Association between Statins and Retinal Vascular Occlusion: A Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
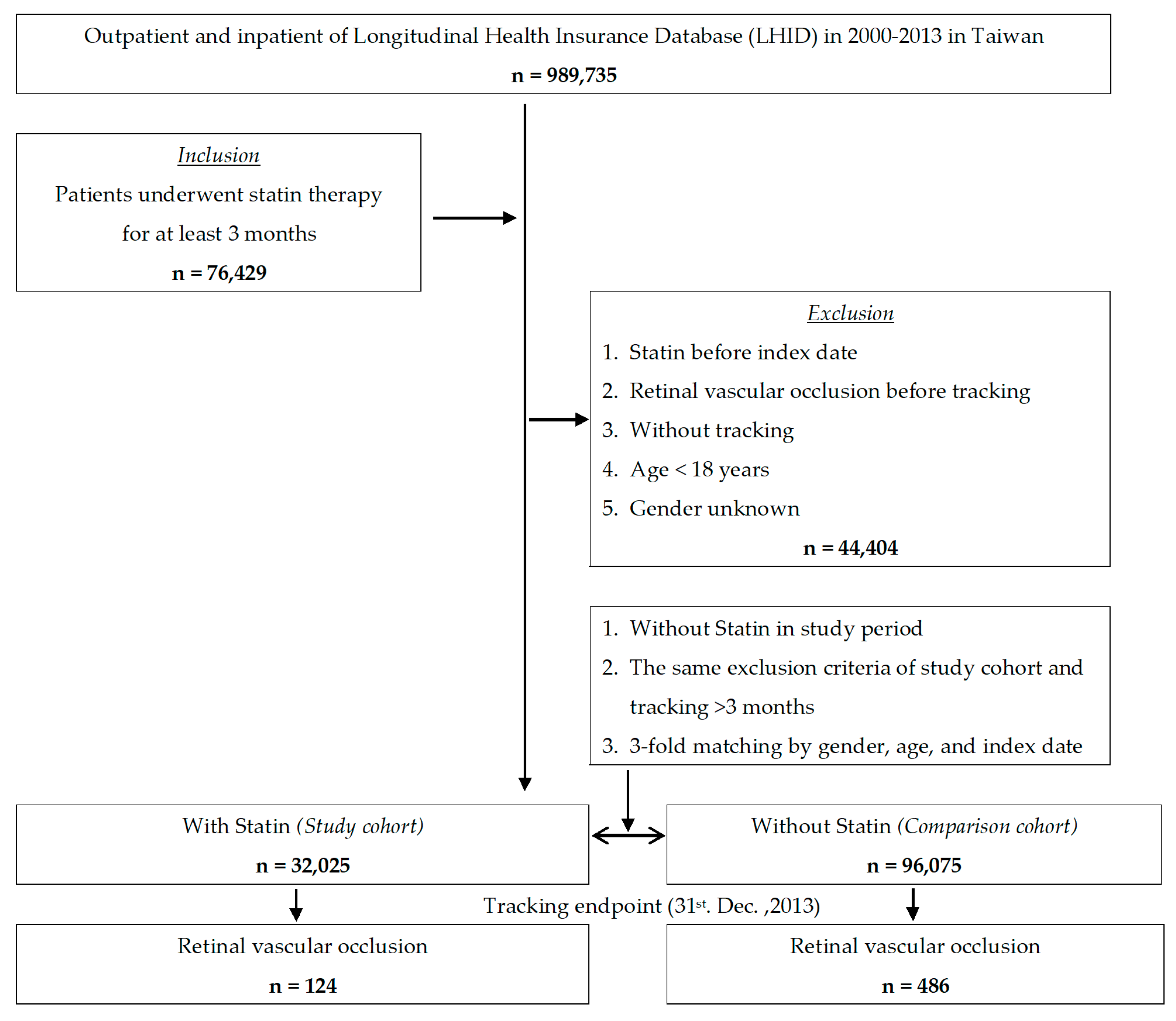
2.2. Inclusion and Exclusion Criteria
2.3. Study Variables
2.4. Study Outcome
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Sample
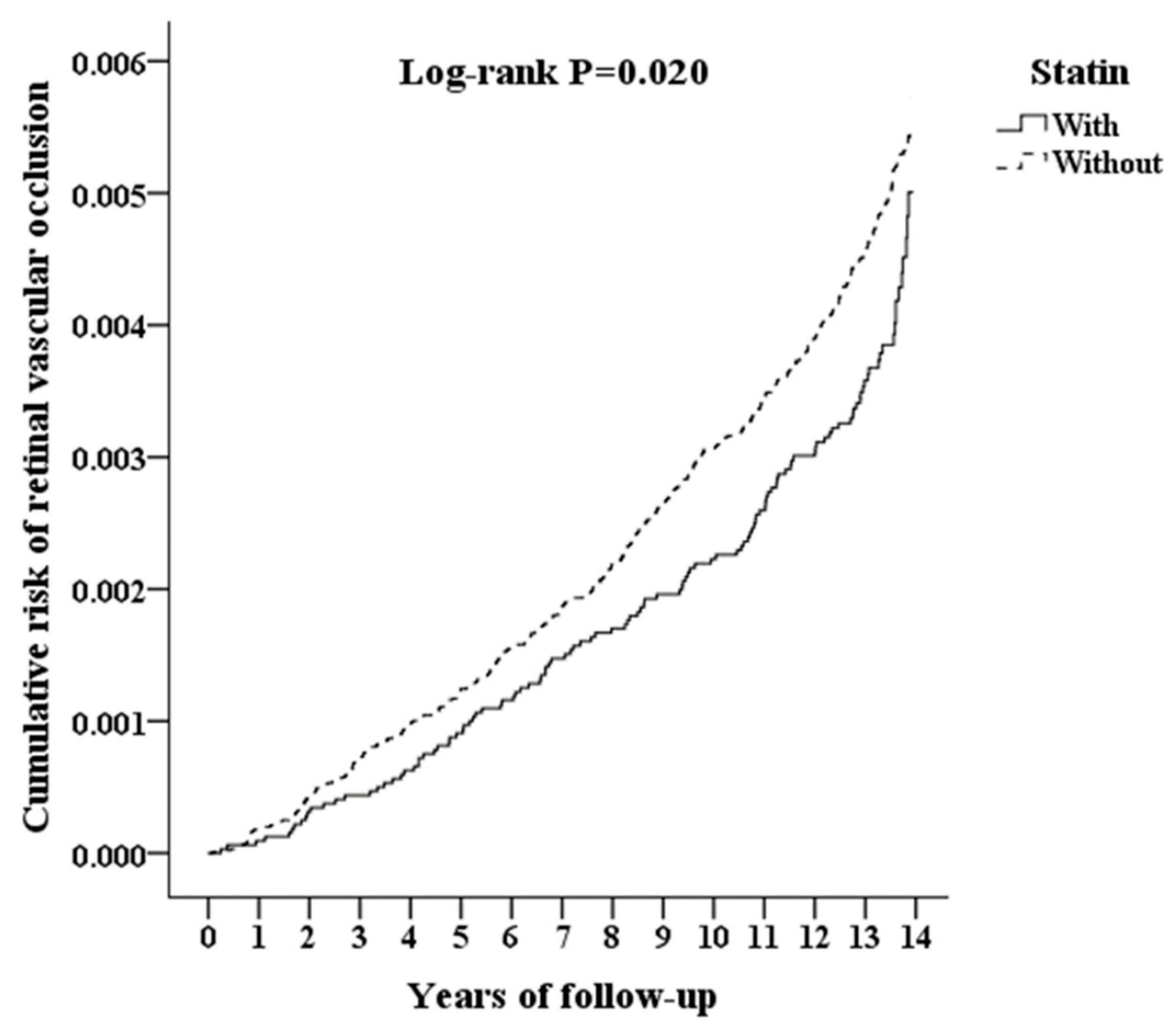
3.2. Cumulative Hazard Curves by the Kaplan–Meier Method
3.3. Univariate and Multivariate Analyses by the Cox Regression Model
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, I.U.; Campochiaro, P.A.; Newman, N.J.; Biousse, V. Retinal vascular occlusions. Lancet 2020, 396, 1927–1940. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B.; Podhajsky, P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am. J. Ophthalmol. 1994, 117, 429–441. [Google Scholar] [CrossRef]
- Wong, T.Y.; Larsen, E.K.; Klein, R.; Mitchell, P.; Couper, D.J.; Klein, B.E.; Hubbard, L.D.; Siscovick, D.S.; Sharrett, A.R. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: The Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology 2005, 112, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch. Ophthalmol. 1996, 114, 545–554. [CrossRef]
- Rehak, M.; Wiedemann, P. Retinal vein thrombosis: Pathogenesis and management. J. Thromb. Haemost. 2010, 8, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar]
- O’Mahoney, P.R.; Wong, D.T.; Ray, J.G. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch. Ophthalmol. 2008, 126, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Dodson, P.M.; Galton, D.J.; Hamilton, A.M.; Blach, R.K. Retinal vein occlusion and the prevalence of lipoprotein abnormalities. Br. J. Ophthalmol. 1982, 66, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.W.; Lee, P.; Wong, T.Y.; Best, J.; Jenkins, A. Retinal vein occlusion: An approach to diagnosis, systemic risk factors and management. Intern. Med. J. 2008, 38, 904–910. [Google Scholar] [CrossRef]
- Prisco, D.; Marcucci, R. Retinal vein thrombosis: Risk factors, pathogenesis and therapeutic approach. Pathophysiol. Haemost. Thromb. 2002, 32, 308–311. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Blazina, I.; Daeges, M.; Jeanne, T.L. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 316, 2008–2024. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Deedwania, P. Reducing morbidity and mortality in high risk patients with statins. Vasc. Health Risk Manag. 2009, 5, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Koh, K.K. Effects of statins on vascular wall: Vasomotor function, inflammation, and plaque stability. Cardiovasc. Res. 2000, 47, 648–657. