Oxidative Stress Parameters in Goitrogen-Exposed Crested Newt Larvae (Triturus spp.): Arrested Metamorphosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Tissue Processing
2.3. Biochemical Analyses
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, S.F. Metamorphosis: The Hormonal Reactivation of Development. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Shibata, Y.; Tanizaki, Y.; Zhang, H.; Lee, H.; Dasso, M.; Shi, Y.B. Thyroid hormone receptor is essential for larval epithelial apoptosis and adult epithelial stem cell development but not adult intestinal morphogenesis during Xenopus tropicalis metamorphosis. Cells 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Lynn, W.G. Types of amphibian metamorphosis. Am. Zool. 1961, 1, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; McGraw Hill: New York, NY, USA, 1986. [Google Scholar]
- Smirnov, S.V. Metamorphosis in the Urodelan amphibians: Patterns, regulation mechanisms, and evolution. Zh. Obshch. Biol. 2006, 67, 323–334. [Google Scholar]
- Weber, R. (Ed.) Biochemistry of amphibian metamorphosis. In The Biochemistry of Animal Development, 1st ed.; Academic Press: New York, NY, USA, 1967; pp. 227–301. [Google Scholar]
- White, B.A.; Nicoll, C.S. Hormonal control of amphibian metamorphosis. In Metamorphosis; Gilbert, L.I., Frieden, E., Eds.; Plenum Press: New York, NY, USA, 1981; pp. 363–396. [Google Scholar]
- Alberch, P.; Gale, E.A.; Larsen, P.R. Plasma T4 and T3 levels in naturally metamorphosing Eurycea bislineata (Amphibia; Plethodontidae). Gen. Comp. Endocrinol. 1986, 61, 153–163. [Google Scholar] [CrossRef]
- Kikuyama, S.; Suzuki, M.R.; Iwamuro, S. Elevation of plasma aldosterone levels of tadpoles at metamorphic climax. Gen. Comp. Endocrinol. 1986, 63, 186–190. [Google Scholar] [CrossRef]
- Baker, B.S.; Tata, J.R. Prolactin prevents the autoinduction of thyroid hormone receptor mRNAs during amphibian metamorphosis. Dev. Biol. 1992, 149, 463–467. [Google Scholar] [CrossRef]
- Hayes, T.B. Steroids as potential modulators of thyroid hormone activity in anuran metamorphosis. Am. Zool. 1997, 37, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Tazawa, I.; Yaoita, Y. Thyroid hormone receptor α- and α-knockout Xenopus tropicalis tadpoles reveal subtype-specific roles during development. Endocrinology 2018, 159, 733–743. [Google Scholar] [CrossRef]
