Metabolic Syndrome and Its Related Factors among Hospital Employees: A Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
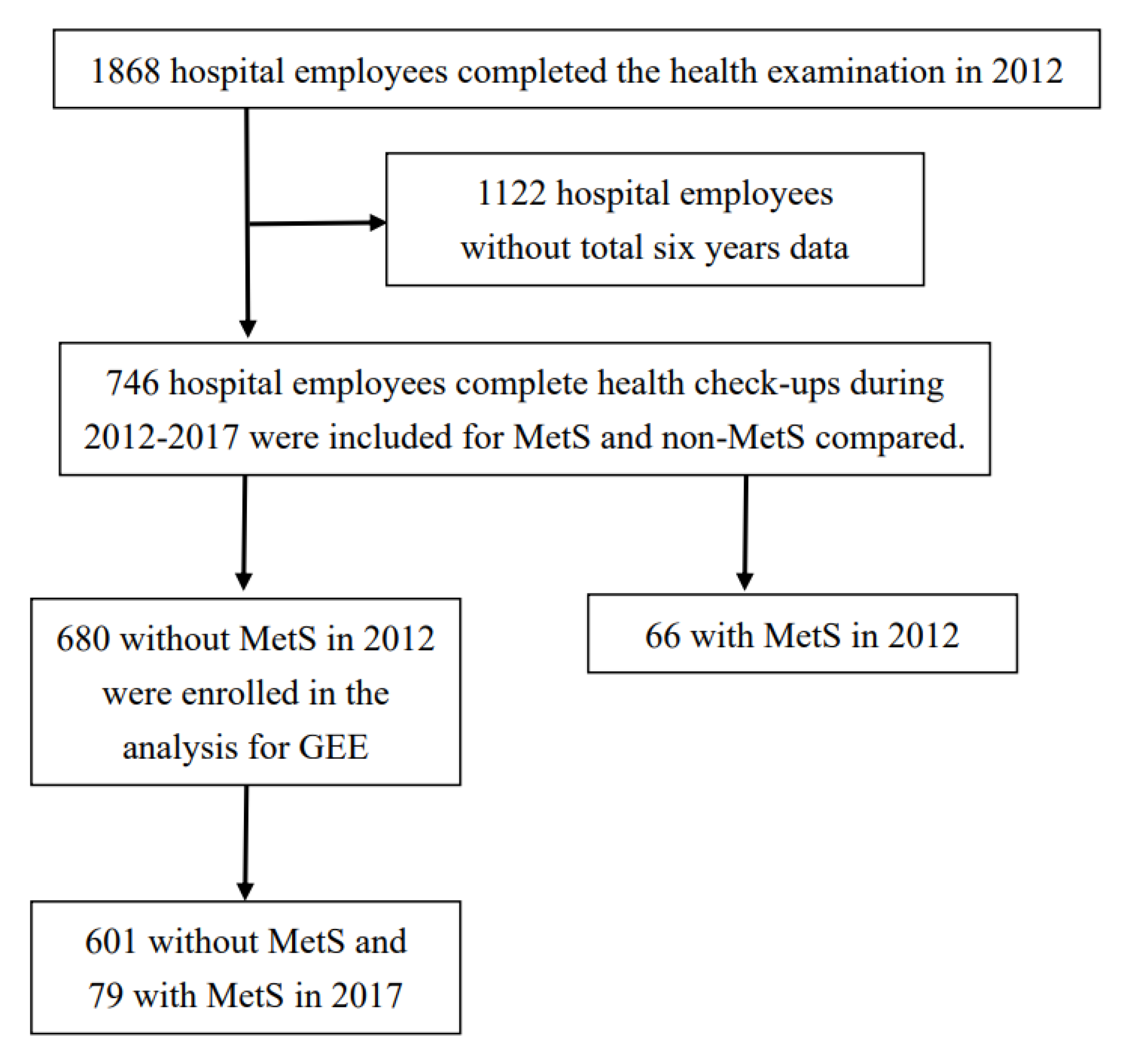
2.3. Study Population
2.4. Statistical Analysis
3. Results
3.1. Comparison of Participant Characteristics by the Presence of Metabolic Syndrome
3.2. Physical and Biochemical Values of the Participants
3.3. Risk Factors for Metabolic Syndrome in Hospital Employees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, T.-Y.; Cheng, J.-F.; Lai, Y.-M. Prevalence of metabolic syndrome and related factors in Taiwanese high-tech industry workers. Clinics 2011, 66, 1531–1535. [Google Scholar] [CrossRef] [Green Version]
- May, E.L. Population health management: Defining the provider’s role. Healthc. Exec. 2013, 28, 20–29. [Google Scholar] [PubMed]
- Chu, C.-H.; Wang, J.-H.; Jan, R.-H.; Huang, C.-H.; Cheng, C.-F. Association between health examination items and body mass index among school children in Hualien, Taiwan. BMC Public Health 2013, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- van Zon, S.K.R.; Amick, B.C., III; de Jong, T.; Brouwer, S.; Bültmann, U. Occupational distribution of metabolic syndrome prevalence and incidence differs by sex and is not explained by age and health behavior: Results from 75 000 Dutch workers from 40 occupational groups. BMJ Open Diabetes Res. Care 2020, 8, e001436. [Google Scholar] [CrossRef] [PubMed]
- Mehrdad, R.; Pouryaghoub, G.; Moradi, M. Association between Metabolic Syndrome and Job Rank. Int. J. Occup. Environ. Med. 2018, 9, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.-H.; Tsai, T.-Y.; Lin, C.-L.; Wu, S.-Y.; Li, C.-Y. Prevalence and Associated Factors for Metabolic Syndrome in Taiwanese Hospital Employees. Asia Pac. J. Public Health 2009, 23, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mottillo, S.; Filion, K.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseley, K.; Noyce, J. Industry Collaboration to Develop Employee Wellness and Health Management Measures. Popul. Health Manag. 2013, 16, 146. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (US). Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Maiello, M.; Zito, A.; Ciccone, M.M.; Palmiero, P. Metabolic syndrome and its components in postmenopausal women living in southern Italy, Apulia region. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 43–46. [Google Scholar] [CrossRef]