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; Pueringer, M.R.; Brue, S.M.; Chamberlain, A.M.; Hodge, D.O. Statin Use and Incident Cataract Surgery: A Case-Control Study. Ophthalmic Epidemiol. 2016, 23, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yan, H. Simvastatin increases circulating endothelial progenitor cells and reduces the formation and progression of diabetic retinopathy in rats. Exp. Eye Res. 2012, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Talwar, N.; Musch, D.C.; Stein, J.D. Association of Daily Dosage and Type of Statin Agent With Risk of Open-Angle Glaucoma. JAMA Ophthalmol. 2017, 135, 263–267. [Google Scholar] [CrossRef]
- Vavvas, D.G.; Daniels, A.B.; Kapsala, Z.G.; Goldfarb, J.W.; Ganotakis, E.; Loewenstein, J.I.; Young, L.H.; Gragoudas, E.S.; Eliott, D.; Kim, I.K.; et al. Regression of Some High-risk Features of Age-related Macular Degeneration (AMD) in Patients Receiving Intensive Statin Treatment. EBioMedicine 2016, 5, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Kang, M.H.; Seong, M.; Cho, H.Y. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br. J. Ophthalmol. 2012, 96, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Mimura, T.; Harino, S.; Hori, S. Aqueous humor levels of vasoactive molecules correlate with vitreous levels and macular edema in central retinal vein occlusion. Eur. J. Ophthalmol. 2010, 20, 402–409. [Google Scholar] [CrossRef]
- Medina, R.J.; O’Neill, C.L.; Devine, A.B.; Gardiner, T.A.; Stitt, A.W. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS ONE 2008, 3, e2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Janabi, A.; Lightman, S.; Tomkins-Netzer, O. Statins in retinal disease. Eye 2018, 32, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Matei, V.M.; Xia, J.Y.; Nguyen, C. Poor outcomes despite aspirin or statin use in high-risk patients with retinal vein occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 761–766. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.L.; Kao, Y.H.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef]
- Fischer, C.; Bruggemann, A.; Hager, A.; Callizo Planas, J.; Roider, J.; Hoerauf, H. Vascular Occlusions following Ocular Surgical Procedures: A Clinical Observation of Vascular Complications after Ocular Surgery. J. Ophthalmol. 2017, 2017, 9120892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Li, J.; Zhang, B.; Lu, P. Association of glaucoma with risk of retinal vein occlusion: A meta-analysis. Acta Ophthalmol. 2019, 97, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Chao, C.C.; Hwang, J.F.; Yang, C.M. Clinical manifestations of central retinal artery occlusion in eyes of proliferative diabetic retinopathy with previous vitrectomy and panretinal photocoagulation. Retina 2014, 34, 1861–1866. [Google Scholar] [CrossRef]
