Recent Trends in Artificial Intelligence-Assisted Coronary Atherosclerotic Plaque Characterization
Abstract
:1. Introduction
- To compare manual grading systems for plaque characterization with various imaging modalities;
- To analyze state-of-the-art artificial intelligence (AI) techniques to characterize plaque;
- To discuss the results and roles of different techniques for plaque characterization; and
- To highlight potential research gaps and future research directions related to plaque characterization using CAD.
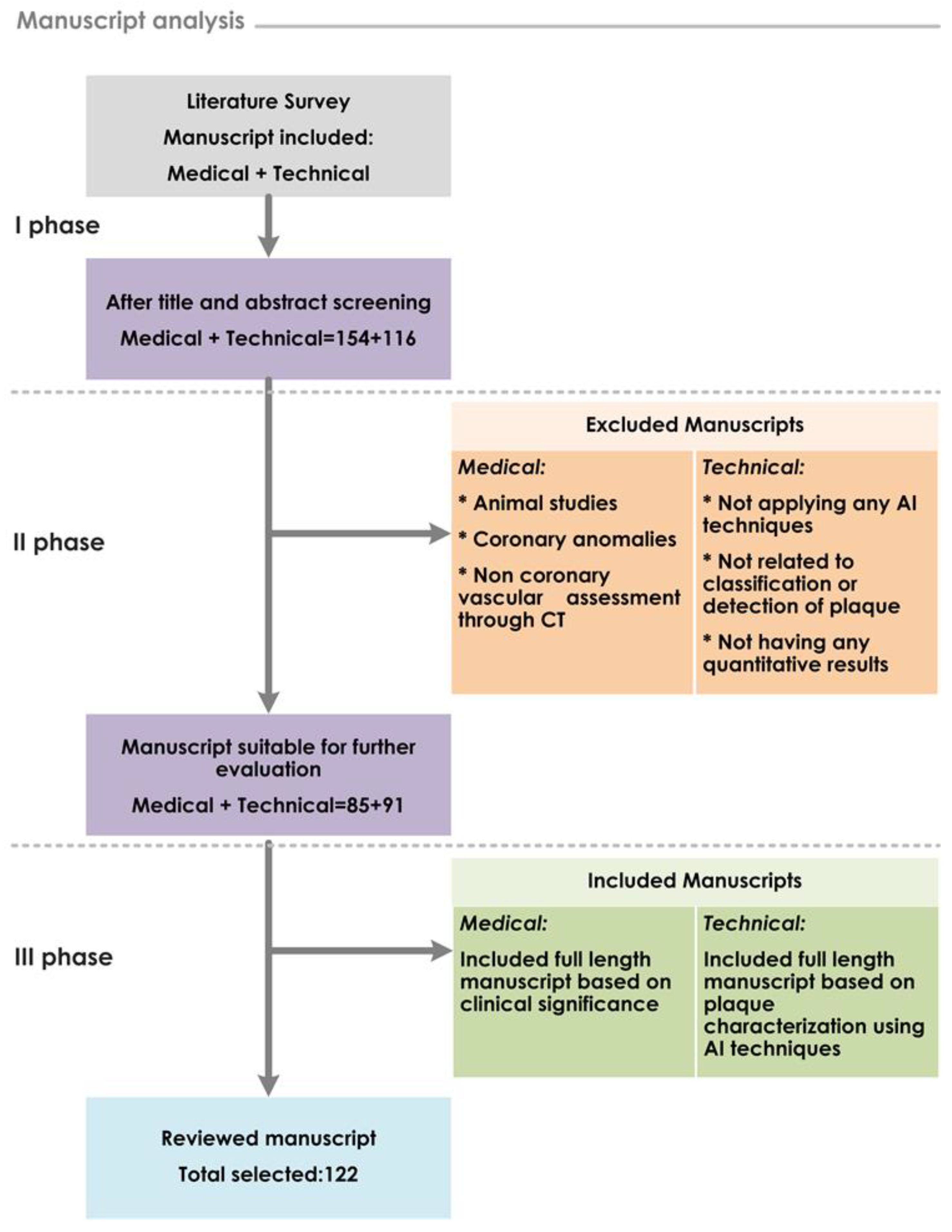
2. Review Process
3. Current Coronary Artery Disease Detection Modalities and Grading System
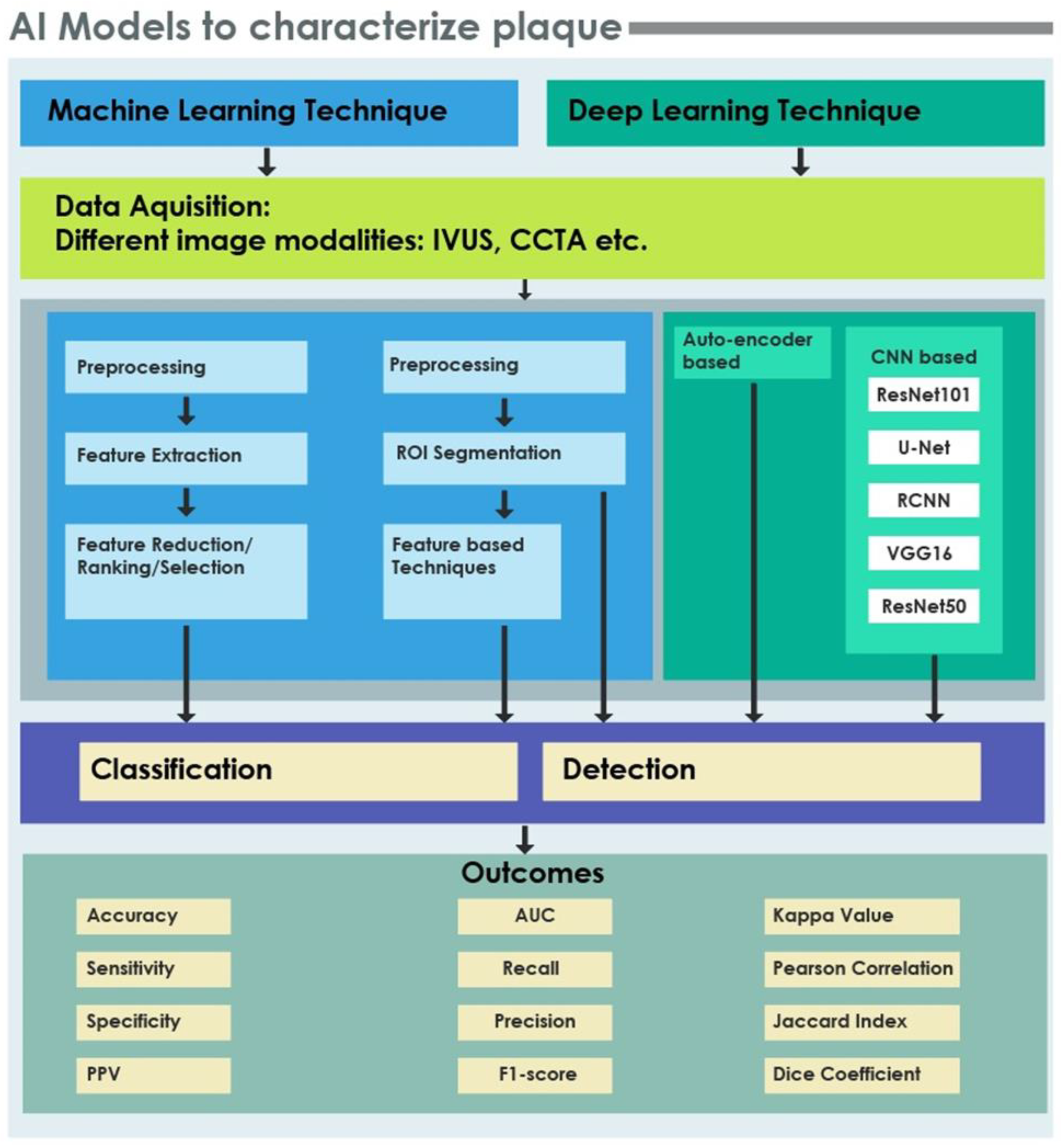
4. Artificial Intelligence (AI): Characterization of Plaque
4.1. ML and DL Techniques in CAD
- Machine Learning Techniques
- Deep Learning Techniques
4.1.1. Preprocessing/Segmentation
4.1.2. Feature Extraction
4.1.3. Feature Reduction/Selection/Ranking/Organization
4.1.4. Classification
5. Discussion
5.1. Role of Various Modalities in Coronary Artery Disease
5.2. Role of CAD in Coronary Artery Disease
5.3. Research Opportunities and Future Direction
5.4. Limitations of the Study
- The present review has been carried out based on manuscripts written in English. Other language manuscripts were not included during the review process;
- The current review process included a plaque grading system using various modalities, and analysis of various AI algorithms to develop CAD for plaque categorization. However, review on grading during plaque deposition and after treatment was not given substantial consideration;
- The specific reasons to select the algorithms based on AI were not mentioned. It was also unclear whether the proposed CAD can improve the survival of patients.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Sofian, H.; Than, J.C.M.; Mohammad, S.; Noor, N.M. Calcification Detection of Coronary Artery Disease in Intravascular Ultrasound Image: Deep Feature Learning Approach. Int. J. Integr. Eng. 2018, 10. Available online: https://publisher.uthm.edu.my/ojs/index.php/ijie/article/view/3473 (accessed on 9 August 2021).