- Tata, J. Amphibian metamorphosis as a model for studying the developmental actions of thyroid hormone. Cell. Res. 1998, 8, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M.; Buchholz, D.R. Insufficiency of thyroid hormone in frog metamorphosis and the role of glucocorticoids. Front. Endocrinol. 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, H.; LaRochelle, F.T., Jr.; Suzuki, M.; Freeman, M.; Frieden, E. Studies on thyroid hormones and their binding in bullfrog tadpole plasma during metamorphosis. Gen. Comp. Endocrinol. 1977, 33, 254–266. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, M. Changes in thyroidal and plasma iodine compounds during and after metamorphosis of the bullfrog, Rana catesbeiana. Gen. Comp. Endocrinol. 1981, 45, 74–81. [Google Scholar] [CrossRef]
- Turani, B.; Aliko, V.; Faggio, C. Amphibian embryos as an alternative model to study the pharmaceutical toxicity of cyclophosphamide and ibuprofen. J. Biol. Res. Boll. Della Soc. Ital. Biol. Sper. 2019, 92, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Kloas, W. Amphibians as a model for the study of endocrine disruptors. Int. Rev. Cytol. 2002, 216, 1–57. [Google Scholar] [PubMed]
- Gudernatsch, J.F. Feeding experiments on tadpoles. Archiv für Entwicklungsmechanik der Organismen 1912, 35, 457–483. [Google Scholar] [CrossRef] [Green Version]
- Joel, T.; D’Angelo, S.A.; Charipper, H.A. The effect of thiourea on the thyroid gland of the winter frog (Rana pipiens) with some observations on the testis. J. Exp. Zool. 1949, 110, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Heyland, A.; Moroz, L.L. Cross-kingdom hormonal signaling: An insight from thyroid hormone functions in marine larvae. J. Exp. Biol. 2005, 208, 4355–4361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutleb, A.C.; Bronkhorst, M.; van den Berg, J.H.; Murk, A.J. Latex laboratory-gloves: An unexpected pitfall in amphibian toxicity assays with tadpoles. Environ. Toxicol. Pharmacol. 2001, 10, 119–121. [Google Scholar] [CrossRef]
- Wang, Z.D.; Yoshida, M.; George, B. Theoretical study on the thermal decomposition of thiourea. Comput. Theor. Chem. 2013, 1017, 91–98. [Google Scholar] [CrossRef]
- Sahu, N.; Patnaik, B.K. Effect of thyroxine (T4) and thiourea on the hepatic oxygen consumption of male garden lizards of three different age groups. Arch. Gerontol. Geriatr. 1989, 8, 55–62. [Google Scholar] [CrossRef]
- Swapna, I.; Rajasekhar, M.; Supriya, A.; Raghuveer, K.; Sreenivasulu, G.; Rasheeda, M.K.; Majumdar, K.C.; Kagawa, H.; Tanaka, H.; Dutta-Gupta, A.; et al. Thiourea-induced thyroid hormone depletion impairs testicular recrudescence in the air-breathing catfish, Clarias gariepinus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 1–10. [Google Scholar] [CrossRef]
