Screening of Particulate Matter Reduction Ability of 21 Indigenous Korean Evergreen Species for Indoor Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Growth and Chlorophyll Fluorescence Analysis
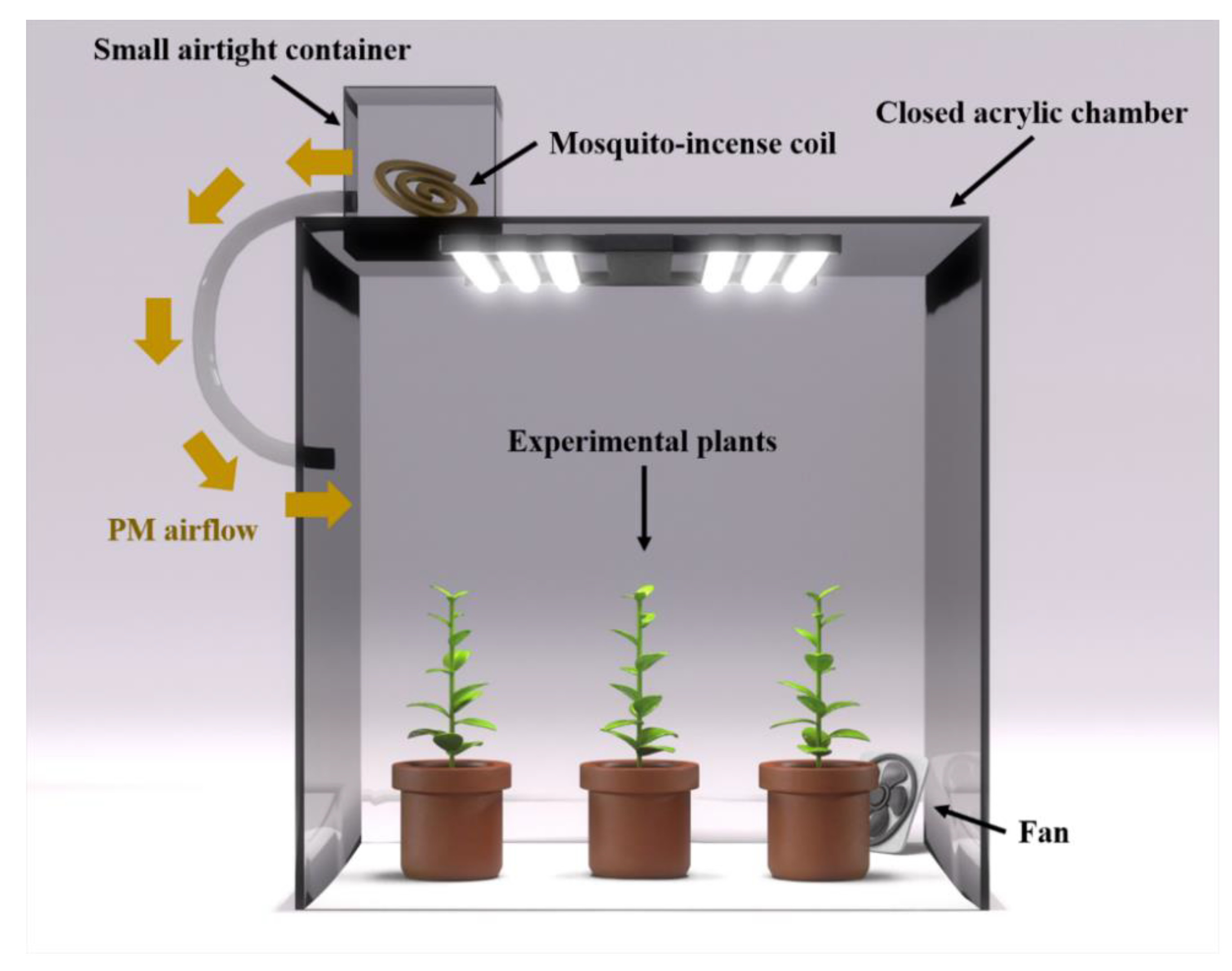
2.3. Particulate Matter (PM) Reduction Experiment
2.4. Particulate Matter (PM) Reduction Effect of Plant Species
2.5. Data Collection and Statistical Analysis
3. Results and Discussion
3.1. Effect of Plant Species on Reduction of PM2.5 and PM10
3.2. Effect of Various Factors on Reduction of PM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosco, M.L.; Varrica, D.; Dongarrà, G. Case study: Inorganic pollutants associated with particulate matter from an area near a petrochemical plant. Environ. Res. 2005, 99, 18–30. [Google Scholar] [CrossRef]
- Wang, L.; Gong, H.; Liao, W.; Wang, Z. Accumulation of particles on the surface of leaves during leaf expansion. Sci. Total Environ. 2015, 532, 420–434. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A. Acute respiratory effects of particulate air pollution. Annu. Rev. Public Health 1994, 15, 107–132. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Colvin, V.L.; Reed, K.L.; Sayes, C.M. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicol. Sci. 2007, 95, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Guo, J.; Wang, L.; Fan, W.; Lu, F.; Guo, M.; Moreno, S.B.R.; Wang, Y.; Wang, H.; Zhou, M.; et al. Higher risk of cardiovascular disease associated with smaller size-fractioned particulate matter. Environ. Sci. Technol. Lett. 2020, 7, 95–101. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawrońska, H.; Gawroński, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Dai, Z.D. Effects of the natural microstructures on the wettability of leaf surfaces. Biosurf. Biotribol. 2016, 2, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Leonard, R.J.; McArthur, C.; Hochuli, D.F. Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban For. Urban Green. 2016, 20, 249–253. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Perini, K.; Ottelé, M.; Giulini, S.; Magliocco, A.; Roccotiello, E. Quantification of fine dust deposition on different plant species in a vertical greening system. Ecol. Eng. 2017, 100, 268–276. [Google Scholar] [CrossRef]
- Lu, S.; Yang, X.; Li, S.; Chen, B.; Jiang, Y.; Wang, D.; Xu, L. Effects of plant leaf surface and different pollution levels on PM2.5 adsorption capacity. Urban For. Urban Green. 2018, 34, 64–70. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Meng, H.; Zhang, T. How does leaf surface micromorphology of different trees impact their ability to capture particulate matter? Forests 2018, 9, 681. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Kim, J.J.; Byeon, H.; Go, T.; Lee, S.J. Removal of fine particulate matter (PM2.5) via atmospheric humidity caused by evapotranspiration. Environ. Pollut. 2019, 245, 253–259. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Qiu, K.; Pott, R. Reduction of urban traffic–related particulate matter—Leaf trait matters. Environ. Sci. Pollut. Res. 2020, 27, 5825–5844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cong, L.; Liu, Y.; Xie, L.; Zhao, S.; Zhang, Z. Rainfall intensity plays an important role in the removal of PM from the leaf surfaces. Ecol. Indic. 2021, 128, 107778. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Lercher, P.; Browning, M.H.E.M.; Stoyanov, D.; Petrova, N.; Novakov, S.; Dimitrova, D.D. Does greenery experienced indoors and outdoors provide an escape and support mental health during the COVID-19 quarantine? Environ. Res. 2021, 196, 110420. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.; Pak, C.H. Changes in leaf variegation and coloration of English ivy and Polka dot plant under various indoor light intensities. HortScience 2012, 22, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Jang, B.K.; Park, K.; Lee, H.M.; Lee, C.H.; Oh, C.J.; Lee, S.Y.; Cho, J.S. The effects of the light intensity on the growth and chlorophyll fluorescence parameters of three evergreen fern species native to Korea. Acta Hortic. 2020, 1291, 131–138. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Hashim, J.H.; Jalaludin, J.; Hashim, Z.; Goldstein, B.D. Mosquito coil emissions and health implications. Environ. Health Perspect. 2003, 111, 1454–1460. [Google Scholar] [CrossRef]
- Thatcher, T.L.; Lai, A.C.K.; Moreno-Jackson, R.; Sextro, R.G.; Nazaroff, W.W. Effects of room furnishings and air speed on particle deposition rates indoors. Atmos. Environ. 2002, 36, 1811–1819. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.S.; Pennisi, S.V.; Son, K.C.; Kays, S.J. Screening indoor plants for volatile organic pollutant removal efficiency. HortScience 2009, 44, 1377–1381. [Google Scholar] [CrossRef]
- Chávez-García, E.; González-Méndez, B. Particulate matter and foliar retention: Current knowledge and implications for urban greening. Air Qual. Atmos. Health 2021, 14, 1433–1454. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Niazi, N.K.; Xiong, T.T.; Farooq, A.B.U.; Khalid, S. Ecotoxicology of Heavy Metal(loid)-Enriched Particulate Matter: Foliar Accumulation by Plants and Health Impacts. In Reviews of Environmental Contamination and Toxicology; De Voogt, P., Ed.; Springer: Cham, Switzerland, 2019; Volume 253, pp. 65–113. [Google Scholar]