- Packard, R.R.; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Ross, R. Mechanisms of disease—Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Slager, C.J.; Wentzel, J.J.; Gijsen, F.J.H.; Thury, A.; Van der Wal, A.C.; Schaar, J.A.; Serruys, P.W. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Shah, P.K.; Fuster, V. Coronary plaque disruption. Circulation 1995, 92, 657–671. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, L.S.; Fotiadis, D.I.; Michalis, L.K. Atherosclerotic Plaque Characterization Methods Based on Coronary Imaging; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arter. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. A Report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Rezaei, Z.; Selamat, A.; Taki, A.; Rahim, M.S.M.; Kadir, M.R.A. Automatic plaque segmentation based on hybrid fuzzy clustering and k nearest neighborhood using virtual histology intravascular ultrasound images. Appl. Soft Comput. 2017, 53, 380–395. [Google Scholar] [CrossRef]
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, H.T.; Jang, I.-K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Kang, D.-H.; Halpern, E.F.; et al. Characterization of Human Atherosclerosis by Optical Coherence Tomography. Circulation 2002, 106, 1640–1645. [Google Scholar] [CrossRef]
- Jang, I.K.; Tearney, G.J.; MacNeill, B.; Takano, M.; Moselewski, F.; Iftima, N.; Shishkov, M.; Houser, S.; Aretz, H.T.; Halpern, E.F.; et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 2005, 111, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, M.; Szilveszter, B.; Merkely, B.; Maurovich-Horvat, P. Plaque imaging with CT-a comprehensive review on coronary CT angiography based risk assessment. Cardiovasc. Diagn. Ther. 2017, 7, 489–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvathi, D.; Emimal, N.; Selvaraj, H. Automated characterization of Atheromatous plaque in intravascular ultrasound images using Neuro fuzzy classifier. Int. J. Electron. Telecommun. 2012, 58, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of Coronary Atherosclerotic Plaques from Computed Tomography Imaging: A Review of Recent Methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.; Puri, R.; Kataoka, Y.; Nicholls, S.J.; Psaltis, P.J. Therapeutic modulation of the natural history of coronary atherosclerosis: Lessons learned from serial imaging studies. Cardiovasc. Diagn. Ther. 2016, 6, 282–303. [Google Scholar] [CrossRef] [Green Version]
- Carlier, S.G.; Tanaka, K. Studying coronary plaque regression with IVUS: A critical review of recent studies. J. Interv. Cardiol. 2006, 19, 11–15. [Google Scholar] [CrossRef]
- Voros, S. Can Computed Tomography Angiography of the Coronary Arteries Characterize Atherosclerotic Plaque Composition? Is the CAT (Scan) Out of the Bag? JACC Cardiovasc. Interv. 2008, 1, 183–185. [Google Scholar] [CrossRef]
- Athanasiou, L.S.; Karvelis, P.S.; Tsakanikas, V.D.; Naka, K.K.; Michalis, L.K.; Bourantas, C.V.; Fotiadis, D.I. A novel Semiautomated atherosclerotic plaque characterization method using Grayscale intravascular ultrasound images: Comparison with virtual histology. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 391–400. [Google Scholar] [CrossRef]
- Olender, M.L.; Athanasiou, L.S.; Michalis, L.K.; Fotiadis, D.I.; Edelman, E.R. A Domain Enriched Deep Learning Approach to Classify Atherosclerosis Using Intravascular Ultrasound Imaging. IEEE J. Sel. Top. Signal Process. 2020, 14, 1210–1220. [Google Scholar] [CrossRef]
- Nair, A.; Margolis, M.P.; Kuban, B.D.; Vince, D.G. Automated coronary plaque characterisation with intravascular ultrasound backscatter: Ex vivo validation. EuroIntervention 2007, 3, 113–120. [Google Scholar] [PubMed]
- Nair, A.; Kuban, B.D.; Tuzcu, E.M.; Schoenhagen, P.; Nissen, S.E.; Vince, D.G. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002, 106, 2200–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.Y.; Lee, J.H.; Na Hwang, Y.; Kim, S.M. A novel intensity-based multi-level classification approach for coronary plaque characterization in intravascular ultrasound images. Biomed. Eng. Online 2018, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Taki, A.; Hetterich, H.; Roodaki, A.; Setarehdan, S.; Unal, G.; Rieber, J.; Navab, N.; König, A. A new approach for improving coronary plaque component analysis based on intravascular ultrasound images. Ultrasound Med. Biol. 2010, 36, 1245–1258. [Google Scholar] [CrossRef]
- Xu, M.; Cheng, J.; Wong, D.W.K.; Taruya, A.; Tanaka, A.; Liu, J.; Foin, N.; Wong, P. Automatic image classification in intravascular optical coherence tomography images. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 1544–1547. [Google Scholar] [CrossRef]
- Gorenoi, V.; Schönermark, M.P.; Hagen, A. CT coronary angiography vs. invasive coronary angiography in CHD. GMS Health Technol. Assess. 2012, 8, Doc02. [Google Scholar] [CrossRef]
- Albus, C.; Barkhausen, J.; Fleck, E.; Haasenritter, J.; Lindner, O.; Silber, S. The diagnosis of chronic coronary heart disease. Dtsch. Aerzteblatt Online 2017, 114, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Murgia, A.; Balestrieri, A.; Crivelli, P.; Suri, J.S.; Conti, M.; Cademartiri, F.; Saba, L. Cardiac computed tomography radiomics: An emerging tool for the non-invasive assessment of coronary atherosclerosis. Cardiovasc. Diagn. Ther. 2020, 10, 2005–2017. [Google Scholar] [CrossRef]
- Infante, T.; Forte, E.; Schiano, C.; Cavaliere, C.; Tedeschi, C.; Soricelli, A.; Salvatore, M.; Napoli, C. An integrated approach to coronary heart disease diagnosis and clinical management. Am. J. Transl. Res. 2017, 9, 3148–3166. [Google Scholar]
- Doh, J.H.; Koo, B.K.; Nam, C.W.; Kim, J.H.; Min, J.K.; Nakazato, R.; Silalahi, T.; Prawira, H.; Choi, H.; Lee, S.Y.; et al. Diagnostic value of coronary CT angiography in comparison with invasive coronary angiography and intravascular ultrasound in patients with intermediate coronary artery stenosis: Results from the prospective multicentre FIGURE-OUT (Functional Imaging criteria for GUiding REview of invasive coronary Angiography, intravascular Ultrasound, and coronary computed Tomographic angiography) study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Mowatt, G.; Cummins, E.; Waugh, N.; Walker, S.; Cook, J.; Jia, X.; Hillis, G.S.; Fraser, C. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. 2008. In NIHR Health Technology Assessment Programme: Executive Summaries; NIHR Journals Library: Southampton, UK, 2003. [Google Scholar]
- Miller, J.M.; Rochitte, C.E.; Dewey, M.; Arbab-Zadeh, A.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 2008, 359, 2324–2336. [Google Scholar] [CrossRef] [Green Version]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals without Known Coronary Artery Disease: Results from the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar]
- Meijboom, W.B.; Meijs, M.F.; Schuijf, J.; Cramer, M.J.; Mollet, N.R.; van Mieghem, C.A.; Nieman, K.; van Werkhoven, J.M.; Pundziute, G.; Weustink, A.C.; et al. Diagnostic Accuracy of 64-Slice Computed Tomography Coronary Angiography: A Prospective, Multicenter, Multivendor Study. J. Am. Coll. Cardiol. 2008, 52, 2135–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajouei, A.S.; Adibi, A.; Maghsodi, Z.; Nejati, M.; Behjati, M. Prognostic value of normal and nonobstructive coronary artery disease based on CT angiography findings. A 12 month follow up study. J. Cardiovasc. Thorac. Res. 2019, 11, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Fujimoto, S.; Kondo, T.; Arai, T.; Sekine, T.; Matsutani, H.; Sano, T.; Kondo, M.; Kodama, T.; Takase, S.; et al. Prevalence of computed tomographic angiography-verified high-risk plaques and significant luminal stenosis in patients with zero coronary calcium score. Int. J. Cardiol. 2012, 158, 272–278. [Google Scholar] [CrossRef]