- Davidson, B.; Soodak, M.; Strout, H.V.; Neary, J.T.; Nakamura, C.; Maloof, F. Thiourea and cyanamide as inhibitors of thyroid peroxidase: The role of iodide. Endocrinology 1979, 104, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, A.; Kiran Kumar, Y. Evaluation of goitrogenic and antithyroidal effect of the fern Adiantum capillus-veneris. Rev. Bras. Farmacogn. 2013, 23, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Edeline, E.; Bardonnet, A.; Bolliet, V.; Dufour, S.; Elie, P. Endocrine control of Anguilla anguilla glass eel dispersal: Effect of thyroid hormones on locomotor activity and rheotactic behavior. Horm. Behav. 2005, 48, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.S.; Goldsmith, E.D.; Charipper, H.A. Effect of thiourea on development of the amphibian. Nature 1943, 154, 504–505. [Google Scholar]
- Krishnapriya, M.V.; Arulvasu, C.; Sheeba, P.; Sujitha, C.S.; Neethu, P.G. Influence of elemental Iodine and thiourea on metamorphosis of Philautus sp. J. Adv. Bot. Zool. 2014, 4, 1–6. [Google Scholar]
- Ajduković, M.; Vučić, T.; Cvijanović, M. Effects of thiourea on the skull of Triturus newts during ontogeny. PeerJ 2021, 9, e11535. [Google Scholar] [CrossRef]
- Smirnov, S.V.; Vasil’eva, A.B. The bony skull of the Siberian salamander Salamandrella keyserlingi (Amphibia: Urodela: Hynobiidae) and the role of thyroid hormones in its development. Dokl. Biol. Sci. 2002, 385, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Denoël, M.; Joly, P.; Whiteman, H.H. Evolutionary ecology of facultative paedomorphosis in newts and salamanders. Biol. Rev. 2005, 80, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Slijepčević, M.D. Evidence for paedomorphosis in Macedonian crested newt (Triturus macedonicus) from Montenegro. Arch. Biol. Sci. 2015, 67, 7–10. [Google Scholar] [CrossRef]
- Denoël, M.; Ivanović, A.; Džukić, G.; Kalezić, M.L. Sexual size dimorphism in the evolutionary context of facultative paedomorphosis: Insights from European newts. BMC Evol. Biol. 2009, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wakahara, M. Heterochrony and neotenic salamanders: Possible clues for understanding the animal development and evolution. Zoolog. Sci. 1996, 13, 765–776. [Google Scholar] [CrossRef]
- Denoël, M.; Ficetola, G.F. Heterochrony in a complex world: Disentangling environmental processes of facultative paedomorphosis in an amphibian. J. Anim. Ecol. 2014, 83, 606–615. [Google Scholar] [CrossRef] [Green Version]
