Polish Cross-Cultural Adaptation of the Lower Limb Functional Index (LLFI) Demonstrates a Valid Outcome Measure for the Lower Limb Region and Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Approval
2.3. Design
2.4. Research Tools
2.4.1. The Lower Limb Functional Index
2.4.2. The Western Ontario and McMaster Universities Osteoarthritis Index
2.4.3. The EuroQol Health Questionnaire 5-Dimensions 5-Level
2.4.4. The 11-Point Pain Numerical Rating Scale (P-NRS)
2.5. Statistical Analysis
2.5.1. Internal Consistency
2.5.2. Test–Retest Reliability
2.5.3. Measurement Error
2.5.4. Construct Validity
2.5.5. Factor Structure
2.5.6. Practical Characteristics
2.5.7. Sample Size
3. Results
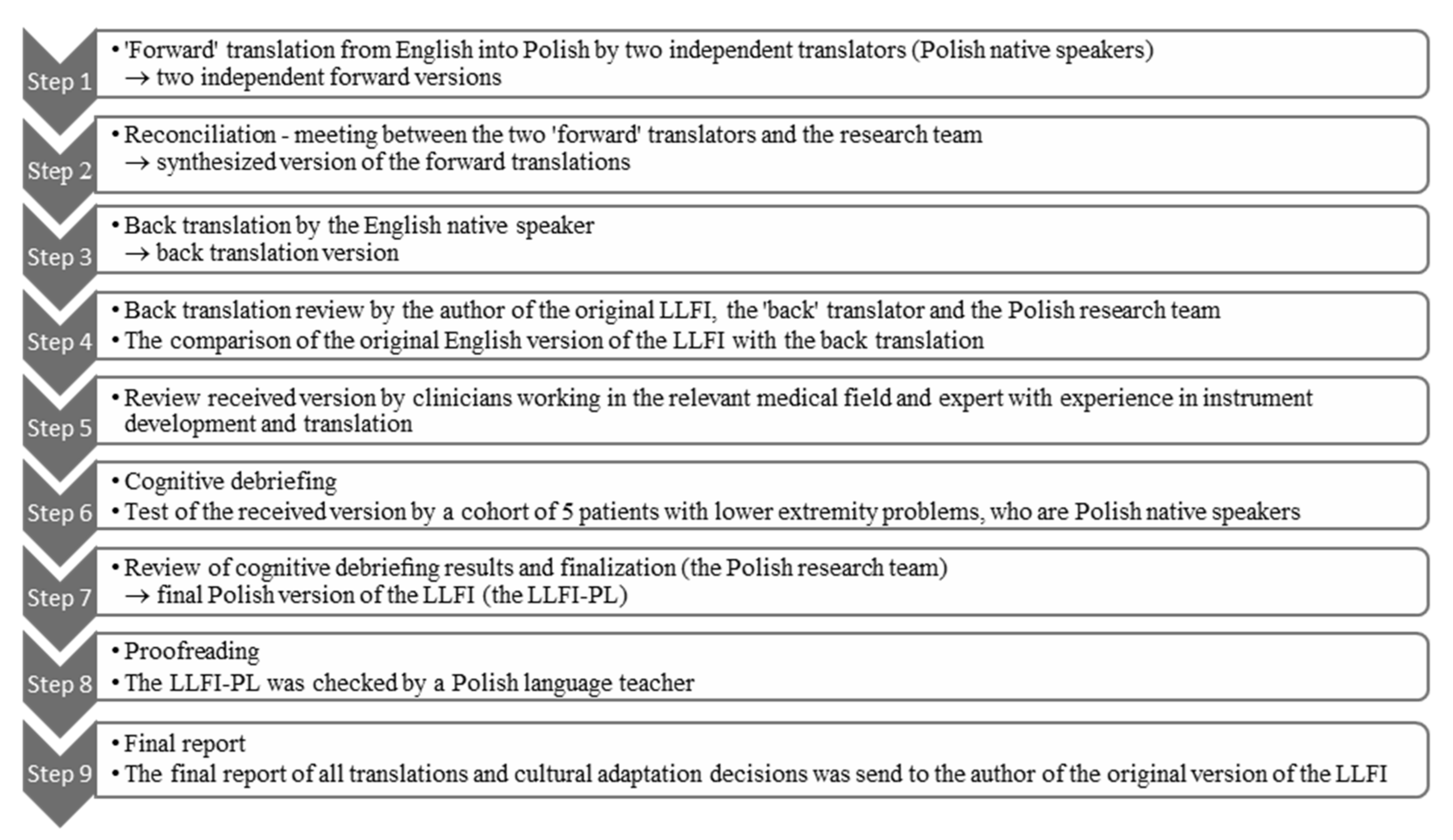
3.1. Stage 1 LLFI Translation and Cross-Cultural Adaptation
3.2. Stage 2 Psychometric Investigation
3.2.1. The Clinical Characteristics of the Patients
3.2.2. The Research Tools Absolute Values
3.2.3. Internal Consistency
3.2.4. Test–Retest Reliability and Measurement Error
3.2.5. Construct Validity
3.2.6. Factor Structure
3.2.7. Practical Considerations
4. Discussion
5. Limitations and Strengths
6. Future Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| LOWER LIMB FUNCTIONAL INDEX—WERSJA POLSKA (LLFI—PL) |
| IMIĘ I NAZWISKO: _______________________________ DATA: _______________________________ URAZ/PROBLEM:__________________________________ LEWA NOGA □ PRAWA NOGA □ |
| ☐ ☐ ☐ 1. Pozostaję w domu przez większość czasu. |
| ☐ ☐ ☐ 2. Często zmieniam pozycję dla większego komfortu. |
| ☐ ☐ ☐ 3. Unikam ciężkich prac (np. sprzątania, podnoszenia rzeczy cięższych niż 5 kg, wykonywania prac w ogrodzie itp.). |
| ☐ ☐ ☐ 4. Częściej odpoczywam. |
| ☐ ☐ ☐ 5. Proszę inne osoby, by zrobiły za mnie niektóre czynności. |
| ☐ ☐ ☐ 6. Odczuwam ból /mam problem prawie przez cały czas. |
| ☐ ☐ ☐ 7. Mam trudności z podnoszeniem i noszeniem (np. toreb, zakupów o wadze do 5 kg). |
| ☐ ☐ ☐ 8. Zmienił mi się apetyt. |
| ☐ ☐ ☐ 9. Chodzenie lub rekreacja lub aktywność sportowa jest utrudniona. |
| ☐ ☐ ☐ 10. Mam trudności z normalnymi domowymi lub rodzinnymi obowiązkami i pracami. |
| ☐ ☐ ☐ 11. Gorzej sypiam. |
| ☐ ☐ ☐ 12. Potrzebuję pomocy w samoobsłudze (np. myciu i utrzymaniu higieny). |
| ☐ ☐ ☐ 13. Moja codzienna aktywność jest utrudniona (praca, kontakty społeczne). |
| ☐ ☐ ☐ 14. Łatwiej się irytuję i/lub wpadam w złość. |
| ☐ ☐ ☐ 15. Czuję się słabszy/-a i/lub zesztywniały/-a. |
| ☐ ☐ ☐ 16. Moje niezależne przemieszczanie się jest utrudnione (prowadzenie samochodu, korzystanie z transportu publicznego). |
| ☐ ☐ ☐ 17. Mam trudności lub potrzebuję pomocy w ubieraniu się (np. spodni, bielizny, butów, skarpetek). |