- Park, E.; Kim, J. Gender- and Age-Specific Prevalence of Metabolic Syndrome among Korean Adults: Analysis of the fifth Korean National Health and Nutrition Examination Survey. J. Cardiovasc. Nurs. 2015, 30, 256–266. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Li, C.-H.; Wu, Y.-S.; Chien, W.-C.; Wang, K.-Y.; Tzeng, W.-C. Gender Differences in the Prevalence of Metabolic Syndrome among Taiwanese Air Force Personnel: A population-based study. J. Cardiovasc. Nurs. 2020, 35, 502–511. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Kim, J.; Son, H. Gender Differences of Health Behaviors in the Risk of Metabolic Syndrome for Middle-Aged Adults: A National Cross-Sectional Study in South Korea. Int. J. Environ. Res. Public Health 2021, 18, 3699. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.C.B.; Filho, F.F.D.; Schnorr, C.C.; Bottega, G.B.; Rodrigues, T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Song, Y.-M. Metabolic syndrome and its components among Korean submariners: A retrospective cross-sectional study. Endocrine 2018, 59, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Weber, F. Metabolic Syndrome in the German Air Force: Prevalence and Associations with BMI and Physical Fitness. Aerosp. Med. Hum. Perform. 2018, 89, 469–472. [Google Scholar] [CrossRef]
- Lee, B.-J.; Chan, M.-Y.; Hsiao, H.-Y.; Chang, C.-H.; Hsu, L.-P.; Lin, P.-T. Relationship of Oxidative Stress, Inflammation, and the Risk of Metabolic Syndrome in Patients with Oral Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9303094. [Google Scholar] [CrossRef]
- Shafique, K.; Zafar, M.; Ahmed, Z.; Khan, N.A.; Mughal, M.A.; Imtiaz, F. Areca nut chewing and metabolic syndrome: Evidence of a harmful relationship. Nutr. J. 2013, 12, 1–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, Y.-C.; Li, W.-C.; Yeh, W.-C.; Ueng, S.W.-N.; Chiu, S.Y.-H.; Chen, J.-Y. The Association between Metabolic Syndrome and Related Factors among the Community-Dwelling Indigenous Population in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 8958. [Google Scholar] [CrossRef]
- Mamun, A.; Kitzman, H.; Dodgen, L. Reducing metabolic syndrome through a community-based life-style intervention in African American women. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1785–1794. [Google Scholar] [CrossRef]
- Gianturco, V.; Gianturco, L.; Regnoli, R.; Bodini, B.D.; Turiel, M.; Trapani, M.; Bini, F.; De Angelis, G. Healthy Promotion for Fighting Metabolic Syndrome: Insights from Multi-Center HeRO-FiT Cohort. Int. J. Environ. Res. Public Health 2020, 17, 5424. [Google Scholar] [CrossRef]
- Farha, R.A.; Alefishat, E. Shift Work and the Risk of Cardiovascular Diseases and Metabolic Syndrome among Jordanian Employees. Oman Med. J. 2018, 33, 235–242. [Google Scholar] [CrossRef]
- Hartopo, A.B.; Arso, I.A.; Setianto, B.Y. Low Plasma Atherogenic Index Associated with Poor Prognosis in Hospitalized Patients with Acute Myocardial Infarction. Acta Med. Indones. 2016, 48, 106–113. [Google Scholar]
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan (R.O.C.). Diagnostic Criteria of Metabolic Syndrome in Taiwan. 2007. Available online: https://www.hpa.gov.tw/Pages/EBook.aspx?nodeid=1175 (accessed on 12 May 2019).
