol consumption since COVID-19 in a UK sample. Drug Alcohol Rev. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Pabst, A.; Bollen, Z.; Creupelandt, C.; Fontesse, S.; Maurage, P. Alcohol consumption changes following COVID-19 lockdown among French-speaking Belgian individuals at risk for alcohol use disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110282. [Google Scholar] [CrossRef]
- Alpers, S.E.; Skogen, J.C.; Mæland, S.; Pallesen, S.; Rabben, Å.K.; Lunde, L.H.; Fadnes, L.T. Alcohol Consumption during a Pandemic Lockdown Period and Change in Alcohol Consumption Related to Worries and Pandemic Measures. Int. J. Environ. Res. Public Health 2021, 18, 1220. [Google Scholar] [CrossRef]
- Evans, S.; Alkan, E.; Bhangoo, J.K.; Tenenbaum, H.; Ng-Knight, T. Effects of the COVID-19 lockdown on mental health, wellbeing, sleep, and alcohol use in a UK student sample. Psychiatry Res. 2021, 298, 113819. [Google Scholar] [CrossRef]
- Schmits, E.; Glowacz, F. Changes in alcohol use during the COVID-19 pandemic: Impact of the lockdown conditions and mental health factors. Int. J. Ment. Health. Addict. 2021, 1–12, epub ahead of print. [Google Scholar] [CrossRef]
- Merlo, A.; Hendriksen, P.A.; Severeijns, N.R.; Garssen, J.; Bruce, G.; Verster, J.C. Alcohol consumption patterns during COVID-19 lockdown and their relationship with perceived immune fitness and reported COVID-19 symptoms. Healthcare 2021, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Public Health and the Environment (RIVM). COVID-19. Available online: https://www.rivm.nl/coronavirus-covid-19 (accessed on 20 July 2021).
| Overall | Men | Women | p-Value | |
|---|---|---|---|---|
| N (%) | 761 (100%) | 292 (38.4%) | 469 (61.6%) | |
| Age (year) | 42.3 (19.0) | 48.0 (19.2) | 38.7 (18.0) * | <0.001 * |
| Variables Assessed | Before Lockdown | During Lockdown | p-Value |
|---|---|---|---|
| Alcoholic drinks/week | 6.0 (8.5) | 5.9 (9.2) | 0.310 |
| Drinking days/week | 2.4 (2.1) | 2.5 (2.2) | 0.179 |
| Variable | N | Before Lockdown | During Lockdown | p-Value (Before vs. During Lockdown) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weekly alcohol consumption | Men | Women | Overall | Men | Women | Overall | Men | Women | Overall | |
