Diagnosis of Muscle Fatigue Using Surface Electromyography and Analysis of Associated Factors in Type 2 Diabetic Patients with Neuropathy: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
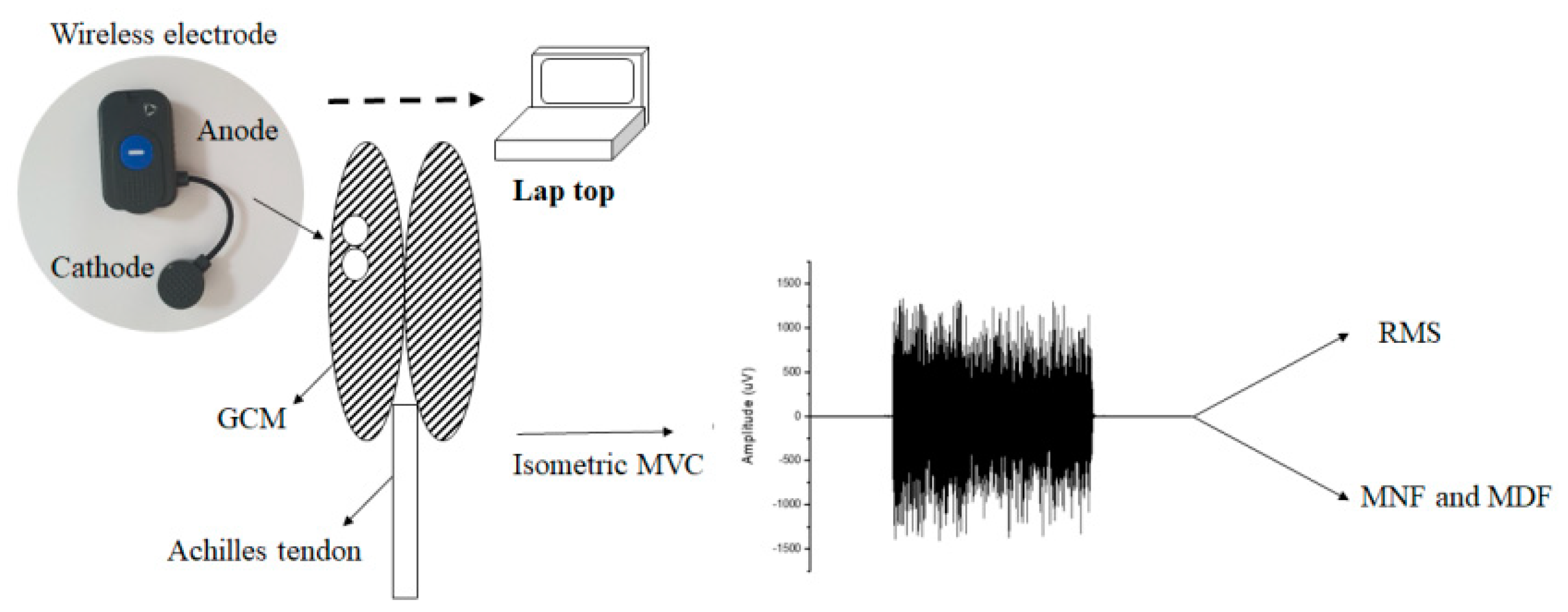
2.2. Exercise Protocol
2.3. Signal Processing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shin, Y.S.; Kim, M.O.; Kim, C.H.; Nam, M.S. Relation of Nerve Conduction Study and Physical Parameters in Diabetic Polyneuropathy. J. Korean Acad. Rehabil. Med. 2009, 33, 112–117. [Google Scholar]
- Juster-Switlyk, K.; Smith, A.G. Updates in diabetic peripheral neuropathy. F1000Research 2016, 5, 738. [Google Scholar] [CrossRef]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.; Kulkarni, J.; Boulton, A.J. Prevalence and Characteristics of Painful Diabetic Neuropathy in a Large Community-Based Diabetic Population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassen, C.S.; Jakobsen, J.; Andersen, H. Muscle Weakness: A Progressive Late Complication in Diabetic Distal Symmetric Polyneuropathy. Diabetes 2006, 55, 806–812. [Google Scholar]
- Fritschi, C.; Quinn, L. Fatigue in patients with diabetes: A review. J. Psychosom. Res. 2010, 69, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, C.H.; Kim, S.H.; Jeong, H.J.; Kim, M.O. Relationship of Diabetic Polyneuropathy Severity with Various Balance Parameters. J. Korean Acad. Rehabil. Med. 2010, 34, 550–553. [Google Scholar]
- Perry, B.D.; Caldow, M.; Brennan-Speranza, T.C.; Sbaraglia, M.; Jerums, G.; Garnham, A.; Wong, C.; Levinger, P.; Haq, M.A.U.; Hare, D.L.; et al. Muscle atrophy in patients with Type 2 Diabetes Mellitus: Roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2016, 22, 94–109. [Google Scholar]
- Singh, R.; Teel, C.; Sabus, C.; McGinnis, P.; Kluding, P. Fatigue in Type 2 Diabetes: Impact on Quality of Life and Predictors. PLoS ONE 2016, 11, e0165652. [Google Scholar] [CrossRef]
- Devulapally, Y.; Negi, D.; Pasula, K. A comparative study of skeletal muscle fatigue in diabetic and non-diabetic human beings. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1529. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, A.; Ma, G.; Ye, Y.; Li, R.; Lu, T. A Comparative Study of EMG Indices in Muscle Fatigue Evaluation Based on Grey Relational Analysis during All-Out Cycling Exercise. BioMed Res. Int. 2018, 2018, 9341215. [Google Scholar] [CrossRef] [Green Version]
- Kuthe, C.D.; Uddanwadiker, R.V.; Ramteke, A.A. Surface electromyography based method for computing muscle strength and fatigue of biceps brachii muscle and its clinical implementation. Inform. Med. Unlocked 2018, 12, 34–43. [Google Scholar] [CrossRef]
- Aragón-Vela, J.; Barranco-Ruiz, Y.; Casals-Vázquez, C.; Plaza-Díaz, J.; Casuso, R.A.; Fontana, L.; Huertas, J.F.R. A novel electromyographic approach to estimate fatigue threshold in maximum incremental strength tests. Motor Control 2018, 22, 170–182. [Google Scholar] [CrossRef]