- Lan, Y.; Mai, Z.; Zhou, S.; Liu, Y.; Li, S.; Zhao, Z.; Duan, X.; Cai, C.; Deng, T.; Zhu, W.; et al. Prevalence of metabolic syndrome in China: An up-dated cross-sectional study. PLoS ONE 2018, 13, e0196012. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Kim, J.-Y.; Chang, E.; Park, S.-Y.; Kang, H.; Kim, J.; Lee, W. Clinical Markers Associated with Metabolic Syndrome Among Military Aviators. Aerosp. Med. Hum. Perform. 2015, 86, 970–975. [Google Scholar] [CrossRef]
- Stout, M.B.; Justice, J.N.; Nicklas, B.J.; Kirkland, J.L. Physiological Aging: Links among Adipose Tissue Dysfunction, Diabetes, and Frailty. Physiology 2017, 32, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, Y.H.; Lee, Y.J.; Kim, K.J.; An, J.H.; Kim, N.H.; Kim, H.Y.; Choi, D.S.; Kim, S.G. Prevalence of metabolic syndrome and its related factors among North Korean refugees in South Korea: A cross-sectional study. BMJ Open 2016, 6, e010849. [Google Scholar] [CrossRef] [PubMed]
- Nikpour, M.; Tirgar, A.; Hajiahmadi, M.; Hosseini, A.; Heidari, B.; Ghaffari, F.; Ebadi, A.; Nasiri-Amiri, F.; Firouzbakht, M. Shift work and metabolic syndrome: A multi-center cross-sectional study on females of reproductive age. Biomed. Rep. 2019, 10, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-W.; Meng, F.-C.; Shih, Y.-L.; Su, F.-Y.; Lin, Y.-P.; Lin, F.; Lin, J.-W.; Chang, W.-K.; Lee, C.-J.; Li, Y.-H.; et al. Sex-Specific Association between Metabolic Abnormalities and Elevated Alanine Aminotransferase Levels in a Military Cohort: The CHIEF Study. Int. J. Environ. Res. Public Health 2018, 15, 545. [Google Scholar] [CrossRef] [Green Version]
- Osadnik, K.; Osadnik, T.; Delijewski, M.; Lejawa, M.; Fronczek, M.; Reguła, R.; Gąsior, M.; Pawlas, N. Calcium and Phosphate Levels are Among Other Factors Associated with Metabolic Syndrome in Patients with Normal Weight. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Vitamin D Status, Calcium Intake and Risk of Developing Type 2 Diabetes: An Unresolved Issue. Nutrients 2019, 11, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, K.; Schow, M.; Shubrook, J.H. Shift Workers at Risk for Metabolic Syndrome. J. Osteopat. Med. 2020, 120, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Givens, M.L.; Malecki, K.C.; Peppard, P.E.; Palta, M.; Said, A.; Engelman, C.D.; Walsh, M.C.; Nieto, F.J. Shiftwork, sleep habits, and metabolic disparities: Results from the survey of the health of Wiscon-sin. Sleep Health 2015, 1, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.-J.; Chen, J.-Q. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in Western countries and possible mechanisms. Food Sci. Hum. Wellness 2013, 2, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Prasain, J.K.; Dai, Y.; Moore, R.; Arabshahi, A.; Barnes, S.; Carlson, S.; Wyss, J.M. Chronic Dietary Kudzu Isoflavones Improve Components of Metabolic Syndrome in Stroke-Prone Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2009, 57, 7268–7273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.W.; Kim, M.K.; Lee, Y.-H.; Shin, D.H.; Shin, M.-H.; Choi, B.Y. Habitual consumption of soy protein and isoflavones and risk of metabolic syndrome in adults ≥40 years old: A prospective analysis of the Korean Multi-Rural Communities Cohort Study (MRCohort). Eur. J. Nutr. 2018, 58, 2835–2850. [Google Scholar] [CrossRef]
| Variables | Total (n = 746) | Metabolic Syndrome | χ2/t | p | |
|---|---|---|---|---|---|
| No (n = 680) | Yes (n = 66) | ||||
| Age (years) | 38.40 ± 9.10 a | 37.97 ± 9.00 a | 42.88 ± 8.96 a | −4.23 | <0.001 |
| Age categories (years) | 15.66 | 0.001 | |||
| >50 | 99 | 83 (12.2) | 16 (24.2) | ||
| 41–50 | 177 | 156 (22.9) | 21 (31.8) | ||
| 31–40 | 308 | 284 (41.8) | 24 (36.4) | ||
| 21–30 | 162 | 157 (23.1) | 5 (7.6) | ||
| Sex | 1.26 | 0.261 | |||
| Male | 226 | 202 (29.7) | 24 (36.4) | ||
| Female | 520 | 478 (70.3) | 42 (63.6) | ||
| Shift work | 6.24 | 0.013 | |||
| Yes | 587 | 543 (79.9) | 44 (66.7) | ||
| No | 159 | 137 (20.1) | 22 (33.3) | ||
| Drinking milk (n = 731) b | 1.76 | 0.185 | |||
| Yes | 158 | 140 (21.0) | 18 (28.1) | ||
| No | 573 | 527 (79.0) | 46 (71.9) | ||
| Eating at least three servings of vegetables and two of fruits (n = 729) b | 0.03 | 0.867 | |||
| Yes | 289 | 263 (39.5) | 26 (40.6) | ||
| No | 440 | 402 (60.5) | 38 (59.4) | ||
| Tooth brushing (n = 727) b | 2.83 | 0.243 | |||
| One time/day | 118 | 104 (15.7) | 14 (21.9) | ||
| Two times/day | 473 | 431 (65.0) | 42 (65.6) | ||
| More than three times/day | 136 | 128 (19.3) | 8 (12.5) | ||
| Smoking status (n = 732) b | 6.31 | 0.012 | |||
| Yes | 41 | 33 (4.9) | 8 (12.5) | ||
| No | 691 | 635 (95.1) | 56 (87.5) | ||
| Alcohol (n = 731) b | 1.66 | 0.198 | |||
| Yes | 244 | 218 (32.7) | 26 (40.6) | ||
| No | 487 | 449 (67.3) | 38 (59.4) | ||
| Chewing betel nut (n = 731) b | 6.15 | 0.013 | |||
| Yes | 5 | 3 (0.4) | 2 (3.1) | ||
| No | 726 | 664 (99.6) | 62 (96.9) | ||
| Number of chronic diseases | 19.27 | <0.001 | |||
| ≥2 | 38 | 28 (4.1) | 10 (15.2) | ||
| 1 | 192 | 170 (25.0) | 22 (33.3) | ||
| 0 | 516 | 482 (70.9) | 34 (51.5) | ||
| Number of family history | 7.92 | 0.095 | |||
| ≥4 | 28 | 24 (3.5) | 4 (6.1) | ||
| 3 | 66 | 56 (8.2) | 10 (15.2) | ||
| 2 | 150 | 133 (19.6) | 17 (25.8) | ||
| 1 | 205 | 189 (27.8) | 16 (24.2) | ||
| 0 | 297 | 278 (40.9) | 19 (28.8) | ||