- Simard, M.; Sirois, C.; Candas, B. Validation of the Combined Comorbidity Index of Charlson and Elixhauser to Predict 30-Day Mortality Across ICD-9 and ICD-10. Med. Care 2018, 56, 441–447. [Google Scholar] [CrossRef]
- Deobhakta, A.; Chang, L.K. Inflammation in retinal vein occlusion. Int. J. Inflam. 2013, 2013, 438412. [Google Scholar] [CrossRef] [Green Version]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef] [Green Version]
- Neumann, F.J.; Ott, I.; Marx, N.; Luther, T.; Kenngott, S.; Gawaz, M.; Kotzsch, M.; Schomig, A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arter. Thromb. Vasc. Biol. 1997, 17, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Ulfhammer, E.; Larsson, P.; Karlsson, L.; Hrafnkelsdottir, T.; Bokarewa, M.; Tarkowski, A.; Jern, S. TNF-alpha mediated suppression of tissue type plasminogen activator expression in vascular endothelial cells is NF-kappaB- and p38 MAPK-dependent. J. Thromb. Haemost. 2006, 4, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Mimura, T.; Harino, S.; Eguchi, S.; Hori, S. Pigment epithelium-derived factor and vascular endothelial growth factor in branch retinal vein occlusion with macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Mimura, T.; Eguchi, S.; Shimada, K. Inflammatory factors in major and macular branch retinal vein occlusion. Ophthalmologica 2012, 227, 146–152. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sonoda, K.H.; Sugahara, M.; Mochizuki, Y.; Enaida, H.; Oshima, Y.; Ueno, A.; Hata, Y.; Yoshida, H.; Ishibashi, T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009, 4, e8158. [Google Scholar] [CrossRef]
- Ehlken, C.; Rennel, E.S.; Michels, D.; Grundel, B.; Pielen, A.; Junker, B.; Stahl, A.; Hansen, L.L.; Feltgen, N.; Agostini, H.T.; et al. Levels of VEGF but not VEGF(165b) are increased in the vitreous of patients with retinal vein occlusion. Am. J. Ophthalmol. 2011, 152, 298–303.e1. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Maloney, J.P.; Gao, L. Proinflammatory Cytokines Increase Vascular Endothelial Growth Factor Expression in Alveolar Epithelial Cells. Mediat. Inflamm. 2015, 2015, 387842. [Google Scholar] [CrossRef] [Green Version]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palinski, W.; Tsimikas, S. Immunomodulatory effects of statins: Mechanisms and potential impact on arteriosclerosis. J. Am. Soc. Nephrol. 