| ☐ ☐ ☐ 18. Mam trudności ze zmienianiem kierunków, skręcaniem lub obracaniem się. |
| ☐ ☐ ☐ 19. Nie jestem w stanie poruszać się tak szybko jak chciałbym/chciałabym. |
| ☐ ☐ ☐ 20. Mam trudności z dłuższym lub przeciągającym się staniem. |
| ☐ ☐ ☐ 21. Mam trudności ze zginaniem się, kucaniem i/lub schylaniem się. |
| ☐ ☐ ☐ 22. Mam trudności z długimi lub przeciągającymi się spacerami. |
| ☐ ☐ ☐ 23. Mam trudności z chodzeniem po schodach. |
| ☐ ☐ ☐ 24. Mam trudności z dłuższym lub przeciągającym się siedzeniem. |
| ☐ ☐ ☐ 25. Mam trudności z utrzymaniem równowagi na nierównej powierzchni i/lub w obuwiu do którego jestem nieprzyzwyczajony/a. |
| Wynik LLFI-PL: W celu obliczenia wyniku—dodaj zaznaczone odpowiedzi: __________WYNIK CAŁKOWITY (punkty LLFI-PL); 100 Skala: 100-(WYNIK CAŁKOWITY × 4) = _________% MDC*(90% CI *): 3,93 % LLFI-PL. Zmiana mniejsza niż podana może wynikać z błędu. |
References
- Dziak, A. Dysfunction of the joints. Acta Clin. 2002, 2, 129–136. [Google Scholar]
- Klose, K.; Kreimeier, S.; Tangermann, U.; Aumann, I.; Damm, K.; on behalf of the RHO Group. Patient- and person-reports on healthcare: Preferences, outcomes, experiences, and satisfaction—An essay. Health Econ. Rev. 2016, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsdotter, A.K.; Lohmander, L.S.; Klässbo, M.; Roos, E.M. Hip disability and osteoarthritis outcome score (HOOS)—Validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradowski, P.T.; Witoński, D.; Kęska, R.; Roos, E. Cross-cultural translation and measurement properties of the Polish version of the knee injury and osteoarthritis outcome score (KOOS) following anterior cruciate ligament reconstruction. Health Qual. Life Outcomes 2013, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Paradowski, P.T.; Kęska, R.; Witoński, D. Validation of the Polish version of the knee injury and osteoarthritis outcome score (KOOS) on patients with osteoarthritis undergoing total knee replacement. BMJ Open 2015, 5, e006947. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, M.; Bejer, A.; Snela, S.; Szymczyk, D.; Jabłoński, J.; Majewska, J. Polish Cross-Cultural Adaptation and Validation of the Knee Outcome Survey Activities of Daily Living Scale (KOS-ADLS) in Patients Undergoing Total Knee Arthroplasty. Med. Sci. Monit. 2018, 24, 5309–5319. [Google Scholar] [CrossRef] [PubMed]
- Piontek, T.; Ciemniewska-Gorzela, K.; Naczk, J.; Cichy, K.; Szulc, A. Linguistic and cultural adaptation into Polish of the IKDC 2000 subjective knee evaluation from and the Lysholm scale. Pol. Orthop. Traumatol. 2012, 7, 115–119. [Google Scholar]