- Iwasaki, K.; Matsumoto, T.; Aono, H.; Furukawa, H.; Samukawa, M. Prevalence of non-calcified coronary plaque on 64-slice computed tomography in asymptomatic patients with zero and low coronary artery calcium. Can. J. Cardiol. 2010, 26, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Uretsky, S.; Rozanski, A.; Singh, P.; Supariwala, A.; Atluri, P.; Bangalore, S.; Pappas, T.; Fisher, E.A.; Peters, M.R. The presence, characterization and prognosis of coronary plaques among patients with zero coronary calcium scores. Int. J. Cardiovasc. Imaging 2011, 27, 805–812. [Google Scholar] [CrossRef]
- Becker, C.R.; Nikolaou, K.; Muders, M.; Babaryka, G.; Crispin, A.; Schoepf, U.J.; Loehrs, U.; Reiser, M.F. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur. Radiol. 2003, 13, 2094–2098. [Google Scholar] [CrossRef]
- Kelly, J.L.; Thickman, D.; Abramson, S.D.; Chen, P.R.; Smazal, S.F.; Fleishman, M.J.; Lingam, S.C. Coronary CT angiography findings in patients without coronary calcification. Am. J. Roentgenol. 2008, 191, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Shimada, K.; Yoshida, K.; Jissyo, S.; Tanaka, H.; Sakamoto, M.; Matsuba, K.; Imanishi, T.; Akasaka, T.; Yoshikawa, J. Non-invasive assessment of plaque rupture by 64-slice multidetector computed tomography—Comparison with intravascular ultrasound. Circ. J. 2008, 72, 1276–1281. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, M.; Tanaka, A.; Kitabata, H.; Tsujioka, H.; Kataiwa, H.; Komukai, K.; Tanimoto, T.; Takemoto, K.; Takarada, S.; Kubo, T.; et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by ultidetector computed tomography. JACC Cardiovasc. Imaging 2009, 2, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Hoffmann, U.; Vorpahl, M.; Nakano, M.; Virmani, R.; Alkadhi, H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc. Imaging 2010, 3, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifarth, H.; Schlett, C.L.; Nakano, M.; Otsuka, F.; Károlyi, M.; Liew, G.; Maurovich-Horvat, P.; Alkadhi, H.; Virmani, R.; Hoffmann, U. Histopathological correlates of the napkin-ring sign plaque in coronary CT angiography. Atherosclerosis 2012, 224, 90–96. [Google Scholar] [CrossRef]
- Schroeder, S. Non-invasive evaluation of atherosclerosis with contrast enhanced 16 slice spiral computed tomography: Results of ex vivo investigations. Heart 2004, 90, 1471–1475. [Google Scholar] [CrossRef]
- Kimura, S.; Yonetsu, T.; Suzuki, K.; Isobe, M.; Iesaka, Y.; Kakuta, T. Characterisation of non-calcified coronary plaque by 16-slice multidetector computed tomography: Comparison with histopathological specimens obtained by directional coronary atherectomy. Int. J. Cardiovasc. Imaging 2011, 28, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.C.; Cater, G.; Vargas, J.; Bluemke, D.A. Beyond Coronary Stenosis: Coronary Computed Tomographic Angiography for the Assessment of Atherosclerotic Plaque Burden. Curr. Cardiovasc. Imaging Rep. 2013, 6, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.F.; Schroeder, S.; Baumbach, A.; Kuettner, A.; Georg, C.; Ohnesorge, B.; Heuschmid, M.; Kuzo, R.; Claussen, C.D. Non-invasive characterisation of coronary lesion morphology and composition by multislice CT: First results in comparison with intracoronary ultrasound. Eur. Radiol. 2001, 11, 1607–1611. [Google Scholar] [CrossRef]
- Schroeder, S.; Kopp, A.F.; Baumbach, A.; Meisner, C.; Kuettner, A.; Georg, C.; Ohnesorge, B.; Herdeg, C.; Claussen, C.D.; Karsch, K.R. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J. Am. Coll. Cardiol. 2001, 37, 1430–1435. [Google Scholar] [CrossRef] [Green Version]
- Leber, A.W.; Knez, A.; Becker, A.; Becker, C.; von Ziegler, F.; Nikolaou, K.; Rist, C.; Reiser, M.; White, C.; Steinbeck, G.; et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: A comparative study with intracoronary ultrasound. J. Am. Coll. Cardiol. 2004, 43, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary atherosclerosis imaging by coronary CT angiography: Current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc. Imaging 2011, 4, 537–554. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.E.W.; Raghavendra, U.; Gudigar, A.; Ping, O.C.; Molinari, F.; Mishra, S.; Mathavan, S.; Raman, R.; Acharya, U. A novel hybrid approach for automated detection of retinal detachment using ultrasound images. Comput. Biol. Med. 2020, 120, 103704. [Google Scholar] [CrossRef]
- Pham, T.-H.; Raghavendra, U.; Koh, J.E.W.; Gudigar, A.; Chan, W.Y.; Hamid, M.T.R.; Rahmat, K.; Fadzli, F.; Ng, K.H.; Ooi, C.P.; et al. Development of breast papillary index for differentiation of benign and malignant lesions using ultrasound images. J. Ambient. Intell. Humaniz. Comput. 2020, 12, 2121–2129. [Google Scholar] [CrossRef]
- Raghavendra, U.; Gudigar, A.; Ciaccio, E.J.; Ng, K.H.; Chan, W.Y.; Rahmat, K.; Acharya, U.R. 2DSM vs. FFDM: A computer aided diagnosis based comparative study for the early detection of breast cancer. Expert Syst. 2019, 38, e12474. [Google Scholar] [CrossRef]
- Raghavendra, U.; Gudigar, A.; Bhandary, S.V.; Rao, T.N.; Ciaccio, E.J.; Acharya, U.R. A Two Layer Sparse Autoencoder for Glaucoma Identification with Fundus Images. J. Med. Syst. 2019, 43, 299. [Google Scholar] [CrossRef]
- Gudigar, A.; Raghavendra, U.; Devasia, T.; Nayak, K.; Danish, S.M.; Kamath, G.; Samanth, J.; Pai, U.M.; Nayak, V.; Tan, R.S.; et al. Global weighted LBP based entropy features for the assessment of pulmonary hypertension. Pattern Recognit. Lett. 2019, 125, 35–41. [Google Scholar] [CrossRef]
- Gudigar, A.; Raghavendra, U.; Ciaccio, E.J.; Arunkumar, N.; Abdulhay, E.; Acharya, U.R. Automated categorization of multi-class brain abnormalities using decomposition techniques with MRI images: A comparative study. IEEE Access 2019, 7, 28498–28509. [Google Scholar] [CrossRef]
- Gudigar, A.; Raghavendra, U.; San, T.R.; Ciaccio, E.J.; Acharya, U.R. Application of multiresolution analysis for automated detection of brain abnormality using MR images: A comparative study. Future Gener. Comput. Syst. 2019, 90, 359–367. [Google Scholar] [CrossRef]
- Molinari, F.; Raghavendra, U.; Gudigar, A.; Meiburger, K.M.; Acharya, U.R. An efficient data mining framework for the characterization of symptomatic and asymptomatic carotid plaque using bidimensional empirical mode decomposition technique. Med. Biol. Eng. Comput. 2018, 56, 1579–1593. [Google Scholar] [CrossRef]
- Raghavendra, U.; Gudigar, A.; Maithri, M.; Gertych, A.; Meiburger, K.M.; Yeong, C.H.; Madla, C.; Kongmebhol, P.; Molinari, F.; Ng, K.H.; et al. Optimized multi-level elongated quinary patterns for the assessment of thyroid nodules in ultrasound images. Comput. Biol. Med. 2018, 95, 55–62. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.; Molinari, F.; Saba, L.; Ho, S.Y.; Ahuja, A.T.; Ho, S.C.; Nicolaides, A.; Suri, J.S. Atherosclerotic risk stratification strategy for carotid arteries using texture-based features. Ultrasound Med. Biol. 2012, 38, 899–915. [Google Scholar] [CrossRef]
- Raghavendra, U.; Bhandary, S.; Gudigar, A.; Acharya, U.R. Novel expert system for glaucoma identification using non-parametric spatial envelope energy spectrum with fundus images. Biocybern. Biomed. Eng. 2018, 38, 170–180. [Google Scholar] [CrossRef]