| BMI (kg/m2) | 23.54 ± 3.91 a | 22.96 ± 3.49 a | 28.97 ± 3.82 a | −13.24 | <0.001 |
| BMI categories (kg/m2) | 147.86 | <0.001 | |||
| ≥30.0 | 50 | 27 (4.0) | 23 (34.8) | ||
| 25.0–29.9 | 183 | 147 (21.6) | 36 (54.5) | ||
| 18.5–24.9 | 473 | 467 (68.7) | 6 (9.1) | ||
| <18.5 | 40 | 39 (5.7) | 1 (1.5) | ||
| Variables | Reference Value | Mean ± Sd (n = 746) | Metabolic Syndrome Mean ± Sd | t | p-Value | |
|---|---|---|---|---|---|---|
| No (n = 680) | Yes (n = 66) | |||||
| Height | -cm | 163.12 ± 7.97 | 163.11 ± 7.97 | 163.24 ± 7.99 | −0.13 | 0.896 |
| Weight | -kg | 62.92 ± 12.71 | 61.50 ± 11.77 | 77.54 ± 12.89 | −10.48 | <0.001 |
| WC | female < 80 cm male < 90 cm | 75.92 ± 10.40 | 74.52 ± 9.29 | 90.39 ± 10.20 | −13.13 | <0.001 |
| SBP | <120 mmHg | 115.69 ± 14.04 | 113.96 ± 12.63 | 133.48 ± 15.53 | −11.73 | <0.001 |
| DBP | <80 mmHg | 70.92 ± 10.70 | 69.65 ± 9.64 | 83.98 ± 12.25 | −9.24 | <0.001 |
| Pulse rate | 60–80/min | 78.34 ± 10.72 | 78.11 ± 10.52 | 80.68 ± 12.44 | −1.86 | 0.063 |
| WBC | 4.5–11 × 103/uL | 6.36 ± 1.69 | 6.26 ± 1.63 | 7.38 ± 2.03 | −4.34 | <0.001 |
| Haemoglobin | female 12.0~16.0 male 13.5~18.0 gm/dL | 13.67 ± 1.54 | 13.62 ± 1.51 | 14.27 ± 1.74 | −3.29 | 0.001 |
| FPG | 70~100 mg/dL | 92.53 ± 18.14 | 90.49 ± 12.17 | 113.55 ± 41.61 | −4.48 | <0.001 |
| Cholesterol | <200 mg/dL | 192.62 ± 34.81 | 191.52 ± 34.62 | 204.05 ± 34.91 | −2.80 | 0.005 |
| Triglycerides | <150 mg/dL | 99.38 ± 61.51 | 92.01 ± 50.11 | 175.41 ± 103.43 | −6.48 | <0.001 |
| AST (GOT) | 8~31 U/L | 19.79 ± 12.51 | 19.49 ± 12.52 | 22.92 ± 12.09 | −2.13 | 0.033 |
| ALT (GPT) | 0~41 U/L | 21.57 ± 24.24 | 20.58 ± 24.07 | 31.77 ± 23.77 | −3.65 | <0.001 |
| BUN | 7~25 mg/dL | 12.53 ± 3.37 | 12.47 ± 3.36 | 13.11 ± 3.47 | −1.46 | 0.144 |
| Total calcium | 8.6~10.2 mg/dL | 9.37 ± 0.34 | 9.36 ± 0.34 | 9.46 ± 0.35 | −2.22 | 0.027 |
| Phosphorus | 2.7~4.5 mg/dL | 3.70 ± 0.49 | 3.71 ± 0.49 | 3.66 ± 0.53 | 0.84 | 0.400 |
| Uric acid | 2.3~7.0 mg/dL | 5.33 ± 1.47 | 5.25 ± 1.44 | 6.16 ± 1.50 | −4.87 | <0.001 |
| Creatinine | 0.5~0.9 mg/dL | 0.75 ± 0.17 | 0.74 ± 0.16 | 0.76 ± 0.19 | −0.61 | 0.546 |
| Alkaline phosphatase | 35~104 U/L | 60.99 ± 16.94 | 60.29 ± 16.62 | 68.11 ± 18.66 | −3.60 | <0.001 |
| Total bilirubin | 0.3~1.0 mg/dL | 0.61 ± 0.27 | 0.61 ± 0.27 | 0.60 ± 0.26 | 0.41 | 0.682 |
| Total protein | 6.6~8.7 g/dL | 7.43 ± 0.37 | 7.42 ± 0.37 | 7.51 ± 0.34 | −1.95 | 0.051 |
| Albumin | 3.5~5.7 g/dL | 4.70 ± 0.25 | 4.70 ± 0.25 | 4.69 ± 0.22 | 0.44 | 0.659 |
| A/G ratio | 1.2~2.4 | 1.75 ± 0.24 | 1.76 ± 0.25 | 1.69 ± 0.23 | 2.07 | 0.039 |
| HDL-C | >65 mg/dL | 69.34 ± 16.56 | 65.56 ± 16.14 | 47.23 ± 10.55 | 12.75 | <0.001 |