| 18–25 years old | 219 | 10.2 (9.3) | 3.9 (5.0) † | 5.5 (7.0) | 7.6 (10.7) | 3.4 (4.2) † | 4.5 (6.8) | 0.001 * | 0.395 | 0.003 * |
| 26–35 years old | 120 | 6.3 (7.2) | 3.8 (4.1) | 4.6 (5.5) | 6.6 (8.6) | 3.7 (3.8) | 4.7 (6.1) | 0.597 | 0.716 | 0.568 |
| 36–45 years old | 54 | 14.6 (17.5) | 3.3 (3.2) † | 8.1 (12.8) | 13.6 (15.3) | 4.2 (4.8) | 8.2 (11.5) | 0.833 | 0.060 | 0.116 |
| 46–55 years old | 88 | 5.6 (6.8) | 3.9 (5.6) | 4.6 (6.2) | 7.3 (9.5) | 3.8 (5.1) | 5.4 (7.6) | 0.243 | 1.000 | 0.446 |
| 56–65 years old | 128 | 9.9 (12.4) | 4.2 (5.4) † | 6.7 (9.5) | 11.0 (16.3) | 4.0 (6.4) † | 7.0 (12.2) | 0.314 | 0.383 | 0.985 |
| 6–75 years old | 85 | 10.1 (13.9) | 5.3 (5.2) | 8.4 (11.9) | 9.8 (14.5) | 5.8 (6.0) | 8.4 (12.3) | 0.582 | 0.306 | 0.888 |
| ≥75 years old | 19 | 7.4 (6.8) | 2.9 (2.7) | 5.3 (5.6) | 7.5 (6.8) | 3.2 (3.2) | 5.5 (5.7) | 0.317 | 0.180 | 0.102 |
| Drinking days per week | ||||||||||
| 18–25 years old | 219 | 2.4 (1.5) | 1.5 (1.2) † | 1.7 (1.3) | 2.2 (1.8) | 1.6 (1.5) | 1.8 (1.6) | 0.274 | 0.159 | 0.627 |
| 26–35 years old | 120 | 2.1 (1.7) | 1.6 (1.1) | 1.8 (1.3) | 2.4 (2.0) | 2.0 (1.7) | 2.2 (1.8) | 0.074 | 0.030 | 0.005 * |
| 36–45 years old | 54 | 3.4 (2.5) | 2.1 (1.8) | 2.6 (2.2) | 3.3 (2.4) | 2.5 (2.2) | 2.9 (2.3) | 0.931 | 0.114 | 0.192 |
| 46–55 years old | 88 | 2.0 (2.2) | 2.1 (2.0) | 2.1 (2.1) | 2.3 (2.5) | 2.0 (2.1) | 2.1 (2.3) | 0.336 | 0.360 | 0.934 |
| 56–65 years old | 128 | 3.7 (2.3) | 2.6 (2.3) † | 3.1 (2.3) | 3.8 (2.5) | 2.4 (2.4) † | 3.0 (2.5) | 0.670 | 0.307 | 0.566 |
| 66–75 years old | 85 | 4.2 (2.5) | 3.5 (2.5) | 4.0 (2.5) | 3.7 (2.6) | 3.8 (2.6) | 3.7 (2.6) | 0.011 | 0.236 | 0.131 |
| ≥ 75 years old | 19 | 4.1 (2.7) | 2.8 (2.8) | 3.5 (2.8) | 4.1 (2.8) | 2.8 (2.8) | 3.5 (2.8) | 1.000 | 1.000 | 1.000 |
| Variables Assessed | Before Lockdown | During Lockdown | p-Value (Before vs. During Lockdown) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Overall | Men | Women | Overall | Men | Women | Overall | |