- Oromi, N.; Michaux, J.; Denoël, M. High gene flow between alternative morphs and the evolutionary persistence of facultative paedomorphosis. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Petrović, T.G.; Vučić, T.Z.; Nikolić, S.Z.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Faggio, C.; Prokić, M.D. The effect of shelter on oxidative stress and aggressive behavior in crested newt larvae (Triturus spp.). Animals 2020, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Petrović, T.G.; Kijanović, A.; Kolarov Tomašević, N.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Vukov, T.; Faggio, C.; Prokić, M.D. Effects of desiccation on metamorphic climax in Bombina variegata: Changes in levels and patterns of oxidative stress parameters. Animals 2021, 11, 953. [Google Scholar] [CrossRef]
- Glücksohn, S. Äußere Entwicklung der Extremitäten und Stadieneinteilung der Larvenperiode von Triton taeniatus Leyd. und Triton cristatus Laur. Wilhelm Roux Arch. Entwicklungsmech. Organismen 1931, 125, 341–405. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.J. Temporal variations in histological appearance of thyroid and pituitary of Salamanders treated with thyroid inhibitors. Biol. Bull. 1953, 104, 250–262. [Google Scholar] [CrossRef]
- Altwegg, R. Multistage density dependence in an amphibian. Oecologia 2003, 136, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Labocha, M.K.; Schutz, H.; Hayes, J.P. Which body condition index is best? Oikos 2014, 123, 111–119. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Pascariello, M.F.; Marinosci, L.; Schettino, T. Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymeactivities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar. Poll. Bull. 2003, 46, 324–330. [Google Scholar] [CrossRef]
- Rossi, M.A.; Cecchini, G.; Dianzani, M.M. Glutathione peroxidase, glutathione reductase and glutathione transferase in two different hepatomas and in normal liver. IRCS Med. Sci. Biochem. 1983, 11, 805. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and simple assay or superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Tamura, M.; Oshino, N.; Chance, B. Some characteristics of hydrogen- and alkylhydroperoxides metabolizing systems in cardiac tissue. J. Biochem. 1982, 92, 1019–1031. [Google Scholar] [CrossRef]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Rehncrona, S.; Smith, D.S.; Akesson, B.; Westerberg, E.; Siesjö, B.K. Peroxidative changes in brain cortical fatty acids and phospholipids, as characterized during Fe2+ and ascorbic acid stimulated lipid peroxidation in vitro. J. Neurochem. 