- Muhammad, S.; Wuyts, K.; Samson, R. Atmospheric net particle accumulation on 96 plant species with contrasting morphological and anatomical leaf characteristics in a common garden experiment. Atmos. Environ. 2019, 202, 328–344. [Google Scholar] [CrossRef]
- Cho, S.; Lee, G.; Park, D.; Kim, M. Study on characteristics of particulate matter resuspension in school classroom through experiments using a simulation chamber: Influence of humidity. Int. J. Environ. Res. Public Health 2021, 18, 2856. [Google Scholar] [CrossRef]
- Mohan, S.M. An overview of particulate dry deposition: Measuring methods, deposition velocity and controlling factors. Int. J. Environ. Sci. Technol. 2016, 13, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, L.; Chen, R.; Tian, G.; Li, D.; Chen, C.; Ge, X.; Ge, G. Effect of relative humidity on the deposition and coagulation of aerosolized SiO2 nanoparticles. Atmos. Res. 2017, 194, 100–108. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Zhai, J.; Cong, L.; Wang, Y.; Ma, W.; Zhang, Z.; Li, C. Comparison of dry and wet deposition of particulate matter in near-surface waters during summer. PLoS ONE 2018, 13, e0199241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Hann, T.; Lee, S.J. Effect of flow and humidity on indoor deposition of particulate matter. Environ. Pollut. 2019, 255, 113263. [Google Scholar] [CrossRef] [PubMed]


| Species | Collection Region | No. of Plants/Chamber | Plant Height (cm) | No. of Leaves/Plant | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | Chlorophyll Content |
|---|---|---|---|---|---|---|---|---|
| Acorus gramineus Ation | Andong | 5 | 17.5 ± 0.86 | 92.9 ± 8.12 | 20.9 ± 0.92 | 0.4 ± 0.02 | 3.6 ± 0.03 | 57.9 ± 2.08 |
| Ardisia crispa (Thunb.) A.DC. | Seogwipo | 5 | 24.3 ± 1.49 | 93.2 ± 11.50 | 7.6 ± 0.23 | 1.9 ± 0.08 | 4.6 ± 0.28 | 38.2 ± 1.58 |
| Ardisia japonica (Thunb.) Blume | Seogwipo | 8 | 11.4 ± 1.35 | 38.7 ± 5.17 | 5.2 ± 0.39 | 2.5 ± 0.12 | 5.1 ± 1.04 | 41.3 ± 1.40 |
| Ardisia pusilla A.DC. | Seogwipo | 5 | 6.3 ± 0.67 | 123.8 ± 16.99 | 2.5 ± 0.07 | 1.6 ± 0.06 | 77.1 ± 12.46 | 38.6 ± 1.52 |
| Camellia japonica L. | Gangjin | 4 | 52.9 ± 2.68 | 39.7 ± 3.36 | 7.4 ± 0.17 | 4.1 ± 0.16 | 15.1 ± 1.40 | 67.3 ± 2.14 |
| Cephalotaxus harringtonii (Knight ex J.Forbes) K.Koch | Naju | 5 | 21.2 ± 1.33 | 541.9 ± 39.82 | 3.8 ± 0.26 | 0.3 ± 0.01 | 0.5 ± 0.07 | 54.5 ± 1.95 |
| Dryopteris lacera (Thunb.) Kuntze | Cheongju | 4 | 17.5 ± 1.15 | 22.3 ± 1.43 | 26.6 ± 0.36 | 10.3 ± 0.30 | 57.1 ± 1.04 | 46.1 ± 1.13 |
| Eriobotrya japonica (Thunb.) Lindl. | Gangjin | 5 | 53.3 ± 3.47 | 17.3 ± 1.71 | 16.9 ± 0.88 | 5.5 ± 0.21 | 57.5 ± 9.70 | 44.5 ± 0.88 |
| Euonymus japonicus Thunb. | Gangjin | 5 | 50.7 ± 1.44 | 54.7 ± 5.10 | 4.1 ± 0.16 | 2.2 ± 0.13 | 5.2 ± 0.04 | 68.4 ± 1.76 |