2002, 13, 1673–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, S.; Stuve, O.; Patarroyo, J.C.; Ruiz, P.J.; Radosevich, J.L.; Hur, E.M.; Bravo, M.; Mitchell, D.J.; Sobel, R.A.; Steinman, L.; et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002, 420, 78–84. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Tiglio, A.; Sadigh, K.; O’Donnell, L.; Collinge, M.; Pardi, R.; Bender, J.R. Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation 2001, 71, 1262–1268. [Google Scholar] [CrossRef]
- Bu, D.X.; Griffin, G.; Lichtman, A.H. Mechanisms for the anti-inflammatory effects of statins. Curr. Opin. Lipidol. 2011, 22, 165–170. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef]
- Gehlbach, P.; Li, T.; Hatef, E. Statins for age-related macular degeneration. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Gupta, V.; Thapar, S.; Bhansali, A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am. J. Ophthalmol. 2004, 137, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Misra, A.; Kumar, A.; Pandey, R.M. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res. Clin. Pract. 2002, 56, 1–11. [Google Scholar] [CrossRef]
- Borkar, D.S.; Tham, V.M.; Shen, E.; Parker, J.V.; Uchida, A.; Vinoya, A.C.; Acharya, N.R. Association between statin use and uveitis: Results from the Pacific Ocular Inflammation study. Am. J. Ophthalmol. 2015, 159, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Statin | With | Without | p | ||
|---|---|---|---|---|---|
| Variables | n | % | n | % | |
| Total | 32,025 | 25.00 | 96,075 | 75.00 | |
| Gender | 0.999 | ||||
| Male | 15,096 | 47.14 | 45,288 | 47.14 | |
| Female | 16,929 | 52.86 | 50,787 | 52.86 | |
| Age (years) | 54.57 ± 13.51 | 52.34 ± 16.51 | 0.088 | ||
| DM | <0.001 * | ||||
| Without | 23,513 | 73.42 | 87,195 | 90.76 | |
| With | 8512 | 26.58 | 8880 | 9.24 | |
| HTN | <0.001 * | ||||
| Without | 23,600 | 73.69 | 85,693 | 89.19 | |
| With | 8425 | 26.31 | 10,382 | 10.81 | |
| Hyperlipidemia | <0.001 * | ||||
| Without | 7027 | 21.94 | 95,078 | 98.96 | |
| With | 24,998 | 78.06 | 997 | 1.04 | |
| IHD | <0.001 * | ||||
| Without | 29,513 | 92.16 | 91,352 | 95.08 | |
| With | 2512 | 7.84 | 4723 | 4.92 | |
| CVD | <0.001 * | ||||
| Without | 28,939 | 90.36 | 90,548 | 94.25 | |
| With | 3086 | 9.64 | 5527 | 5.75 | |
| Renal disease | <0.001 * | ||||
| Without | 29,180 | 91.12 | 94,770 | 98.64 | |
| With | 2845 | 8.88 | 1305 | 1.36 | |
| Tumor | <0.001 * | ||||
| Without | 31,019 | 96.86 | 94,077 | 97.92 | |
| With | 1006 | 3.14 | 1998 | 2.08 | |