- Santini, G.; Della Latta, D.; Martini, N.; Valvano, G.; Gori, A.; Ripoli, A.; Susini, C.L.; Landini, L.; Chiappino, D. An automatic deep learning approach for coronary artery calcium segmentation. In EMBEC & NBC 2017; Eskola, H., Väisänen, O., Viik, J., Hyttinen, J., Eds.; Springer: Singapore, 2016; Volume 65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, H.; Guo, X.; Molony, D.; Guo, X.; Samady, H.; Giddens, D.P.; Athanasiou, L.; Nie, R.; Cao, J.; et al. Convolution neural networks and support vector machines for automatic segmentation of intracoronary optical coherence tomography. Mol. Cell. Biomech. 2019, 16, 153–161. [Google Scholar] [CrossRef]
- Candemir, S.; White, R.D.; Demirer, M.; Gupta, V.; Bigelow, M.T.; Prevedello, L.; Erdal, B.S. Automated coronary artery atherosclerosis detection and weakly supervised localization on coronary CT angiography with a deep 3-dimensional convolutional neural network. Comput. Med. Imaging Graph. 2020, 83, 101721. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.M.; Riaz, A.; Rajani, R.; Reyes-Aldasoro, C.C.; Slabaugh, G. Framework for detection and localization of coronary non-calcified plaques in cardiac CTA using mean radial profiles. Comput. Biol. Med. 2017, 89, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Vercio, L.L.; Orlando, J.I.; Del Fresno, M.; Larrabide, I. Assessment of image features for vessel wall segmentation in intravascular ultrasound images. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1397–1407. [Google Scholar] [CrossRef]
- Balakrishna, C.; Dadashzadeh, S.; Soltaninejad, S. Automatic detection of lumen and media in the IVUS images using U-Net with VGG16 Encoder. arXiv 2018, arXiv:1806.07554. [Google Scholar]
- Lee, J.; Prabhu, D.; Kolluru, C.; Gharaibeh, Y.; Zimin, V.N.; Dallan, L.A.P.; Bezerra, H.G.; Wilson, D.L. Fully automated plaque characterization in intravascular OCT images using hybrid convolutional and lumen morphology features. Sci. Rep. 2020, 10, 2596. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, B.; Chen, F.; Cao, X.; Yi, H.; Hou, Y.; He, X.; Liang, J. An automatic multi-class coronary atherosclerosis plaque detection and classification framework. Med. Biol. Eng. Comput. 2019, 57, 245–257. [Google Scholar] [CrossRef]
- Gudigar, A.; Raghavendra, U.; Hegde, A.; Kalyani, M.; Ciaccio, E.J.; Acharya, U.R. Brain pathology identification using computer aided diagnostic tool: A systematic review. Comput. Methods Programs Biomed. 2020, 187, 105205. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, U.; Gudigar, A.; Rao, T.N.; Ciaccio, E.J.; Ng, E.Y.K.; Acharya, U.R. Computer-aided diagnosis for the identification of breast cancer using thermogram images: A comprehensive review. Infrared Phys. Technol. 2019, 102, 103041. [Google Scholar] [CrossRef]
- Gudigar, A.; Raghavendra, U.; Hegde, A.; Menon, G.; Molinari, F.; Ciaccio, E.; Acharya, U.R. Automated Detection and Screening of Traumatic Brain Injury (TBI) Using Computed Tomography Images: A Comprehensive Review and Future Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 6499. [Google Scholar] [CrossRef]
- Raghavendra, U.; Bhat, N.S.; Gudigar, A.; Acharya, U.R. Automated system for the detection of thoracolumbar fracture using a CNN architecture. Future Gener. Comput. Syst. 2018, 85, 184–189. [Google Scholar] [CrossRef]
- Raghavendra, U.; Fujita, H.; Bhandary, S.; Gudigar, A.; Tan, J.H.; Acharya, U.R. Deep convolution neural network for accurate diagnosis of glaucoma using digital fundus images. Inf. Sci. 2018, 441, 41–49. [Google Scholar] [CrossRef]
- Huang, W.; Huang, L.; Lin, Z.; Huang, S.; Chi, Y.; Zhou, J.; Zhang, J.; Tan, R.-S.; Zhong, L. Coronary Artery Segmentation by Deep Learning Neural Networks on Computed Tomographic Coronary Angiographic Images. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 608–611. [Google Scholar] [CrossRef]
- Taki, A.; Najafi, Z.; Roodaki, A.; Setarehdan, S.K.; Zoroofi, R.A.; König, A.; Navab, N. Automatic segmentation of calcified plaques and vessel borders in IVUS images. Int. J. Comput. Assist. Radiol. Surg. 2008, 3, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Pang, Y.; Niu, S.; Yang, H.; Dong, J.; Zhou, J.; Chen, Y. Automatic identification of vulnerable plaque based on flexible neural tree. In Proceedings of the 2018 International Conference on Security, Pattern Analysis, and Cybernetics (SPAC), Jinan, China,, 14–17 December 2018; pp. 242–245. [Google Scholar] [CrossRef]
- Athanasiou, L.S.; Bourantas, C.V.; Rigas, G.A.; Exarchos, T.P.; Sakellarios, A.I.; Siogkas, P.K.; Papafaklis, M.I.; Naka, K.K.; Michalis, L.K.; Prati, F.; et al. Fully automated calcium detection using optical coherence tomography. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1430–1433. [Google Scholar] [CrossRef]
- Athanasiou, L.S.; Bourantas, C.V.; Rigas, G.; Sakellarios, A.; Exarchos, T.P.; Siogkas, P.K.; Ricciardi, A.; Naka, K.; Papafaklis, M.; Michalis, L.K.; et al. Methodology for fully automated segmentation and plaque characterization in intracoronary optical coherence tomography images. J. Biomed. Opt. 2014, 19, 026009. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, C.; Wang, J.; Miao, Y.; Zhu, T.; Zhou, P.; Li, Z. Intravascular optical coherence tomography image segmentation based on support vector machine algorithm. MCB Mol. Cell. Biomech. 2018, 15, 117–125. [Google Scholar]
- Liu, X.; Du, J.; Yang, J.; Xiong, P.; Liu, J.; Lin, F. Coronary Artery Fibrous Plaque Detection Based on Multi-Scale Convolutional Neural Networks. J. Signal Process. Syst. 2020, 92, 325–333. [Google Scholar] [CrossRef]
- Dehnavi, S.M.; Babu, M.S.P.; Yazchi, M.; Basij, M. Automatic soft and hard plaque detection in IVUS images: A textural approach. In Proceedings of the 2013 IEEE Conference on Information & Communication Technologies, Thuckalay, India, 11–12 April 2013; pp. 214–219. [Google Scholar] [CrossRef]
- Wang, Z.; Kyono, H.; Bezerra, H.G.; Wang, H.; Gargesha, M.; Alraies, C.; Xu, C.; Schmitt, J.M.; Wilson, D.L.; Costa, M.A.; et al. Semiautomatic segmentation and quantification of calcified plaques in intracoronary optical coherence tomography images. J. Biomed. Opt. 2010, 15, 061711. [Google Scholar] [CrossRef] [PubMed]
- Giannoglou, V.G.; Stavrakoudis, D.G.; Theocharis, J.B. IVUS-based characterization of atherosclerotic plaques using feature selection and SVM classification. In Proceedings of the 2012 IEEE 12th International Conference on Bioinformatics & Bioengineering (BIBE), Larnaca, Cyprus, 11–13 November 2012; pp. 715–720. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujisaku, K.; Sasaki, K.; Yuasa, T.; Shibuya, K. Semi-automatic detection of calcified plaque in coronary CT angiograms with 320-MSCT. In Proceedings of the 2016 24th European Signal Processing Conference (EUSIPCO), Budapest, Hungary, 29 August–2 September 2016; pp. 1703–1707. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, W.; Liu, X.; Huang, W.; Zhang, H.; Tan, N.; Hau, W.K.; Zhang, Y.; Liu, H. Automated Detection Framework of the Calcified Plaque with Acoustic Shadowing in IVUS Images. PLoS ONE 2014, 9, e109997. [Google Scholar] [CrossRef] [Green Version]
- Taki, A.; Roodaki, A.; Setarehdan, S.K.; Avansari, S.; Unal, G.; Navab, N. An IVUS image-based approach for improvement of coronary plaque characterization. Comput. Biol. Med. 2013, 43, 268–280. [Google Scholar] [CrossRef]