| Anxiety | 1.6 (1.9) | 2.1 (2.5) | 2.0 (2.3) | 2.7 (2.6) | 3.2 (2.8) | 3.1 (2.7) | <0.001 * | <0.001 * | <0.001 * |
| Depression | 1.5 (1.9) | 2.2 (2.4) † | 2.0 (2.3) | 2.5 (2.6) | 3.3 (3.0) | 3.1 (2.9) | <0.001 * | <0.001 * | <0.001 * |
| Loneliness | 1.8 (2.1) | 2.1 (2.2) | 2.0 (2.2) | 3.6 (2.9) | 3.7 (2.9) | 3.7 (2.9) | <0.001 * | <0.001 * | <0.001 * |
| Fatigue | 3.7 (2.6) | 4.9 (2.6) † | 4.5 (2.7) | 3.9 (2.6) | 4.9 (2.5) † | 4.6 (2.6) | 0.406 | 0.652 | 0.418 |
| Hostile | 0.9 (1.5) | 0.9 (1.8) | 0.9 (1.7) | 1.6 (2.4) | 1.2 (2.2) | 1.3 (2.2) | <0.001 * | <0.001 * | <0.001 * |
| Happy | 7.3 (1.4) | 6.9 (1.7) | 7.0 (1.6) | 6.4 (1.7) | 6.0 (2.0) | 6.1 (1.9) | <0.001 * | <0.001 * | <0.001 * |
| Stress | 3.5 (2.6) | 4.6 (2.5) † | 4.3 (2.6) | 4.2 (2.7) | 5.1 (2.5) † | 4.8 (2.6) | 0.010 | 0.005 * | <0.001 * |
| Optimism | 3.1 (0.8) | 2.9 (1.0) | 3.0 (1.0) | 2.8 (0.8) | 2.6 (1.0) | 2.6 (1.0) | 0.025 | <0.001 * | <0.001 * |
| Being active | 6.9 (2.0) | 6.7 (2.0) | 6.8 (2.0) | 5.5 (2.2) | 5.5 (2.3) | 5.5 (2.3) | <0.001 * | <0.001 * | <0.001 * |
| Coping with stress | 6.0 (1.6) | 5.3 (1.7) | 5.5 (1.7) | 5.6 (1.8) | 5.0 (1.8) | 5.1 (1.8) | 0.026 | 0.029 | 0.003 * |
| Perceived immune fitness | 7.8 (1.5) | 7.4 (1.5) | 7.5 (1.5) | 7.5 (1.6) | 7.3 (1.6) | 7.4 (1.6) | 0.012 | 0.286 | 0.022 |
| COVID-19 symptoms severity | 3.0 (3.1) | 4.3 (4.0) † | 3.9 (3.8) | 3.0 (3.4) | 4.4 (4.2) † | 4.0 (4.1) | 0.538 | 0.474 | 0.737 |
| COVID-19 symptoms presence | 2.3 (2.0) | 3.0 (2.2) | 2.8 (2.2) | 2.3 (2.0) | 2.9 (2.2) † | 2.7 (2.1) | 0.438 | 0.915 | 0.645 |
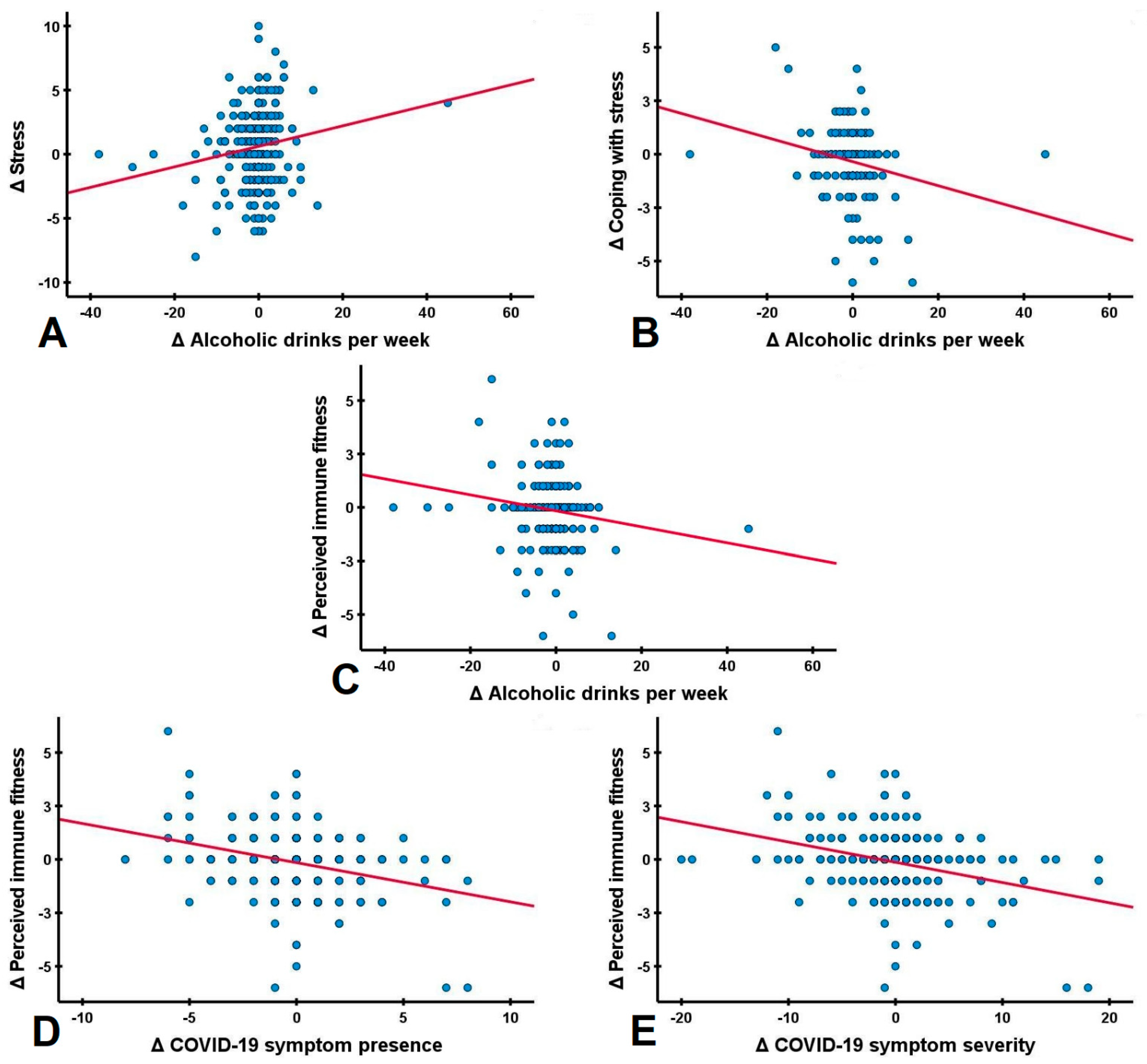
| Variables | Sex | Δ Stress | Δ Coping with Stress | Δ Alcoholic Drinks/Week | Δ Drinking Days/Week | Δ Perceived Immune Fitness | Δ COVID-19 Symptoms Severity | Δ COVID-19 Symptoms Presence |
|---|---|---|---|---|---|---|---|---|
| Δ Anxiety | Men | 0.503 * | −0.309 | 0.064 | 0.242 | −0.343 * | −0.024 | −0.088 |
| Women | 0.535 * | −0.433 * | 0.128 | 0.007 | −0.256 * | 0.044 | 0.016 | |
| Overall | 0.528 * | −0.407 * | 0.088 | 0.075 | −0.278 * | 0.029 | −0.010 | |
| Δ Depression | Men | 0.339 * | −0.604 * | 0.058 | 0.059 | −0.436 * | 0.199 | 0.167 |