| MetS | <0.001 * | ||||
| Without | 29,671 | 92.65 | 93,333 | 97.15 | |
| With | 2354 | 7.35 | 2742 | 2.85 | |
| Hypercoagulable state | 0.004 * | ||||
| Without | 31,639 | 98.79 | 95,104 | 98.99 | |
| With | 386 | 1.21 | 971 | 1.01 | |
| Ischemic stroke | <0.001 * | ||||
| Without | 29,040 | 90.68 | 90,623 | 94.33 | |
| With | 2985 | 9.32 | 5452 | 5.67 | |
| Cataract | <0.001 * | ||||
| Without | 30,710 | 95.89 | 92,749 | 96.54 | |
| With | 1315 | 4.11 | 3326 | 3.46 | |
| Glaucoma | <0.001 * | ||||
| Without | 29,240 | 91.30 | 89,064 | 92.70 | |
| With | 2785 | 8.70 | 7011 | 7.30 | |
| Diabetic retinopathy | <0.001 * | ||||
| Without | 31,243 | 97.56 | 95,084 | 98.97 | |
| With | 782 | 2.44 | 991 | 1.03 | |
| AMD | <0.001 * | ||||
| Without | 31,379 | 97.98 | 94,931 | 98.81 | |
| With | 646 | 2.02 | 1144 | 1.19 | |
| CCI | 0.05 ± 0.18 | 0.03 ± 0.15 | <0.001 * | ||
| Variables | Crude HR | 95% CI | 95% CI | p | Adjusted HR | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Statin | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.792 | 0.650 | 0.965 | 0.020 * | 0.704 | 0.591 | 0.873 | <0.001 * |
| Gender | ||||||||
| Male | 0.953 | 0.812 | 1.118 | 0.552 | 0.970 | 0.822 | 1.111 | 0.703 |
| Female | Reference | Reference | ||||||
| Age (yrs) | 1.037 | 1.031 | 1.042 | <0.001 * | 1.026 | 1.019 | 1.031 | <0.001 * |
| DM | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.798 | 1.546 | 1.986 | <0.001 * | 1.714 | 1.512 | 1.888 | <0.001 * |
| HTN | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.068 | 1.573 | 2.676 | <0.001 * | 1.976 | 1.501 | 2.630 | <0.001 * |
| Hyperlipidemia | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.234 | 1.004 | 1.308 | 0.046 * | 0.819 | 0.478 | 1.335 | 0.586 |
| IHD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.462 | 1.297 | 1.682 | <0.001 * | 1.369 | 1.194 | 1.617 | <0.001 * |
| CVD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.350 | 1.104 | 1.553 | <0.001 * | 1.271 | 1.043 | 1.495 | 0.007 * |
| Renal disease | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.668 | 1.321 | 2.095 | <0.001 * | 1.609 | 1.225 | 1.962 | <0.001 * |
| Tumor | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.032 | 0.862 | 1.345 | 0.184 | 1.031 | 0.860 | 1.352 | 0.176 |
| MetS | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.065 | 1.482 | 2.798 | <0.001 * | 1.531 | 1.045 | 2.088 | 0.003 * |
| Hypercoagulable state | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.526 | 0.862 | 2.060 | 0.289 | 1.306 | 0.671 | 1.884 | 0.462 |