- Glinkowski, W.; Żukowska, A.; Dymitrowicz, M.; Wołyniec, E.; Glinkowska, B.; Kozioł-Kaczorek, D. Translation, Cross-Cultural Adaptation, and Psychometric Properties of the Polish Version of the Hip Disability and Osteoarthritis Outcome Score (HOOS). Medicina 2019, 20, 614. [Google Scholar] [CrossRef] [Green Version]
- Gojło, M.K.; Paradowski, P.T. Polish adaptation and validation of the hip disability and osteoarthritis outcome score (HOOS) in osteoarthritis patients undergoing total hip replacement. Health Qual. Life Outcomes 2020, 18, 135. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L. Validation study of WOMAC: A health status instrument for measuring clinically-important patient-relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J. Orthop. Rheumatol. 1998, 1, 95–108. [Google Scholar]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale Development, Measurement Properties, and Clinical Application. Phys. Ther. 1999, 79, 371–383. [Google Scholar]
- Gabel, C.P.; Melloh, M.; Burkett, B.; Michener, L.A. Lower Limb Functional Index: Development and clinimetric properties. Phys. Ther. 2012, 92, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta-Vargas, A.I.; Gabel, C.P.; Bennett, P. Cross cultural adaptation and validation of a Spanish version of the lower limb functional index. Health Qual. Life Outcomes 2014, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Duruturk, N.; Tonga, E.; Gabel, C.P.; Acar, M.; Tekindal, A. Cross-cultural adaptation, reliability and validity of the Turkish version of the Lower Limb Functional Index. Disabil. Rehabil. 2015, 37, 2439–2444. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.W.M.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C.W. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vet, H.C.W.; Terwee, C.B.; Mokkink, L.B.; Knol, D.L. Measurement in Medicine: A Practical Guide; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Golicki, D.; Jakubczyk, M.; Niewada, M.; Wrona, W.; Busschbach, J.J.V. Valuation of EQ-5D Health States in Poland: First TTO-Based Social Value Set in Central and Eastern Europe. Value Health 2010, 13, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.W.; Bagraith, K.S.; Khan, A.; Deen, M.; Strong, J. Comparative responsiveness of Verbal and Numerical Rating Scales to measure pain intensity in patients with chronic pain. J. Pain 2013, 14, 1653–1662. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson Prentice Hall Health: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Williams, B.; Brown, T.; Onsman, A. Exploratory factor analysis: A five-step guide for novices. J. Emerg. Prim. Health Care 2010, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; 2009. Available online: https://www.fda.gov/media/77832/download (accessed on 21 May 2011).