| Farfugium japonicum (L.) Kitam. | Seogwipo | 5 | 17.1 ± 1.11 | 5.1 ± 0.56 | 6.7 ± 0.40 | 11.1 ± 0.75 | 73.5 ± 5.79 | 41.7 ± 1.30 |
| Ilex × wandoensis C.F.Mill. & M.Kim | Wando | 5 | 22.6 ± 0.96 | 54.6 ± 5.70 | 6.4 ± 0.18 | 2.9 ± 0.09 | 10.4 ± 0.43 | 57.8 ± 1.14 |
| Liriope muscari (Decne.) L.H.Bailey | Andong | 5 | 20.5 ± 0.84 | 96.2 ± 5.05 | 21.3 ± 0.56 | 0.4 ± 0.02 | 1.6 ± 0.11 | 62.4 ± 1.30 |
| Machilus thunbergii Siebold & Zucc. | Gangjin | 5 | 52.0 ± 1.64 | 38.9 ± 3.51 | 9.1 ± 0.43 | 3.6 ± 0.21 | 18.9 ± 0.76 | 41.0 ± 0.94 |
| Neolitsea sericea (Blume) Koidz. | Wando | 4 | 50.1 ± 2.55 | 36.6 ± 4.39 | 10.4 ± 0.29 | 4.2 ± 0.21 | 13.6 ± 1.16 | 41.1 ± 0.92 |
| Pittosporum tobira (Thunb.) W.T.Aiton | Gangjin | 5 | 37.2 ± 1.39 | 59.0 ± 8.12 | 5.0 ± 0.25 | 2.2 ± 0.09 | 7.0 ± 1.26 | 49.3 ± 1.65 |
| Polypodium vulgare L. | Cheongju | 5 | 7.3 ± 0.72 | 10.1 ± 1.35 | 11.7 ± 0.38 | 5.3 ± 0.24 | 11.2 ± 0.26 | 30.8 ± 2.11 |
| Quercus acuta Thunb. | Wando | 5 | 29.7 ± 1.54 | 31.2 ± 2.03 | 9.1 ± 0.51 | 2.9 ± 0.23 | 11.9 ± 0.55 | 36.4 ± 1.21 |
| Rhaphiolepis indica var. umbellata (Thunb.) H.Ohashi. | Gangjin | 5 | 59.6 ± 1.92 | 56.2 ± 4.78 | 8.1 ± 0.45 | 2.7 ± 0.10 | 9.5 ± 1.19 | 61.9 ± 1.97 |
| Rhododendron brachycarpum D.Don ex G.Don | Seogwipo | 4 | 22.2 ± 0.94 | 45.4 ± 4.80 | 10.7 ± 0.53 | 3.6 ± 0.13 | 15.2 ± 1.00 | 49.0 ± 1.27 |
| Ternstroemia gymnanthera (Wight & Arn.) Sprague | Wando | 4 | 28.1 ± 2.93 | 30.8 ± 4.85 | 8.8 ± 2.12 | 4.8 ± 2.08 | 6.2 ± 0.47 | 37.6 ± 2.33 |
| Torreya nucifera (L.) Siebold & Zucc. | Naju | 5 | 34.7 ± 1.46 | 1303.4 ± 68.92 | 2.3 ± 0.09 | 0.3 ± 0.01 | 0.3 ± 0.01 | 67.1 ± 1.79 |
| Species | Category | Leaf Shape | Leaf Area/ Plant (cm2) | Leaf Area/ Chamber (cm2) | ≥90% RH 2 (time) | PM2.5 Reduction after 8 h (%) | PM10 Reduction after 8 h (%) |
|---|---|---|---|---|---|---|---|
| Acorus gramineus | Herb | Linear | 335.5 ± 27.66 hi 1 | 1677.5 ± 138.28 e–h | 100 | 77.4 ± 2.21 c–f | 96.3 ± 0.80 a–d |
| Ardisia crispa | Shrub | Lanceolate | 432.6 ± 50.52 f–h | 2162.8 ± 252.59 c–g | 140 | 76.4 ± 2.64 c–f | 96.3 ± 0.47 a–d |
| Ardisia japonica | Shrub | Ovate | 195.7 ± 38.34 jk | 1565.4 ± 306.75 f–h | 120 | 77.9 ± 0.87 c–e | 97.2 ± 0.32 a–c |
| Ardisia pusilla | Subshrub | Elliptic | 164.8 ± 22.43 jk | 824.2 ± 112.16 ij | 180 | 75.3 ± 0.88 d–f | 97.1 ± 0.30 a–c |
| Camellia japonica | Subtree | Ovate | 588.2 ± 19.55 de | 2352.9 ± 78.18 c–e | 180 | 80.1 ± 0.63 b–d | 97.4 ± 0.50 a–c |
| Cephalotaxus harringtonii | Shrub | Linear | 244.1 ± 17.24 i–k | 1220.4 ± 86.20 h–j | 80 | 80.4 ± 1.89 b–d | 98.3 ± 0.23 ab |
| Dryopteris lacera | Herb/ferns | pinnate | 1274.4 ± 48.10 a | 5097.7 ± 192.41 a | 60 | 86.8 ± 0.82 a | 91.7 ± 2.54 e |