| Ischemic stroke | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.398 | 1.167 | 1.592 | <0.001 * | 1.288 | 1.050 | 1.516 | 0.001 * |
| Cataract | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.411 | 1.086 | 1.702 | 0.004 * | 1.352 | 1.015 | 1.670 | 0.032 * |
| Glaucoma | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.525 | 1.126 | 1.978 | <0.001 * | 1.488 | 1.034 | 1.826 | 0.018 * |
| Diabetic retinopathy | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.279 | 1.089 | 1.465 | <0.001 * | 1.186 | 1.003 | 1.395 | 0.046 * |
| AMD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.382 | 0.979 | 1.891 | 0.072 | 1.297 | 0.875 | 1.742 | 0.088 |
| CCI_R | 1.234 | 1.125 | 1.290 | <0.001 * | 1.200 | 1.093 | 1.248 | <0.001 * |
| Statin | With | Without (Reference) | With vs. Without (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retinal Vascular Occlusion Subgroups | Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | p |
| Overall | 124 | 413,862.59 | 29.96 | 486 | 1,235,055.74 | 39.35 | 0.704 | 0.591 | 0.873 | <0.001 * |
| Central Retinal Artery Occlusion | 31 | 413,862.59 | 7.49 | 126 | 1,235,055.74 | 10.20 | 0.679 | 0.564 | 0.841 | <0.001 * |
| Arterial Branch Occlusion | 27 | 413,862.59 | 6.52 | 119 | 1,235,055.74 | 9.64 | 0.620 | 0.518 | 0.779 | <0.001 * |
| Central Retinal Vein Occlusion | 34 | 413,862.59 | 8.22 | 124 | 1,235,055.74 | 10.04 | 0.758 | 0.640 | 0.952 | 0.008 * |
| Branch Retinal Vein Occlusion | 32 | 413,862.59 | 7.73 | 117 | 1,235,055.74 | 9.47 | 0.743 | 0.629 | 0.939 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, C.-C.; Chen, P.-H.; Chung, C.-H.; Sun, C.-A.; Chien, W.-C.; Chien, K.-H. Association between Statins and Retinal Vascular Occlusion: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 9864. https://doi.org/10.3390/ijerph18189864
Chien C-C, Chen P-H, Chung C-H, Sun C-A, Chien W-C, Chien K-H. Association between Statins and Retinal Vascular Occlusion: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(18):9864. https://doi.org/10.3390/ijerph18189864
Chicago/Turabian StyleChien, Chien-Cheng, Po-Huang Chen, Chi-Hsiang Chung, Chien-An Sun, Wu-Chien Chien, and Ke-Hung Chien. 2021. "Association between Statins and Retinal Vascular Occlusion: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 18, no. 18: 9864. https://doi.org/10.3390/ijerph18189864
APA StyleChien, C.-C., Chen, P.-H., Chung, C.-H., Sun, C.-A., Chien, W.-C., & Chien, K.-H. (2021). Association between Statins and Retinal Vascular Occlusion: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 18(18), 9864. https://doi.org/10.3390/ijerph18189864