1980, 34, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Darlington, R.B.; Weinberg, S.L.; Walberg, H.J. Canonical variate analysis and related techniques. Rev. Educ. Res. 1973, 43, 433–454. [Google Scholar] [CrossRef]
- Menon, J.; Rozman, R. Oxidative stress, tissue remodeling and regression during amphibian metamorphosis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Rudneva, I.I. Antioxidant system of Black Sea animals in early development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1999, 122, 265–271. [Google Scholar] [CrossRef]
- Prokić, M.D.; Petrović, T.G.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Faggio, C.; Saičić, Z.S. Comparative assessment of the antioxidative defense system in subadult and adult anurans: A lesson from the Bufotes viridis toad. Zoology 2018, 130, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Prokić, M.D.; Gavrić, J.P.; Petrović, T.G.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Krizmanić, I.; Pavlović, S.Z. Oxidative stress in Pelophylax esculentus complex frogs in the wild during transition from aquatic to terrestrial life. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 234, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jehle, R.; Thiesmeier, B.; Foster, J. The Crested Newt: A Dwindling Pond Dweller; Laurenti-Verlag: Bielefeld, Germany, 2011. [Google Scholar]
- Lukanov, S.; Tzankov, N.; Handschuh, S.; Heiss, E.; Naumov, B.; Natchev, N. On the amphibious food uptake and prey manipulation behavior in the Balkan-Anatolian crested newt (Triturus ivanbureschi, Arntzen and Wielstra, 2013). Zoology 2016, 119, 224–231. [Google Scholar] [CrossRef]
- Amicarelli, F.; Falone, S.; Cattani, F.; Alamanou, M.T.; Bonfigli, A.; Zarivi, O.; Miranda, M.; Ragnelli, A.M.; Di Ilio, C. Amphibian transition to the oxidant terrestrial environment affects the expression of glutathione S-transferases isoenzymatic pattern. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1691, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aceto, A.; Amicarelli, F.; Sacchetta, P.; Dragani, B.; Bucciarelli, T.; Masciocco, L.; Miranda, M.; Di Ilio, C. Developmental aspects of detoxifying enzymes in fish (Salmo iridaeus). Free Radic. Res. 1994, 21, 285–294. [Google Scholar] [CrossRef]