| LDL-C | <120 mg/dL | 113.40 ± 29.87 | 112.11 ± 29.45 | 120.59 ± 31.28 | −2.69 | 0.008 |
| AIP | −0.3~0.1 | 0.15 ± 0.29 | 0.11 ± 0.27 | 0.53 ± 0.25 | −12.96 | <0.001 |
| Variable | Beta (95% CI) | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|
| Sex | |||
| Male | 0.019 (−0.509~0.547) | 0.943 | 1.020 (0.601~1.729) |
| Female | |||
| Shift work | |||
| Yes | 0.059 (−0.410~0.527) | 0.806 | 1.060 (0.664~1.694) |
| No | |||
| Number of chronic diseases | |||
| ≥2 | 0.036 (−0.557~0.628) | 0.906 | 1.036 (0.573~1.874) |
| 1 | −0.080 (−0.460~0.300) | 0.680 | 0.923 (0.631~1.350) |
| 0 | |||
| Number of family history | |||
| ≥4 | 0.373 (−0.260~1.006) | 0.248 | 1.452 (0.771~2.734) |
| 3 | 0.235 (−0.271~0.741) | 0.363 | 1.265 (0.763~2.097) |
| 2 | 0.133 (−0.590~0.325) | 0.570 | 0.876 (0.554~1.384) |
| 1 | −0.217 (−0.654~0.220) | 0.331 | 0.805 (0.520~1.247) |
| 0 | |||
| Smoking status | |||
| Yes | −0.404 (−1.259~0.450) | 0.354 | 0.667 (0.284~1.569) |
| No | |||
| Alcohol | |||
| Yes | −0.185 (−0.612~0.243) | 0.398 | 0.831(0.542~1.275) |
| No | |||
| Age categories (years) | |||
| >50 | 2.333 (1.126~3.541) | <0.001 | 10.312 (3.083~34.493) |
| 41–50 | 2.063 (0.877~3.248) | 0.001 | 7.866 (2.405~25.729) |
| 31–40 | 1.525 (0.373~2.677) | 0.009 | 4.596 (1.452~14.544) |
| 21–30 | |||
| BMI categories (kg/m2) | |||
| ≥30 | 2.580 (2.009~3.151) | <0.001 | 13.197 (7.459~23.351) |
| 25.0–29.9 | 1.370 (0.906~1.834) | <0.001 | 3.934 (2.474~6.256) |
| <24.9 | |||
| Chewing betel nut | |||
| Yes | 1.260 (−0.140~2.661) | 0.078 | 3.526 (0.869~14.307) |
| No | |||
| White blood cell | 0.177 (0.074~0.280) | 0.001 | 1.194 (1.077~1.324) |
| Alanine aminotransferase | 0.013 (0.005~0.021) | 0.002 | 1.013 (1.005~1.021) |
| Uric acid | 0.223 (0.069~0.378) | 0.005 | 1.250 (1.072~1.459) |
| Haemoglobin | 0.001 (−0.161~0.163) | 0.988 | 1.001 (0.851~1.177) |
| Total calcium | 0.487 (−0.038~1.012) | 0.069 | 1.628 (0.963~2.751) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-S.; Tzeng, W.-C.; Chu, C.-M.; Wang, W.-Y. Metabolic Syndrome and Its Related Factors among Hospital Employees: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 9826. https://doi.org/10.3390/ijerph18189826
Wu Y-S, Tzeng W-C, Chu C-M, Wang W-Y. Metabolic Syndrome and Its Related Factors among Hospital Employees: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(18):9826. https://doi.org/10.3390/ijerph18189826
Chicago/Turabian StyleWu, Yi-Syuan, Wen-Chii Tzeng, Chi-Ming Chu, and Wei-Yun Wang. 2021. "Metabolic Syndrome and Its Related Factors among Hospital Employees: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 18, no. 18: 9826. https://doi.org/10.3390/ijerph18189826