- Wang, R.; Fukuda, D.H.; Stout, J.R.; Robinson, E.H.; Miramonti, A.A.; Townsend, J.R.; Mangine, G.T.; Jajtner, A.R.; Wells, A.J.; Gonzalez, A.M.; et al. Evaluation of EMG Frequency Domain Changes during a Three-Minute Maximal Effort Cycling Test. Med. Sci. Sports Exerc. 2014, 46, 939. [Google Scholar] [CrossRef]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojic, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef]
- Lien, A.S.; Hwang, J.; Jiang, Y. Diabetes related fatigue sarcopenia, frailty. J. Diabetes Investig. 2017, 9, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyck, P.J.; O’Brien, P.C.; Litchy, W.J.; Harper, C.M.; Daube, J.R.; Dyck, P.J.B. Use of percentiles and normal deviates to express nerve conduction and other test abnormalities. Muscle Nerve 2001, 24, 307–310. [Google Scholar] [CrossRef]
- Dyck, P.J.; Litchy, W.J.; Daube, J.R.; Harper, C.M.; Dyck, P.J.B.; Davies, J.; O’Brien, P.C. Individual attributes versus composite scores of nerve conduction abnormality: Sensitivity, reproducibility, and concordance with impairment. Muscle Nerve 2003, 27, 202–210. [Google Scholar] [CrossRef]
- Vieira, T.M.; Botter, A.; Muceli, S.; Farina, D. Specificity of surface EMG recordings for gastrocnemius during upright standing. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, B.; Lu, J.; Jiang, L.; Zhang, Y.; Shen, Y.; Wang, C.; Jia, W. Serum albumin is associated with peripheral nerve function in patients with type 2 diabetes. Endocrine 2015, 50, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Przybilla, M.; Koniar, B.; Whitley, C.B. RTB lectin-mediated delivery of lysosomal α-L-iduronidase mitigates disease manifestations systemically including the central nervous system. Mol. Genet. Metab. 2018, 123, 105–111. [Google Scholar] [CrossRef]
- Aggarwal, H.K.; Sood, S.; Jain, D.; Kaverappa, V.; Yadav, S. Evaluation of spectrum of peripheral neuropathy in predialysis patients with chronic kidney disease. Ren. Fail. 2013, 35, 1323–1329. [Google Scholar] [CrossRef]
- Allen, M.D.; Kimpinski, K.; Doherty, T.J.; Rice, C.L. Length dependent loss of motor axons and altered motor unit properties in human diabetic polyneuropathy. Clin. Neurophysiol. 2014, 125, 836–843. [Google Scholar] [CrossRef]
- Javed, S.; Petropoulos, I.N.; Alam, U.; Malik, R. Treatment of painful diabetic neuropathy. Ther. Adv. Chronic Dis. 2014, 6, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.K. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, S.; Khobragade, A.; Langade, D. Methylcobalamin, Pyridoxine and Nicotinamide in Diabetic Neuropathy: A Review. Indian J. Clin. Pract. 2009, 20, 17–21. [Google Scholar]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.-M.; Hahm, J.-R.; Kim, T.-K.; Choi, W.-H. Factors Affecting Fatigue in Patients with Type II Diabetes Mellitus in Korea. Asian Nurs. Res. 2015, 9, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garssen, M.P.J.; Van Doorn, P.A.; Visser, G.H. Nerve conduction studies in relation to residual fatigue in Guillain-Barré syndrome. J. Neurol. 2006, 253, 851–856. [Google Scholar] [CrossRef]