- Athanasiou, L.S.; Exarchos, T.P.; Naka, K.K.; Michalis, L.K.; Prati, F.; Fotiadis, D.I. Atherosclerotic plaque characterization in Optical Coherence Tomography images. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Baltimore, MD, USA, 2–4 June 2011; pp. 4485–4488. [Google Scholar] [CrossRef]
- Giannoglou, V.G.; Stavrakoudis, D.G.; Theocharis, J.B.; Petridis, V. Genetic fuzzy rule-based classification systems for tissue characterization of intravascular ultrasound images. In Proceedings of the 2012 IEEE International Conference on Fuzzy Systems, Brisbane, QLD, Australia, 10–15 June 2012; pp. 1–8. [Google Scholar] [CrossRef]
- Giannoglou, V.G.; Stavrakoudis, D.; Theocharis, J.B.; Petridis, V. Genetic fuzzy rule based classification systems for coronary plaque characterization based on intravascular ultrasound images. Eng. Appl. Artif. Intell. 2015, 38, 203–220. [Google Scholar] [CrossRef]
- Acharya, U.R.; Meiburger, K.M.; Koh, J.E.W.; Vicnesh, J.; Ciaccio, E.J.; Lih, O.S.; Tan, S.K.; Aman, R.R.A.R.; Molinari, F.; Ng, K.H. Automated plaque classification using computed tomography angiography and Gabor transformations. Artif. Intell. Med. 2019, 100, 101724. [Google Scholar] [CrossRef]
- Lee, J.; Na Hwang, Y.; Kim, G.Y.; Kwon, J.Y.; Kim, S.M. Automated classification of dense calcium tissues in gray-scale intravascular ultrasound images using a deep belief network. BMC Med. Imaging 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahadevi, C.; Sivakumar, S. Performance of Coronary Plaque Feature Extraction and Identification of Plaque Severity for Intravascular Ultrasound B-Mode Images. In International Conference on Mining Intelligence and Knowledge Exploration; Springer: Berlin, Germany, 2019; pp. 221–233. [Google Scholar]
- Hwang, Y.N.; Lee, J.H.; Kim, G.Y.; Shin, E.S.; Kim, S.M. Characterization of coronary plaque regions in intravascular ultrasound images using a hybrid ensemble classifier. Comput. Methods Programs Biomed. 2018, 153, 83–92. [Google Scholar] [CrossRef]
- Araki, T.; Ikeda, N.; Shukla, D.; Londhe, N.D.; Shrivastava, V.; Banchhor, S.K.; Saba, L.; Nicolaides, A.; Shafique, S.; Laird, J.R.; et al. A new method for IVUS-based coronary artery disease risk stratification: A link between coronary & carotid ultrasound plaque burdens. Comput. Methods Programs Biomed. 2016, 124, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Z.; Selamat, A.; Taki, A.; Rahim, M.S.M.; Kadir, M.R.A.; Penhaker, M.; Krejcar, O.; Kuca, K.; Herrera-Viedma, E.; Fujita, H. Thin Cap Fibroatheroma Detection in Virtual Histology Images Using Geometric and Texture Features. Appl. Sci. 2018, 8, 1632. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Gharaibeh, Y.; Kolluru, C.; Zimin, V.N.; Dallan, L.A.P.; Kim, J.N.; Bezerra, H.G.; Wilson, D.L. Segmentation of Coronary Calcified Plaque in Intravascular OCT Images Using a Two-Step Deep Learning Approach. IEEE Access 2020, 8, 225581–225593. [Google Scholar] [CrossRef]
- Gessert, N.; Lutz, M.; Heyder, M.; Latus, S.; Leistner, D.M.; Abdelwahed, Y.S.; Schlaefer, A. Automatic Plaque Detection in IVOCT Pullbacks Using Convolutional Neural Networks. IEEE Trans. Med. Imaging 2019, 38, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Liu, J.; Sun, Z.; Cui, Y.; He, Y.; Yang, Z. Deep learning analysis in coronary computed tomographic angiography imaging for the assessment of patients with coronary artery stenosis. Comput. Methods Programs Biomed. 2020, 196, 105651. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.J.; Kang, S.-J.; Lee, J.-G.; Kweon, J.; Na, W.; Kang, D.; Kim, D.; Kim, D.; Kim, Y.-H. Automated detection of vulnerable plaque in intravascular ultrasound images. Med. Biol. Eng. Comput. 2019, 57, 863–876. [Google Scholar] [CrossRef] [Green Version]
- Ughi, G.J.; Adriaenssens, T.; Sinnaeve, P.; Desmet, W.; D’hooge, J. Automated tissue characterization of in vivo atherosclerotic plaques by intravascular optical coherence tomography images. Biomed. Opt. Express 2013, 4, 1014–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolluru, C.; Prabhu, D.; Gharaibeh, Y.; Bezerra, H.; Guagliumi, G.; Wilson, D. Deep neural networks for A-line-based plaque classification in coronary intravascular optical coherence tomography images. J. Med. Imaging 2018, 5, 044504. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, L.S.; Olender, M.L.; Hernandez, J.M.D.L.T.; Ben-Assa, E.; Edelman, E.R. A deep learning approach to classify atherosclerosis using intracoronary optical coherence tomography. In Medical Imaging 2019: Computer-Aided Diagnosis; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 109500N. [Google Scholar] [CrossRef]
- Zreik, M.; van Hamersvelt, R.W.; Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. Automatic Detection and Characterization of Coronary Artery Plaque and Stenosis Using a Recurrent Convolutional Neural Network in Coronary CT Angiography; European Society of Radiology: Vienna, Austria, 2018. [Google Scholar]
- Zreik, M.; Van Hamersvelt, R.W.; Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. A Recurrent CNN for Automatic Detection and Classification of Coronary Artery Plaque and Stenosis in Coronary CT Angiography. IEEE Trans. Med. Imaging 2019, 38, 1588–1598. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, C.; Feng, J.; Du, Y.; Lu, J.; Zhou, J. A Vessel-Focused 3D Convolutional Network for Automatic Segmentation and Classification of Coronary Artery Plaques in Cardiac CTA. In International Workshop on Statistical Atlases and Computational Models of the Heart; Springer: Berlin, Germany, 2018; pp. 131–141. [Google Scholar]
- Acharya, U.R.; Meiburger, K.M.; Koh, J.E.W.; Ciaccio, E.J.; Vicnesh, J.; Tan, S.K.; Wong, J.H.D.; Aman, R.R.A.R.; Ng, K.H. Automated detection of calcified plaque using higher-order spectra cumulant technique in computer tomography angiography images. Int. J. Imaging Syst. Technol. 2020, 30, 285–297. [Google Scholar] [CrossRef]
- Filho, E.D.S.; Saijo, Y.; Tanaka, A.; Yambe, T.; Li, S.; Yoshizawa, M. Automated Calcification Detection and Quantification in Intravascular Ultrasound Images by Adaptive Thresholding. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Seoul, Korea, 27 August–1 September 2006; pp. 1421–1425. [Google Scholar]
- Sofian, H.; Ng, A.; Than, J.; Mohamad, S.; Noor, N.M. Calcification boundary detection in coronary artery using intravascular ultrasound images. In Proceedings of the TENCON 2017—2017 IEEE Region 10 Conference, Penang, Malaysia, 5–8 November 2017; pp. 2835–2839. [Google Scholar] [CrossRef]
- Roy, A.G.; Conjeti, S.; Carlier, S.G.; Houissa, K.; König, A.; Dutta, P.K.; Laine, A.F.; Navab, N.; Katouzian, A.; Sheet, D. Multiscale distribution preserving autoencoders for plaque detection in intravascular optical coherence tomography. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016; pp. 1359–1362. [Google Scholar] [CrossRef]
- Sofian, H.; Ming, J.T.C.; Mohamad, S.; Noor, N.M. Calcification Detection Using Deep Structured Learning in Intravascular Ultrasound Image for Coronary Artery Disease. In Proceedings of the 2018 2nd International Conference on BioSignal Analysis, Processing and Systems (ICBAPS), Kuching, Malaysia, 24–26 July 2018; pp. 47–52. [Google Scholar] [CrossRef]
- Sofian, H.; Ming, J.T.C.; Muhammad, S.; Noor, N.M. Calcification detection using convolutional neural network architectures in intravascular ultrasound images. J. Electr. Eng. Comput. Sci. 2019, 17, 1313–1321. [Google Scholar] [CrossRef]