- Angelucci, S.; Sacchetta, P.; De Luca, A.; Moio, P.; Amicarelli, F.; Di Ilio, C. Glutathione transferase isoenzymes from frog (Xenopus laevis) liver and embryo. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 81–85. [Google Scholar] [CrossRef]
- Trijuno, D.D.; Yoseda, K.; Hirokawa, J.; Tagawa, M.; Tanaka, M. Effects of thyroxine and thiourea on the metamorphosis of coral trout grouper Plectropomus leopardus. Fish. Sci. 2002, 68, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Ruthsatz, K.; Dausmann, K.H.; Reinhardt, S.; Robinson, T.; Sabatino, N.M.; Peck, M.A.; Glos, J. Endocrine disruption alters developmental energy allocation and performance in Rana temporaria. Integr. Comp. Biol. 2019, 59, 70–88. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Dausmann, K.H.; Drees, C.; Becker, L.I.; Hartmann, L.; Reese, J.; Reinhardt, S.; Robinson, T.; Sabatino, N.M.; Peck, M.A.; et al. Altered thyroid hormone levels affect the capacity for temperature-induced developmental plasticity in larvae of Rana temporaria and Xenopus laevis. J. Therm. Biol. 2020, 90, 102599. [Google Scholar] [CrossRef]
- Carlsson, G. Effect-based environmental monitoring for thyroid disruption in Swedish amphibian tadpoles. Environ. Monit. Assess. 2019, 191, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oktay, S.; Uslu, L.; Emekli, N. Effects of altered thyroid states on oxidative stress parameters in rats. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 159–165. [Google Scholar] [CrossRef]
- Bhanja, S.; Chainy, G.B.N. PTU-induced hypothyroidism modulates antioxidant defence status in the developing cerebellum. Int. J. Dev. Neurosci. 2010, 28, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R.; Lips, F.; Martinez, P.G.; Pipe, R.K. Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar. Biol. 1992, 112, 265–276. [Google Scholar] [CrossRef]
- Onderwater, R.C.; Commandeur, J.N.; Groot, E.J.; Sitters, A.; Menge, W.M.; Vermeulen, N.P. Cytotoxicity of a series of mono-and di-substituted thiourea in freshly isolated rat hepatocytes: A preliminary structure-toxicity relationship study. Toxicology 1998, 125, 117–129. [Google Scholar] [CrossRef]
- Takamura, A.E.; de Oliveira, M.A.; Vigoya, A.A.; De Stéfani, M.V.; Ribeiro Filho, O.P. Thiourea effects on the metamorphosis and development of bullfrog tadpole (Lithobates catesbeianus). Int. J. Res. Eng. Sci. 2020, 8, 30–41. [Google Scholar]
- Gutleb, A.C.; Schriks, M.; Mossink, L.; Van den Berg, J.H.J.; Murk, A.J. A synchronized amphibian metamorphosis assay as an improved tool to detect thyroid hormone disturbance by endocrine disruptors and apolar sediment extracts. Chemosphere 2007, 70, 93–100. [Google Scholar] [CrossRef]