- Mokhtarinia, H.R.; Hosseini, A.; Maleki-Ghahfarokhi, A.; Gabel, C.P.; Zohrabi, M. Cross-cultural adaptation, validity, and reliability of the Persian version of the Spine Functional Index. Health Qual. Life Outcomes 2018, 16, 95. [Google Scholar] [CrossRef] [Green Version]
- Mokhtarinia, H.R.; Zareiyan, A.; Gabel, C.P. Cross-cultural adaptation, validity, and reliability of the Persian version of the Upper Limb Functional Index. Hand Ther. 2021, 26, 43–52. [Google Scholar] [CrossRef]
- Gittings, P.M.; Heberlien, N.; Devenish, N.; Parker, M.; Phillips, M.; Wood, F.M.; Edgar, D.W. The Lower Limb Functional Index—A reliable and valid functional outcome assessment in burns. Burns 2016, 42, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Prodinger, B.; Hammond, A.; Tennant, A.; Prior, Y.; Tyson, S. Revisiting the disabilities of the arm, shoulder and Hand (DASH) and QuickDASH in rheumatoid arthritis. BMC Musculoskelet. Disord. 2019, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Irwing, P.; Booth, T.; Hughes, D.J. The Wiley Handbook of Psychometric Testing: A Multidisciplinary Reference on Survey Scale, and Test Development; Wiley Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Rinaldi, M.; Ranavolo, A.; Conforto, S.; Martino, G.; Draicchio, F.; Conte, C.; Varrecchia, T.; Bini, F.; Casali, C.; Pierelli, F.; et al. Increased lower limb muscle coactivation reduces gait performance and increases metabolic cost in patients with hereditary spastic paraparesis. Clin. Biomech. 2017, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]

| Subregion | a Diagnosis | n (%) |
|---|---|---|
| HIP | Osteoarthritis Total hip arthroplasty | 42 (33.6) 38 (90.5) 4 (9.5) |
| UPPER LEG | Muscle strain: Biceps femoris Quadriceps femoris Adductors | 7 (5.6) 7 (100) 2 (28.6) 3 (42.9) 2 (28.6) |
| KNEE | Osteoarthritis Ligament injury: ACL MCL Patellar chondromalacia Meniscus repair Knee arthroplasty | 53 (42.4) 27 (50.9) 5 (9.4) 5 (9.4) 2 (3.8) 4 (7.5) 10 (18.9) |
| LOWER LEG | Muscle strain: Triceps surae Tibialis anterior Achilles tendon Injury (contusion/hematoma) | 11 (8.8) 7 (63.6) 5 (45.5) 1 (9.1) 1 (9.1) 4 (34.4) |
| ANKLE | Injury of ligaments Osteoarthritis | 12 (9.6) 11 (91.7) 1 (8.3) |
| FOOT | A joint Hallux | 4 (3.2) 2 (50.0) 2 (50.0) |
| WHOLE LIMB | Neurological reasons | 5 (4.0) 5 (100.0) |
| MULTIPLE AREAS | Diagnoses were included above | 9 (7.2) |
| PAIN | Acute ˃ 4–6 weeks | 40 (32.0) |
| CHARACTERISTIC | Subacute 6–12 weeks | 13 (10.4) |
| Chronic ≥ 12 weeks | 72 (57.6) |
| Questionnaire | ± SD | Me | Range |
|---|---|---|---|
| LLFI -PL I (0–100) | 60.7 ± 25.1 | 66.0 | 6.0–100.0 |
| LLFI-PL II (0–100) | 66.4 ± 24.4 | 72 | 6.0–100.0 |
| NRS I (0–10) | 5.2 ± 1.8 | 5 | 2–9 |
| NRS II (0–10) | 4.6 ± 1.75 | 4 | 1–9 |
| WOMAC I (0–100) | 61.7 ± 21.6 | 61.0 | 16.0–95.0 |
| EQ-5D-5L Index value I (1–5) | 1.7 ± 1.17 | 1.8 | 1.1–2.0 |
| EQ-5D-5L—VAS I (0-100) | 60.2 ± 19.8 | 60.0 | 15.0–95.0 |