| Women | 0.522 * | −0.528 * | 0.157 | 0.167 | −0.368 * | 0.071 | 0.099 | |
| Overall | 0.482 * | −0.535 * | 0.106 | 0.138 | −0.375 * | 0.092 | 0.111 | |
| Δ Loneliness | Men | 0.185 | −0.109 | −0.141 | 0.054 | −0.242 | 0.086 | 0.076 |
| Women | 0.277 * | −0.377 * | −0.021 | 0.035 | −0.176 * | 0.076 | 0.062 | |
| Overall | 0.252 * | −0.318 * | −0.075 | 0.038 | −0.195 * | 0.077 | 0.066 | |
| Δ Fatigue | Men | 0.429 * | −0.421 * | 0.161 | 0.112 | −0.152 | 0.063 | 0.039 |
| Women | 0.492 * | −0.561 * | 0.146 | 0.133 | −0.382 * | 0.141 | 0.158 | |
| Overall | 0.477 * | −0.530 * | 0.135 | 0.122 | −0.327 * | 0.123 | 0.128 | |
| Δ Hostile | Men | 0.171 | −0.320 | −0.136 | 0.003 | −0.143 | −0.011 | −0.013 |
| Women | 0.338 * | −0.327 * | 0.069 | 0.106 | −0.166 | 0.049 | 0.067 | |
| Overall | 0.284 * | −0.326 * | −0.047 | 0.058 | −0.162 * | 0.029 | 0.040 | |
| Δ Happy | Men | −0.400 * | 0.210 | −0.076 | 0.064 | −0.451 * | −0.144 | −0.086 |
| Women | −0.534 * | 0.567 * | −0.071 | −0.101 | 0.311 * | −0.080 | −0.091 | |
| Overall | −0.503 * | 0.496 * | −0.064 | −0.089 | 0.339 * | −0.092 | −0.090 | |
| Δ Optimistic | Men | −0.530 * | 0.467 * | −0.155 | −0.258 | 0.396 * | −0.109 | −0.152 |
| Women | −0.522 * | 0.569 * | −0.195 | −0.164 | 0.257 * | −0.065 | −0.090 | |
| Overall | −0.524 * | 0.544 * | −0.157 | −0.190 | 0.280 * | −0.075 | −0.104 | |
| Δ Being active | Men | −0.105 | 0.288 | −0.064 | −0.182 | 0.280 * | 0.004 | 0.069 |
| Women | −0.272 * | 0.421 * | −0.042 | −0.065 | 0.435 * | −0.123 | −0.075 | |
| Overall | −0.227 * | 0.385 * | −0.046 | −0.100 | 0.393 * | −0.090 | −0.035 |
| Δ Stress | Δ Coping with Stress | |||||
|---|---|---|---|---|---|---|
| Men | Women | Overall | Men | Women | Overall | |
| Δ Alcoholic drinks/week | 0.192 | 0.169 * | 0.163 * | −0.156 | −0.351 * | −0.225 * |
| Δ Drinking days/week | 0.105 | 0.110 | 0.106 | −0.265 | −0.298 * | −0.279 * |
| Δ Perceived immune fitness | −0.321 * | −0.271 * | −0.285 * | 0.470 * | 0.394 * | 0.411 * |
| Δ COVID-19 symptoms severity | −0.044 | 0.094 | 0.061 | −0.379 * | −0.096 | −0.160 |