- Chiba, C.; Yamada, S.; Tanaka, H.; Inae-Chiba, M.; Miura, T.; Casco-Robles, M.M.; Yoshikawa, T.; Inami, W.; Mizuno, A.; Islam, M.R.; et al. Metamorphosis inhibition: An alternative rearing protocol for the newt, Cynops pyrrhogaster. Zool. Sci. 2012, 29, 293–298. [Google Scholar] [CrossRef]
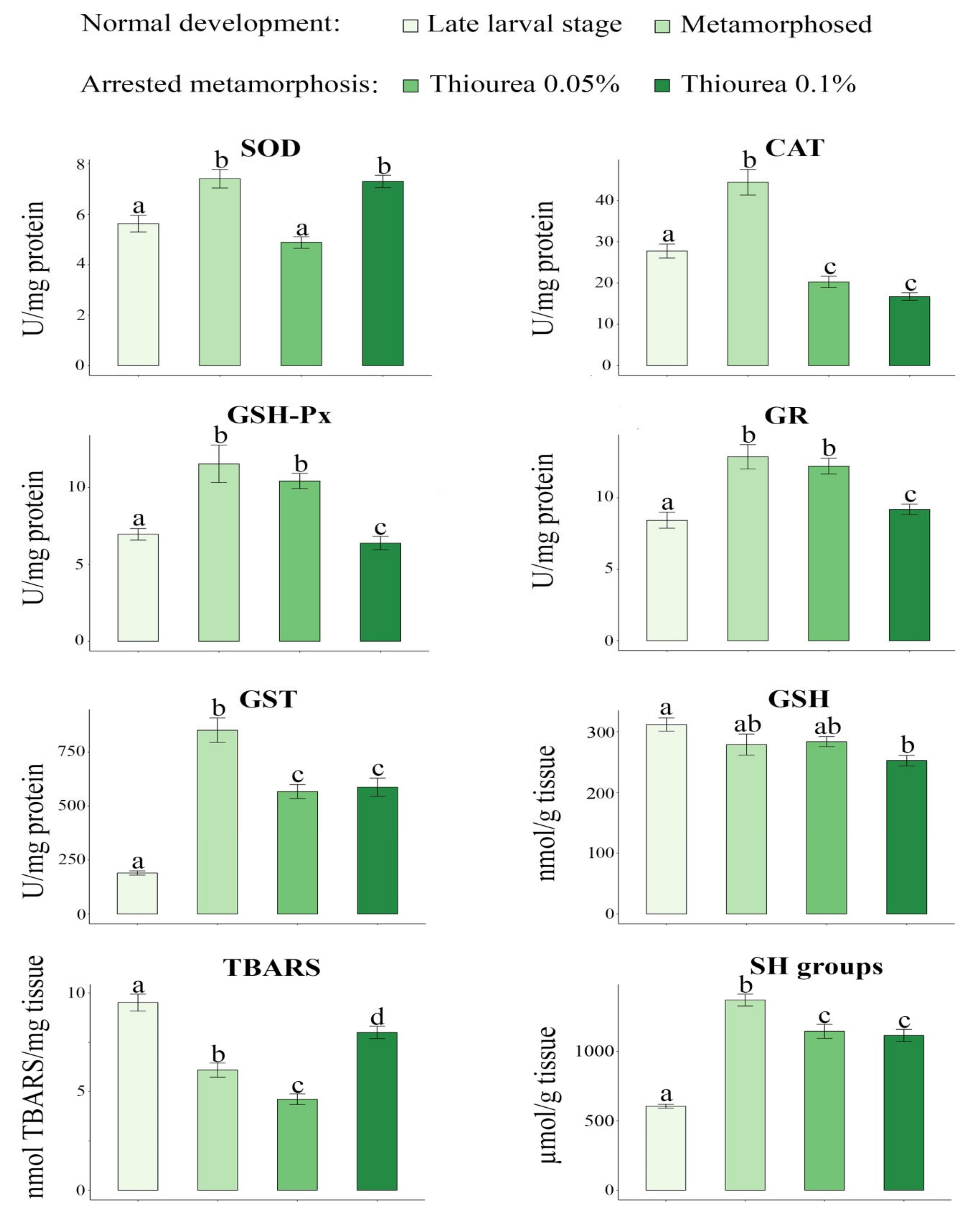
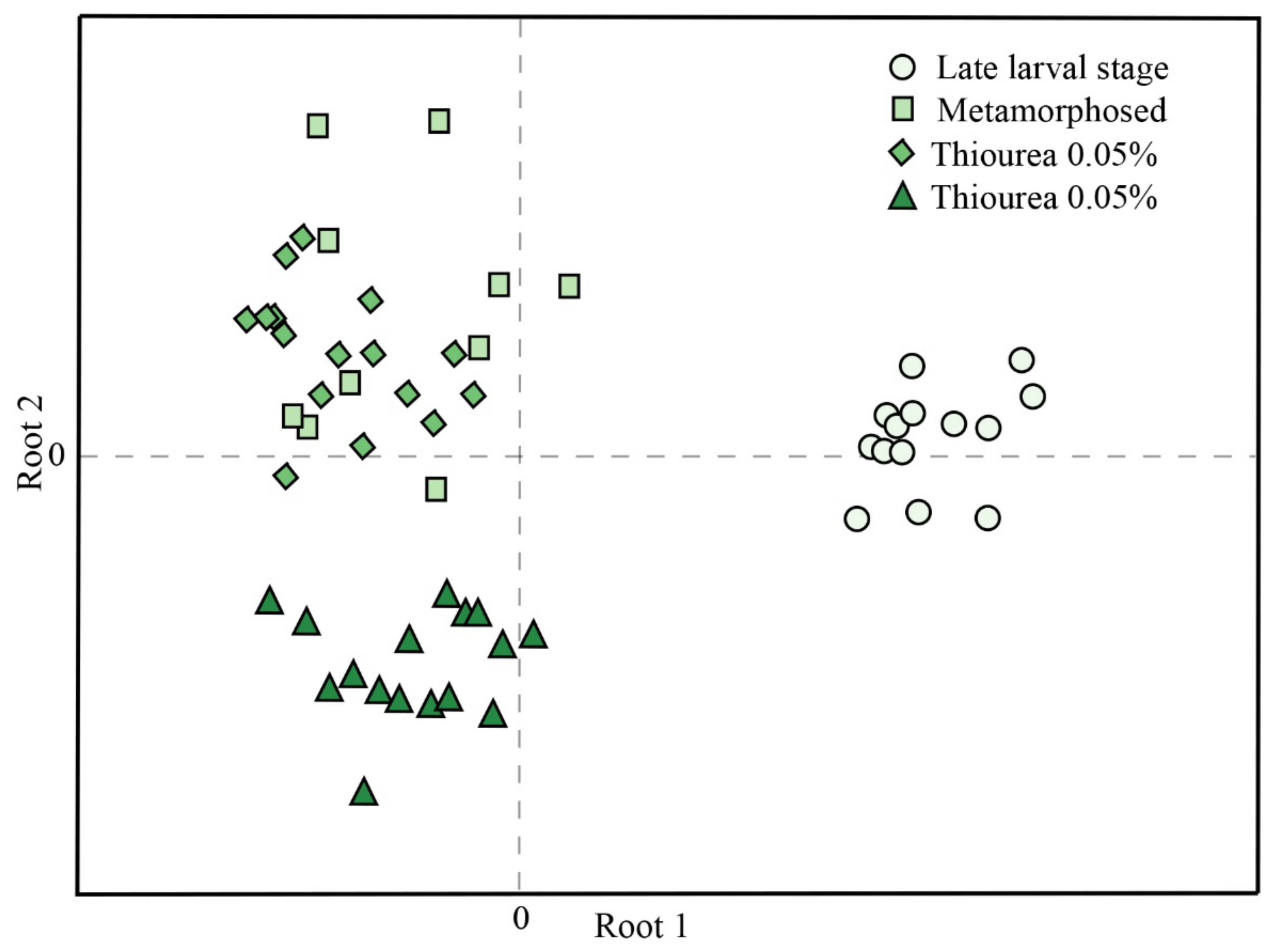
| Late Larval Stage | Newly Metamorphosed | Thiourea 0.05% | Thiourea 0.1% | |
|---|---|---|---|---|
| SVL (mm) | 20.69 ± 0.38 a | 34.47 ± 0.80 b | 33.56 ± 0.69 b | 32.85 ± 0.75 b |
| BM (g) | 0.45 ± 0.03 a | 1.69 ± 0.10 b | 1.93 ± 0.12 b | 1.81 ± 0.11 b |
| CI | 0.12 ± 0.01 a | 0.28 ± 0.01 b | 0.30 ± 0.01 b | 0.29 ± 0.01 b |
| Variable | F | p |
|---|---|---|
| CI | 79.99 | 0.000000 |
| SOD | 18.89 | 0.000000 |
| CAT | 43.98 | 0.000000 |
| GSH-Px | 16.56 | 0.000000 |
| GR | 14.34 | 0.000001 |
| GST | 50.14 | 0.000000 |
| GSH | 5.60 | 0.002025 |
| TBARS | 38.36 | 0.000000 |
| SH groups | 55.44 | 0.000000 |
| Variable | Root 1 | Root 2 |
|---|---|---|
| SH groups | −0.778 | −0.310 |
| CAT | 0.718 | 0.766 |
| TBARS | 0.620 | −0.198 |
| GST | −0.494 | 0.202 |
| GSH | 0.392 | 0.081 |
| SOD | −0.222 | −0.957 |
| GR | −0.134 | 0.192 |
| GSH-Px | 0.014 | 0.439 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Petrović, T.G.; Ajduković, M.; Vučić, T.; Cvijanović, M.; Faggio, C.; Prokić, M.D. Oxidative Stress Parameters in Goitrogen-Exposed Crested Newt Larvae (Triturus spp.): Arrested Metamorphosis. Int. J. Environ. Res. Public Health 2021, 18, 9653. https://doi.org/10.3390/ijerph18189653
Gavrić JP, Despotović SG, Gavrilović BR, Radovanović TB, Petrović TG, Ajduković M, Vučić T, Cvijanović M, Faggio C, Prokić MD. Oxidative Stress Parameters in Goitrogen-Exposed Crested Newt Larvae (Triturus spp.): Arrested Metamorphosis. International Journal of Environmental Research and Public Health. 2021; 18(18):9653. https://doi.org/10.3390/ijerph18189653
Chicago/Turabian StyleGavrić, Jelena P., Svetlana G. Despotović, Branka R. Gavrilović, Tijana B. Radovanović, Tamara G. Petrović, Maja Ajduković, Tijana Vučić, Milena Cvijanović, Caterina Faggio, and Marko D. Prokić. 2021. "Oxidative Stress Parameters in Goitrogen-Exposed Crested Newt Larvae (Triturus spp.): Arrested Metamorphosis" International Journal of Environmental Research and Public Health 18, no. 18: 9653. https://doi.org/10.3390/ijerph18189653