| Questionnaire | Internal Consistency n = 125 | Test–Retest Reliability n = 94 | Error Score (0–100%) n = 94 | Error Score (73–100%) n = 47 | ||||
| Cronbach’s Alpha | (ICC2.1) | 95% CI LB UB | SEM | 90%CI MDC | SEM | 90%CI MDC | ||
| LLFI-PL | 0.936 | 0.962 | 0.941 | 0.975 | 4.83 | 11.3 | 1.69 | 3.93 |
| Questionnaire | LLFI-PL (n = 125) |
|---|---|
| PCC | |
| WOMAC Total | r = 0.81, p < 0.001 * |
| WOMAC Pain | r = 0.77, p < 0.001 * |
| WOMAC Stiffness | r = 0.45, p < 0.001 * |
| WOMAC Function | r = 0.81, p < 0.001 * |
| EQ-5D-5L Index value | r = −0.63, p < 0.001 * |
| EQ-5D-5L—VAS | r = 0.57, p < 0.001 * |
| NRS Pain | r = −0.39, p < 0.001 * |
| Factor | Total | % of Variance | Cumulative % |
|---|---|---|---|
| 1 | 9.919 | 39.676 | 39.676 |
| 2 | 2.034 | 8.136 | 47.812 |
| 3 | 0.930 | 3.722 | 51.534 |
| 4 | 0.960 | 3.842 | 55.375 |
| 5 | 0.784 | 3.136 | 58.512 |
| LLFI-PL Items | Factor 1 |
|---|---|
| LLFI-PL_7 | 0825 |
| LLFI-PL_25 | 0799 |
| LLFI-PL_4 | 0795 |
| LLFI-PL_16 | 0785 |
| LLFI-PL_1 | 0782 |
| LLFI-PL_11 | 0769 |
| LLFI-PL_18 | 0759 |
| LLFI-PL_6 | 0729 |
| LLFI-PL_10 | 0727 |
| LLFI-PL_13 | 0724 |
| LLFI-PL_15 | 0721 |
| LLFI-PL _5 | 0680 |
| LLFI-PL_24 | 0661 |
| LLFI-PL_3 | 0631 |
| LLFI-PL_17 | 0579 |
| LLFI-PL_14 | 0501 |
| LLFI-PL_2 | 0496 |
| LLFI-PL_21 | 0478 |
| LLFI-PL_12 | 0475 |
| LLFI-PL_8 | 0424 |
| LLFI-PL_19 | 0374 |
| LLFI-PL_20 | 0371 |
| LLFI-PL_9 | 0366 |
| LLFI-PL_23 | 0327 |
| LLFI-PL_22 | 0,298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejer, A.; Bieś, A.; Kyc, S.; Lorenc, M.; Mataczyński, P.; Domka-Jopek, E.; Melloh, M.; Gabel, C.P. Polish Cross-Cultural Adaptation of the Lower Limb Functional Index (LLFI) Demonstrates a Valid Outcome Measure for the Lower Limb Region and Joints. Int. J. Environ. Res. Public Health 2021, 18, 9894. https://doi.org/10.3390/ijerph18189894
Bejer A, Bieś A, Kyc S, Lorenc M, Mataczyński P, Domka-Jopek E, Melloh M, Gabel CP. Polish Cross-Cultural Adaptation of the Lower Limb Functional Index (LLFI) Demonstrates a Valid Outcome Measure for the Lower Limb Region and Joints. International Journal of Environmental Research and Public Health. 2021; 18(18):9894. https://doi.org/10.3390/ijerph18189894
Chicago/Turabian StyleBejer, Agnieszka, Agnieszka Bieś, Sylwia Kyc, Magdalena Lorenc, Piotr Mataczyński, Elżbieta Domka-Jopek, Markus Melloh, and Charles Philip Gabel. 2021. "Polish Cross-Cultural Adaptation of the Lower Limb Functional Index (LLFI) Demonstrates a Valid Outcome Measure for the Lower Limb Region and Joints" International Journal of Environmental Research and Public Health 18, no. 18: 9894. https://doi.org/10.3390/ijerph18189894
APA StyleBejer, A., Bieś, A., Kyc, S., Lorenc, M., Mataczyński, P., Domka-Jopek, E., Melloh, M., & Gabel, C. P. (2021). Polish Cross-Cultural Adaptation of the Lower Limb Functional Index (LLFI) Demonstrates a Valid Outcome Measure for the Lower Limb Region and Joints. International Journal of Environmental Research and Public Health, 18(18), 9894. https://doi.org/10.3390/ijerph18189894