- De Vos, B.D.; Wolterink, J.M.; Leiner, T.; De Jong, P.A.; Lessmann, N.; Isgum, I. Direct Automatic Coronary Calcium Scoring in Cardiac and Chest CT. IEEE Trans. Med. Imaging 2019, 38, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, R.; Foldyna, B.; Eslami, P.; Weiss, J.; Alexander, I.; Taron, J.; Parmar, C.; Alvi, R.M.; Banerji, D.; Uno, M.; et al. Deep convolutional neural networks to predict cardiovascular risk from computed tomography. Nat. Commun. 2021, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Balocco, S.; González, M.; Ñanculef, R.; Radeva, P.; Thomas, G. Calcified Plaque Detection in IVUS Sequences: Preliminary Results Using Convolutional Nets. In International Workshop on Artificial Intelligence and Pattern Recognition; Springer: Berlin/Heidelberg, Germany, 2018; pp. 34–42. [Google Scholar]
- Liu, S.; Neleman, T.; Hartman, E.M.; Ligthart, J.M.; Witberg, K.T.; van der Steen, A.F.; Wentzel, J.J.; Daemen, J.; van Soest, G. Automated Quantitative Assessment of Coronary Calcification Using Intravascular Ultrasound. Ultrasound Med. Biol. 2020, 46, 2801–2809. [Google Scholar] [CrossRef]
- Li, Y.-C.; Shen, T.-Y.; Chen, C.-C.; Chang, W.-T.; Lee, P.-Y.; Huang, C.-C.J. Automatic Detection of Atherosclerotic Plaque and Calcification from Intravascular Ultrasound Images by Using Deep Convolutional Neural Networks. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2021, 68, 1762–1772. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, G.; Shu, H. Calcified coronary plaques detection in CTA based-on automatic scale selection and fuzzy C means. In Proceedings of the 2016 International Conference on Machine Learning and Cybernetics (ICMLC), Jeju, Korea, 10–13 July 2016; pp. 807–813. [Google Scholar] [CrossRef]
- Mirunalini, P.; Aravindan, C.; Nambi, A.T.; Poorvaja, S.; Priya, V.P. Segmentation of Coronary Arteries from CTA axial slices using Deep Learning techniques. In Proceedings of the TENCON 2019—2019 IEEE Region 10 Conference (TENCON), Kochi, India, 17–20 October 2019; pp. 2074–2080. [Google Scholar] [CrossRef]
- Mirunalini, P.; Aravindan, C.; Jaisakthi, S.M. Automatic stenosis detection using SVM from CTA projection images. Multimed. Syst. 2019, 25, 83–93. [Google Scholar] [CrossRef]
- Chow, B.J.; Abraham, A.; Wells, G.A.; Chen, L.; Ruddy, T.D.; Yam, Y.; Govas, N.; Galbraith, P.D.; Dennie, C.; Beanlands, R.S. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ. Cardiovasc. Imaging 2009, 2, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.; Schmidt, M.; Magedanz, A.; Voigtländer, T.; Schmermund, A. Coronary CT angiography in managing atherosclerosis. Int. J. Mol. Sci. 2015, 16, 3740–3756. [Google Scholar] [CrossRef]
- Leber, A.W.; Knez, A.; von Ziegler, F.; Becker, A.; Nikolaou, K.; Paul, S.; Wintersperger, B.; Reiser, M.; Becker, C.R.; Steinbeck, G.; et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: A comparative study with quantitative coronary angiography and intravascular ultrasound. J. Am. Coll. Cardiol. 2005, 46, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkadhi, H.; Scheffel, H.; Desbiolles, L.; Gaemperli, O.; Stolzmann, P.; Plass, A.; Goerres, G.W.; Luescher, T.F.; Genoni, M.; Marincek, B.; et al. Dual-source computed tomography coronary angiography: Influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur. Heart J. 2008, 29, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, U.; Bamberg, F.; Chae, C.U.; Nichols, J.H.; Rogers, I.S.; Seneviratne, S.K.; Truong, Q.A.; Cury, R.C.; Abbara, S.; Shapiro, M.D.; et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: The ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J. Am. Coll. Cardiol. 2009, 53, 1642–1650. [Google Scholar] [CrossRef] [Green Version]
- Song, F.X.; Zhou, J.; Zhou, J.J.; Shi, Y.X.; Zeng, M.S.; Zhang, Z.Y.; Lv, P.; Sheng, R.F. The diagnosis of coronary plaque stability by multi-slice computed tomography coronary angiography. J. Thorac. Dis. 2018, 10, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Sehovic, S. Diagnostic Capabilities of 64 Slice CT Coronography Compared to Classic in Coronary Disease Detection. Acta Inform. Med. 2013, 21, 208–210. [Google Scholar] [CrossRef] [Green Version]
- Mowatt, G.; Cook, J.; Hillis, G.S.; Walker, S.; Fraser, C.; Jia, X.; Waugh, N. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: Systematic review and meta-analysis. Heart 2008, 94, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.T.; Ko, B.S.; Cameron, J.D.; Nerlekar, N.; Leung, M.C.; Malaiapan, Y.; Crossett, M.; Leong, D.P.; Worthley, S.G.; Troupis, J.; et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: A comparison with fractional flow reserve. J. Am. Coll. Cardiol. 2013, 61, 1271–1279. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Choi, J.H.; Kim, J.H.; Park, K.W.; Doh, J.H.; Kim, Y.J.; Koo, B.K.; Min, J.K.; Erglis, A.; Gwon, H.C.; et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: Diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc. Imaging 2012, 5, 1088–1096. [Google Scholar]
- Gonzalez, J.A.; Lipinski, M.J.; Flors, L.; Shaw, P.W.; Kramer, C.M.; Salerno, M. Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am. J. Cardiol. 2015, 116, 1469–1478. [Google Scholar] [CrossRef] [Green Version]
- Baumann, S.; Renker, M.; Hetjens, S.; Fuller, S.R.; Becher, T.; Loßnitzer, D.; Lehmann, R.; Akin, I.; Borggrefe, M.; Lang, S.; et al. Comparison of coronary computed tomography angiography-derived vs invasive fractional flow reserve assessment. Meta-analysis with subgroup evaluation of intermediate stenosis. Acad. Radiol. 2016, 23, 1402–1411. [Google Scholar] [CrossRef]
- Fischer, C.; Hulten, E.; Belur, P.; Smith, R.; Voros, S.; Villines, T.C. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: A meta-analysis. J. Cardiovasc. Comput. Tomogr. 2013, 7, 256–266. [Google Scholar] [CrossRef]
- Kolossváry, M.; Kellermayer, M.; Merkely, B.; Maurovich-Horvat, P. Cardiac Computed Tomography Radiomics: A Comprehensive Review on Radiomic Techniques. J. Thorac. Imaging 2018, 33, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kigka, V.I.; Sakellarios, A.; Kyriakidis, S.; Rigas, G.; Athanasiou, L.; Siogkas, P.; Tsompou, P.; Loggitsi, D.; Benz, D.C.; Buechel, R.; et al. A three-dimensional quantification of calcified and non-calcified plaques in coronary arteries based on computed tomography coronary angiography images: Comparison with expert’s annotations and virtual histology intravascular ultrasound. Comput. Biol. Med. 2019, 113, 103409. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, J.; Xie, H.; Zhao, Y.; Zhang, S.; Gu, L. Automatic detection of coronary artery stenosis by convolutional neural network with temporal constraint. Comput. Biol. Med. 2020, 118, 103657. [Google Scholar] [CrossRef]


| Dataset | Preprocessing/ROI Segmentation | Feature Extraction | Feature Reduction/Feature Selection/ Feature Ranking/Organization | Detection | Classification | Task | Outcomes * | |
|---|---|---|---|---|---|---|---|---|
| [11] | 599 VH-IVUS images of 10 patients | Thresholding + HFCM-kNN model | CLBT + OLBT | SVM with radial basis function (RBF) | Multiclass (PIT, TCFA and CaTCFA) and binary (TCFA and non-TCFA) | For binary: Pqacc.: 81.03 Pqsen.: 84.81 Pqspec.: 84.81 Precision: 84.81 For multiclass Pqavg.acc.: 98.42 + 0.01 Kappa: 0.9198 | ||
| [15] | IVUS images | Neuro Fuzzy | Atheromatous plaque (fibrotic, lipidic, calcified, and normal) | Pqavg.acc.: 98.9 | ||||
| [21] | 300 IVUS frames of 10 patients | Deformable models + Estimation borders by experts | Co-occurrence matrix + LBP+ Mean value + Entropy + Geometrical features | t-test | RF | Multiclass (DC, NC, FT, and FFT) | Pqacc.: 85.65 | |