| Eriobotrya japonica | Subtree | Elliptic | 989.4 ± 144.89 b | 4947.1 ± 724.44 a | 60 | 81.0 ± 1.51 bc | 96.9 ± 0.88 a–c |
| Euonymus japonicus | Shrub | Elliptic | 284.8 ± 37.52 ij | 1423.9 ± 187.58 g–i | 120 | 76.0 ± 1.32 c–f | 96.2 ± 1.33 a–d |
| Farfugium japonicum | Herb | Reniform | 366.7 ± 27.08 g–i | 1833.7 ± 135.41 e–h | 120 | 76.7 ± 1.71 c–f | 95.9 ± 1.06 a–d |
| Ilex × wandoensis | Shrub | Oblong | 567.4 ± 37.88 d–f | 2836.9 ± 189.39 c | 60 | 84.9 ± 0.67 ab | 98.5 ± 0.28 a |
| Liriope muscari | Herb | Linear | 156.9 ± 12.97 jk | 784.5 ± 64.84 ij | 120 | 80.5 ± 1.52 b–d | 97.2 ± 0.25 a–c |
| Machilus thunbergii | Tree | Elliptic | 731.6 ± 28.20 c | 3658.0 ± 140.98 b | 60 | 84.3 ± 0.54 ab | 98.5 ± 0.12 a |
| Neolitsea sericea | Tree | Elliptic | 486.3 ± 32.25 e–g | 1945.0 ± 129.01 d–h | 220 | 75.2 ± 0.90 d–f | 94.5 ± 1.20 c–e |
| Pittosporum tobira | Shrub | Obovate | 388.0 ± 28.64 g–i | 1940.0 ± 143.21 d–h | 160 | 69.6 ± 3.58 g | 93.5 ± 0.43 de |
| Polypodium vulgare | Herb/ferns | pinnate | 112.6 ± 2.24 k | 562.8 ± 11.21 j | 180 | 74.5 ± 1.64 ef | 94.9 ± 1.10 b–d |
| Quercus acuta | Tree | Ovate | 369.2 ± 6.47 g–i | 1846.0 ± 32.35 e–h | 120 | 74.8 ± 2.24 ef | 97.6 ± 0.64 a–c |
| Rhaphiolepis indica var. umbellata | Shrub | Obovate | 532.1 ± 59.65 ef | 2660.6 ± 298.23 cd | 80 | 80.4 ± 1.33 b–d | 97.2 ± 0.92 a–c |
| Rhododendron brachycarpum | Shrub | Elliptic | 679.5 ± 41.53 cd | 2717.8 ± 166.11 c | 100 | 84.0 ± 1.55 ab | 98.3 ± 0.34 ab |
| Ternstroemia gymnanthera | Subtree | Elliptic | 191.6 ± 15.97 jk | 766.3 ± 63.90 ij | 240 | 72.5 ± 0.81 fg | 91.7 ± 1.98 e |
| Torreya nucifera | Tree | Linear | 448.9 ± 26.99 e–h | 2244.4 ± 134.95 c–f | 60 | 78.3 ± 0.46 c–e | 95.9 ± 0.47 a–d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, B.-K.; Park, K.; Lee, S.Y.; Lee, H.; Yeon, S.H.; Ji, B.; Lee, C.H.; Cho, J.-S. Screening of Particulate Matter Reduction Ability of 21 Indigenous Korean Evergreen Species for Indoor Use. Int. J. Environ. Res. Public Health 2021, 18, 9803. https://doi.org/10.3390/ijerph18189803
Jang B-K, Park K, Lee SY, Lee H, Yeon SH, Ji B, Lee CH, Cho J-S. Screening of Particulate Matter Reduction Ability of 21 Indigenous Korean Evergreen Species for Indoor Use. International Journal of Environmental Research and Public Health. 2021; 18(18):9803. https://doi.org/10.3390/ijerph18189803
Chicago/Turabian StyleJang, Bo-Kook, Kyungtae Park, Sang Yeob Lee, Hamin Lee, Soo Ho Yeon, Boran Ji, Cheol Hee Lee, and Ju-Sung Cho. 2021. "Screening of Particulate Matter Reduction Ability of 21 Indigenous Korean Evergreen Species for Indoor Use" International Journal of Environmental Research and Public Health 18, no. 18: 9803. https://doi.org/10.3390/ijerph18189803