| Δ COVID-19 symptoms presence | −0.078 | 0.127 | 0.074 | −0.387 * | −0.120 | −0.183 |
| Δ Alcoholic Drinks/Week | Δ Drinking Days/Week | |||||
|---|---|---|---|---|---|---|
| Men | Women | Overall | Men | Women | Overall | |
| Δ Perceived immune fitness | −0.221 | −0.149 | −0.165 * | 0.009 | −0.137 | −0.086 |
| Δ COVID-19 symptoms severity | 0.053 | −0.002 | 0.020 | −0.186 | 0.014 | 0.037 |
| Δ COVID-19 symptoms presence | 0.029 | 0.049 | 0.037 | −0.161 | 0.024 | −0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlo, A.; Severeijns, N.R.; Benson, S.; Scholey, A.; Garssen, J.; Bruce, G.; Verster, J.C. Mood and Changes in Alcohol Consumption in Young Adults during COVID-19 Lockdown: A Model Explaining Associations with Perceived Immune Fitness and Experiencing COVID-19 Symptoms. Int. J. Environ. Res. Public Health 2021, 18, 10028. https://doi.org/10.3390/ijerph181910028
Merlo A, Severeijns NR, Benson S, Scholey A, Garssen J, Bruce G, Verster JC. Mood and Changes in Alcohol Consumption in Young Adults during COVID-19 Lockdown: A Model Explaining Associations with Perceived Immune Fitness and Experiencing COVID-19 Symptoms. International Journal of Environmental Research and Public Health. 2021; 18(19):10028. https://doi.org/10.3390/ijerph181910028
Chicago/Turabian StyleMerlo, Agnese, Noortje R Severeijns, Sarah Benson, Andrew Scholey, Johan Garssen, Gillian Bruce, and Joris C Verster. 2021. "Mood and Changes in Alcohol Consumption in Young Adults during COVID-19 Lockdown: A Model Explaining Associations with Perceived Immune Fitness and Experiencing COVID-19 Symptoms" International Journal of Environmental Research and Public Health 18, no. 19: 10028. https://doi.org/10.3390/ijerph181910028
APA StyleMerlo, A., Severeijns, N. R., Benson, S., Scholey, A., Garssen, J., Bruce, G., & Verster, J. C. (2021). Mood and Changes in Alcohol Consumption in Young Adults during COVID-19 Lockdown: A Model Explaining Associations with Perceived Immune Fitness and Experiencing COVID-19 Symptoms. International Journal of Environmental Research and Public Health, 18(19), 10028. https://doi.org/10.3390/ijerph181910028