| [22] | 553 IVUS frames of eight patients | ROI Extraction + Otsu’s automatic thresholding + Pathological tissue detection | CNN | Multiclass (DC, NC, FT, FFT, Media) | Overall accuracy: 93.5Pqacc.:DC: 98.5NC: 88.6FT: 91.1FFT: 90.0Media: 99.4 | |||
| [25] | IVUS images from 11 patients | Manual segmentation by expert | LBP + FOS +GLCM + LEM + Extended GLRLM + Intensity | PCA | RF | Multiclass(DC, NC, FT, and FFT) | AUC: 0.845, 0.704, 0.783 Pqacc.: 85.1,71.9,77.2 Pqsen.: 82,81.2, 80.6 Pqspec.: 87.1, 59.6, 75.9 (Respectively for Net1: FT/FFT or NC/DC Net2: FT or FFT Net3: NC or DC) | |
| [27] | 1000 IVOCT images from 47 patients | Anisotropic diffusion + Polar Transformation+ Hough Transform | Intensity + HOG + LBP + FV + k-means clustering | SVM | Multiclass (normal, fibrous plaque, fibro-atheroma, plaque rupture, fibro-calcific plaque) | Pqavg.acc.: 90 With standard deviation of 0.02 | ||
| [67] | 770 OCT images of 5 patients | ROI Extraction | LBP+GLCM | CNN (U Net) | Multiclass (lipid tissue, fibrous tissue, background) | Pqacc.: 95.8 | ||
| [70] | 435 IVUS images | Polar Transformation + Gaussian filter +Median filter+ Anisotropic Diffusion filter | Haralick’s +Laws’ textural feature | SVM | Two class (fibrotic plaque and normal) | AUC: 0.97 Jaccard Index: 0.85 | ||
| [72] | 6556 OCT images from 49 patients | ROI+ Dynamic programming + Gaussian filter | CNN + Morphological features | Wilcoxon signed rank test | RF | Binary class: (fibro-lipidic and fibrocalcific plaque) | Fibro-lipidic plaque: Pqsen.: 84.8 Pqspec.: 97.8 Fibro-calcific plaque: Pqsen.: 91.4 Pqspec.: 95.7 | |
| [73] | 18 CTA images Total: 1786 cross sections with Non calcified plaque (NCP): 729, Calcified plaque (CP): 511, Mixed plaques: 546. | DRLSE | RRS feature vector | SVM (Gaussian kernel) | Multiclass(calcified, non calcified and mixed plaques) | Average precision: 92.6±1.9 Average recall: 94.3±2.1 | ||
| [80] | 60 IVUS images of 7 patients | Anisotropic diffusion filter + Thresholding | Deformable models | Bayesian | Two class (calcified and non-calcified plaque) | AUC: 0.943 Pqspec.: 98.5 Pqsen.: 92.67 | ||
| [83] | 27 OCT pullbacks of 22 patients | Gaussian filter + Thresholding + k-means | LBP + GLCM | WRP | RF | Multiclass (calcium, lipid pool, fibrous tissue, and mixed Tissue) | Pearson’s correlation coefficient: 0.97 (FT) | |
| [84] | IVOCT images of 11 patients | Gaussian filter + Otsu threshold filtering | Attenuation coefficient + GLCM | SVM (RBF) | Multiclass (fibrous, calcification and lipid tissue) | Pqacc.: 83 | ||
| [88] | IVUS images of 7 patients | Multilevel discrete wavelet frame decomposition | FOS + GLCM + LBP + RL + Wavelet Intensity values | FuzCoC | SVM (RBF) | Multiclass (calcium, necrotic core, fibrous, and fibro-fatty) | Pqavg.acc.: 81 | |
| [91] | In-vivo dataset: VH-IVUS 2263 images of 10 patients Ex-vivo dataset:64 images | Shadow detection using threshold | NGL + LBP + MRL | SVM and ECOC | Multiclass(calcium, necrotic core, and fibro fatty) | Kappa values: 0.639 (in-vivo) and 0.628 (ex-vivo) | ||
| [92] | 50 OCT images from 3 patients | Co-occurrence matrix + LBP+ Entropy + Mean value | RF | Multiclass (calcium, lipid pool, fibrous tissue, and mixed plaque) | Pqacc.: 80.41 | |||
| [93] | 300 IVUS images of 7 patients | Multilevel discrete wavelet frames decomposition + SOFM | FOS + GLCM + RL + LBP + wavelets + LISA | FaIRLiC | Multiclass (DC, NC, FT, and FFT) | Testing accuracy: 76.16% | ||
| [94] | IVUS images of 7 patients | Border detection + 2-D Kohonen’s self-organizing feature map (SOFM) | FOS + GLCM + WF + RL + LBP | FaIRLiC | Multiclass(calcium, necrotic core, fibrous and fibro lipid) | Average classification Accuracy on each frame: 73.67 | ||
| [95] | 2646 Coronary Tomography Angiography (CTA) images of 73 patients (CP: 28, NCP: 15, Normal: 30) | Adaptive Histogram Equalization | Gabor Transform + Entropy | ANOVA | SVM (RBF and polynomial kernel) | Multiclass (normal, non calcified and calcified) | Pqacc.: 89.09 PqPPV: 91.70 Pqsen.: 91.83 Pqspec.: 83.70 | |
| [96] | 316 IVUS images of 26 patients | Thresholding + Polar transformation + Morphological operations | FOS + FD (Box counting) + GLCM + GLRLM + LTE | PCA | Deep belief network | Multiclass (DC, NC, FT, and FFT) | Pqsen.: 92.8 ± 0.1, Pqspec.: 85.1 ± 0.1, Pqacc.: 88.4 ± 0.1, PqPPV: 86 ± 0.1 PqNPV: 91.2 ± 0.1 (p < 0.05). | |
| [97] | IVUS images of 7 patients | Adjacent pattern algorithm + Color moments of histogram + Statistical features | SVM based CNN | Multiclass (mild, moderate and severe) | Pqacc.: 98.80, 98.80, 97.59 Pqsen.: 100, 100, 100 Pqspec.: 98.70, 98.70, 97.40 Precision: 85.71, 85.71, 75 Recall: 100, 100, 100 F-score: 0.92, 0.92, 0.99 (Respectively for Mild, moderate and severe) | |||
| [98] | IVUS images from 11 patients | Manual border segmentation | FOS + GLCM + GLRLM + LBP + Intensity + Discrete wavelet features +LTE | Genetic algorithm | Hybrid ensemble classifier(FFNN+ RF+ Ada boost) | Multiclass (DC, NC, FT, and FFT) | Pqacc.: 82.8, 71.6, 77 AUC: 0.832, 0.697, 0.787 Pqsen.: 84.4, 81.9, 74.9 Pqspec.: 81.9, 57.6, 82.4 PqPPV: 71.2, 72.4, 91.7 PqNPV: 90.8, 70.1, 55.9 (Respectively for Net1: FT/FFT or NC/DC Net2: FT or FFT Net3: NC or DC) | |
| [99] | 2685 IVUS images of 15 patients | ImgTracer software | GLCM + GLRLM + IH + GLDS + NGTDM + IM + Statistical feature matrix | SVM (polynomial kernel 2nd order) | Coronary and carotid plaque | Pqacc.: 94.95 AUC: 0.95 Pqsen.: 92.88 Pqspec.: 96.61 PqPPV: 96.69 | ||
| [100] | 588 VH-IVUS images of 10 patients | Fuzzy c means and k means with particle swarm optimization | LBP + GLCM + MRL | PCA | SVM (RBF) | TCFA and Non-TCFA | Pqacc.: 98.61 | |
| [102] | 4000 IVOCT images from 49 patients | Cartesian Transformation | CNN from ImageNet ResNet50-v2 and DenseNet-121 | Binary class: plaque (calcified plaque and lipid/fibrous plaque) and no plaque | Pqacc.: 91.7 Pqsen.: 90.9 Pqspec.: 92.4 | |||
| [103] | CCTA of 150 patients | CNN (U Net + V Net) | Stenosis Detection and Plaque classification (calcified, partially calcified, noncalcified and no plaque) | Stenosis identification: CCTA AI (p<0.001) AUC: 0.870 Pqacc.: 86 Pqsen.: 88 Pqspec.: 85 PqPPV: 73 PqNPV: 94 Plaque classification: AUC: 0.750 | ||||
| [104] | 12,325 IVUS images from 100 patients | IVUS and OCT registration + ROI segmentation | CNN | Binary (thin cap fibro-atherma and normal) | AUC: 0.911 Pqspec.: 82.81 Pqsen.: 87.31 | |||
| [105] | 64 IVOCT images from 49 patients | Otsu’s method + morphological operation | Attenuation + Texture | RF | Multiclass (fibrotic, calcified, and lipid rich) | Pqacc.: 81.5 | ||
| [106] | 4469 IOCT images of 48 patients | Edge detection + Gaussian filter | t-test | CNN followed by post processing (Conditional Random Field + Morphological processing) | Multiclass (fibrocalcific, fibro-lipidic and other classes) | Pqacc.: 77.7 ± 4.1, 86.5 ± 2.3, 85.3 ± 2.5 Pqsen.: 80, 85, 84 Pqspec.: 95, 92, 92 (Respectively for fibrocalcific, fibro-lipidic, other classes) p-value: 0.00027 | ||
| [107] | 700 OCT images of 28 patients | CNN | Multiclass (calcium, lipid tissue, fibrous tissue, mixed tissue, media and no visible tissue) | Pqacc.: 96 | ||||
| [108] | CCTA scans of 131 patients | 3D Recurrent Convolutional Neural Network | Multiclass (no plaque, non-calcified, mixed, calcified) and stenosis (no stenosis, non- significant, significant) | Plaque analysis: Pqacc.: 72, F1 score: 0.61 Cohen’s kappa: 0.60 Stenosis analysis: Pqacc.: 81 F1 score: 0.78 Cohen’s kappa: 0.70 Pqsen.: 61 PqPPV: 83 | ||||
| [109] | CCTA scans of 163 patients | Recurrent convolutional neural network | Multiclass(calcified, non-calcified and mixed) | Plaque detection: Pqacc.: 77 F1 score: 0.61 Cohen’s kappa: 0.61 Stenosis detection: Accuracy: 80% F1 score: 0.75 Cohen’s kappa: 0.68 Pqsen.: 61 PqPPV: 65 | ||||
| [110] | CTA scans from 25 patients | Multiplanar reformation technique | 3D CNN U-Net (Encoder-decoder) | Multiclass (calcified plaque, non-calcified plaque and mixed calcified plaque) | Dice scores: 0.83, 0.73, 0.68 Pqsen.: 85, 76,72 PqPPV: 82, 69, 62 Respectively for CAP, NCAP, MCAP | |||
| [111] | 2060 CTA images from 60 patients | Higher-order spectra cumulants | Multiple factor analysis + t-test | SVM(RBF) | Binary (calcified, noncalcified) | Pqacc.: 95.83 Pqsen.: 94.54 Pqspec.: 97.13 PqPPV: 97.05 | ||
| Dataset | Preprocessing/ROI Segmentation | Feature Extraction | Feature Reduction/Feature Selection/ Feature Ranking/Organization | Detection | Classification | Task | Outcome * | |
|---|---|---|---|---|---|---|---|---|
| [2] | 2175 IVUS images of 10 patients 530 images with calcification 1645 images without calcification | Original image resized and converted to RGB | ResNet50, ResNet101, Inception-v3 | SVM and DA | Calcified plaque detection | Pqacc.: 100 Pqsen.: 100 Pqspec.: 100 | ||
| [66] | CT images of 56 patients | Thresholding | CNN (ConvNet) | Calcification identification | Pqsen.: 91.24 Pqspec.: 95.37 PqPPV: 90.5 Pearson coefficient: 0.983 Cohen’s kappa: 0.879 | |||
| [68] | CCTA of 493 patients | Centerline extraction + Clamping technique | 3D CNN | Atherosclerosis detection | Pqavg.acc.: 90.9 PqPPV: 58.8 Pqsen.: 68.9 Pqspec.: 93.6 PqNPV: 96.1 Average AUC: 0.91 | |||
| [69] | 32 datasets of CTA | Normalization of high intensity calcified plaque | Fisher method | Mean radial profile | SVM Gaussian RBF | Soft plaque detection | Pqacc.: 88.4 Pqsen.: 93.2 Pqspec.: 80.3 Dice coefficient: 0.832 | |
| [71] | 435 IVUS scan images | CNN (U-Net + VGG16 encoder) | Detection of lumen and media | For media: Avg Jaccard measure: 0.8085 Avg Dice score: 0.8825 For Lumen: Avg Jaccard measure:0.7982 Avg Dice score:.8846 | ||||
| [79] | 78 CCTA images of 18 patients | 3D U-Net CNN | Coronary artery lumen segmentation (for grading stenosis) | Dice: 0.8291 | ||||
| [81] | 1000 OCT images | Polar Transformation + Anisotropic diffusion | Flexible neural tree | Vulnerable plaque detection | Pqacc.: 90.80 | |||
| [82] | 27 OCT images from 10 patients | Gaussian filter + Thresholding | k-means clustering | Calcified plaque detection | Pqsen.: 83 PqPPV: 74 Pearson correlation: 0.434 | |||
| [85] | 1000 OCT images | Hough Transform + Polar transformation | CNN | Fibrous plaque detection | Pqacc.: 94.12 Recall: 94.12 | |||
| [86] | 60 IVUS images from 7 patients | Polar Transformation | GLCM + FCM + ROI selection + Morphological processing + Curve fitting | Detection (Hard plaque and soft plaque) | Pqspec.: 83 Pqsen.: 91 | |||
| [87] | 106 IOCT images of 8 patients | Otsu’s thresholding + Edge detection | Intensity + level-set model | Segmentation of calcified plaque | Pqacc.: 78 ± 9 | |||
| [89] | CCTA of 7 patients | Thresholding + 3D region growing | Blob enhancing filter | Stenosis by Calcified plaque | Precision:94.4 | |||
| [90] | 996 in-vivo IVUS images of 8 patients | Rayleigh mixture model | Markov random field and Graph searching algorithm | Calcified plaque detection | Pqsen.: 94.68 Pqspec.: 95.82 | |||
| [101] | 8231 IOCT images of 68 patients | Dynamic programming + semantic segmentation method + Gaussian filter | Wilcoxon signed rank test | 3D CNN + SegNet | Calcified plaque segmentation | Pqsen.: 86.2 Precision: 75.8 F1 score: 0.781 | ||
| [112] | 20 IVUS images | Adaptive thresholding | Priori information of the acoustic Shadow | Calcification detection | Pqspec.: 88 Pqsen.: 84 AUC: 0.87 | |||
| [113] | 2175 IVUS images of 10 patients | Otsu thresholding + Morphological operation + Empirical threshold | Detection of calcification boundary | Pqacc.: 82 Pqsen.: 80 Pqspec.: 84 PqPPV: 83 | ||||
| [114] | 30 OCT images | DPAE–NN | Binary (detection of plaque and normal tissues) | AUC: 0.9132 Pqacc.: 93.6 Kappa score: 0.62 | ||||
| [115] | 2175 IVUS image of 10 patients with 530 calcified images and 1645 without calcification | Original image resized and converted into RGB | CNN architecture ResNet101 | NB | Calcification detection | Pqacc.: 99.95 Pqsen.: 99.81 Pqspec.: 100 PqPPV: 100 PqNPV: 99.94 | ||
| [116] | 2175 IVUS images of 10 patients Calcified images: 530 Noncalcified: 1645 | Images resized and converted to RGB | Inception-ResNet-v2 | NB | Calcification detection | Pqsen.: 100 Pqspec.: 95.87 PqPPV: 88.63 Pqacc.: 96.87 AUC: 0.9967 | ||
| [117] | 903 CT scans | ConvNet | Calcium scoring | Cohen’s kappa: 0.95 Precision:77 Recall: 85 Pqacc.: 99 Dice score: 0.81 | ||||
| [118] | CT scans of 20084 individuals | CNN (U-Net) | Assessment of calcium score | AUC: 0.74 | ||||
| [119] | 8914 IVUS images of 80 patients | CNN | Calcified plaque detection | Average F1 score: 0.67 Average precision: 77 Average recall: 83 | ||||
| [120] | 105 IVUS pullback dataset | Polar transformation | SVM (RBF) | Calcified plaque detection | Pqacc. > 90 Precision: 96 Recall: 93 | |||
| [121] | 713 grayscale IVUS images of 18 patients | CNN U-Net architecture | Detection (media adventitia, lumen and calcium regions) | Average precision: 73 Pqsen.: 72 Pqspec.: 99 Mean Dice score function (DSC): 0.67Spearman’s correlation: 0.92 | ||||
| [122] | CTA of 12 patients | Thresholding + Difference of Gaussian filter (DOG) | Fuzzy c means + Median filter | Calcified plaque detection | Recall: 94 Precision: 94 | |||
| [123] | 2D axial CTA images of 50 patients | U-Net | CNN-RNN model | CNN | Segmentation of coronary artery (stenosis detection) | Recall:95.9 Precision: 97.9 Pqacc.: 96.1 | ||
| [124] | CTA images of 30 patients | Hessian matrix+ Thresholding+ Morphological operations | SVM | Stenosis detection | Average Recall: 94.08 Precision: 88.59 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudigar, A.; Nayak, S.; Samanth, J.; Raghavendra, U.; A J, A.; Barua, P.D.; Hasan, M.N.; Ciaccio, E.J.; Tan, R.-S.; Rajendra Acharya, U. Recent Trends in Artificial Intelligence-Assisted Coronary Atherosclerotic Plaque Characterization. Int. J. Environ. Res. Public Health 2021, 18, 10003. https://doi.org/10.3390/ijerph181910003
Gudigar A, Nayak S, Samanth J, Raghavendra U, A J A, Barua PD, Hasan MN, Ciaccio EJ, Tan R-S, Rajendra Acharya U. Recent Trends in Artificial Intelligence-Assisted Coronary Atherosclerotic Plaque Characterization. International Journal of Environmental Research and Public Health. 2021; 18(19):10003. https://doi.org/10.3390/ijerph181910003
Chicago/Turabian StyleGudigar, Anjan, Sneha Nayak, Jyothi Samanth, U Raghavendra, Ashwal A J, Prabal Datta Barua, Md Nazmul Hasan, Edward J. Ciaccio, Ru-San Tan, and U. Rajendra Acharya. 2021. "Recent Trends in Artificial Intelligence-Assisted Coronary Atherosclerotic Plaque Characterization" International Journal of Environmental Research and Public Health 18, no. 19: 10003. https://doi.org/10.3390/ijerph181910003
APA StyleGudigar, A., Nayak, S., Samanth, J., Raghavendra, U., A J, A., Barua, P. D., Hasan, M. N., Ciaccio, E. J., Tan, R.-S., & Rajendra Acharya, U. (2021). Recent Trends in Artificial Intelligence-Assisted Coronary Atherosclerotic Plaque Characterization. International Journal of Environmental Research and Public Health, 18(19), 10003. https://doi.org/10.3390/ijerph181910003