- Gerdle, B.; Karlsson, S.; Crenshaw, A.G.; Elert, J.; Fridén, J. The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 81, 2–10. [Google Scholar] [CrossRef] [PubMed]
- King, W.; Kissel, J.T.; Montes, J.; De Vivo, D.C.; Finkel, R.S. Six-Minute Walk Test Demonstrates Motor Fatigue in Spinal Muscular Atrophy. Neurology 2010, 75, 1121–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Median Motor Nerve | Median Sensory Nerve | Ulnar Motor Nerve | Ulnar Sensory Nerve |
| L > 4.0 ms | L > 3.5 ms | L > 3.8 ms | L > 3.4 ms |
| A < 5.0 mV | A < 10.0 μV | A < 5.0 mV | A < 7.5 μV |
| CV < 49.0 m/s | CV < 49.0 m/s | ||
| MF > 24.2 ms | MF > 24.8 ms | ||
| Peroneal Motor Nerve | Superficial Peroneal Sensory Nerve | Tibial Motor Nerve | Sural Sensory Nerve |
| L > 4.5 ms | L > 3.5 ms | L > 5.0 ms | L > 3.5 ms |
| A < 1.0 mV | A < 3.7 μV | A < 5.0 mV | A < 5.0 μV |
| CV < 40.0 m/s | CV < 40.0 m/s | ||
| MF > 45.0 ms | MF > 45.3 ms |
| Characteristic | DM − DN (n = 10) | DM + DN (n = 15) | p-Value |
|---|---|---|---|
| Sex, men/women | 5/5 | 8/7 | |
| Age (years) | 45.67 ± 11.44 | 48.00 ± 16.24 | 0.615 |
| Duration of DM, years | 5.23 ± 5.89 | 10.43 ± 6.47 | 0.066 |
| Height (cm) | 165.63 ±11.24 | 166.16 ± 8.20 | 0.897 |
| Weight, kg | 76.09 ± 18.88 | 68.99 ± 19.18 | 0.394 |
| BMI (kg/m2) | 27.62 ± 5.78 | 24.73 ± 5.33 | 0.233 |
| Albumin (g/dL) | 4.52 ± 0.35 | 4.01 ± 0.425 | 0.007 * |
| Creatinine (mg/dL) | 0.96 ± 0.45 | 2.09 ± 3.35 | 0.328 |
| HbA1c (%) | 10.29 ± 2.48 | 10.35 ± 3.30 | 0.963 |
| FPG (mg/dL) | 192.33 ± 61.22 | 191.57 ± 91.71 | 0.983 |
| TG (mg/dL) | 180.56 ± 70.47 | 200.50 ± 170.85 | 0.744 |
| HDL-cholesterol (mg/dL) | 46.56 ± 8.23 | 45.71 ± 14.70 | 0.878 |
| LDL-cholesterol (mg/dL) | 106.00 ± 38.12 | 105.38 ± 39.71 | 0.968 |
| Characteristic | DM − DN (n = 10) | DM + DN (n = 15) | p-Value |
|---|---|---|---|
| CS | 0.07 ± 0.10 | 0.70 ± 0.45 | 0.000 * |
| RMS (μV) | 191.79 ± 103.79 | 212.53 ± 86.23 | 0.608 |
| MDF (Hz) | 175.10 ± 37.38 | 131.99 ± 45.85 | 0.028 * |
| MNF (Hz) | 190.88 ± 40.48 | 147.27 ± 44.13 | 0.027 * |
| Components | Coefficient | CS | RMS (μV) | MDF (Hz) | MNF (Hz) |
|---|---|---|---|---|---|
| BMI | r | −0.334 | 0.248 | −0.061 | −0.086 |
| Creatinine | r | 0.601 * | 0.172 | −0.424 | −0.418 |
| Albumin | r | −0.447 | 0.264 | −0.229 | −0.204 |
| FPG | r | 0.134 | 0.308 | 0.581 * | 0.628 * |
| TG | r | −0.204 | −0.376 | 0.313 | 0.324 |
| HDL | r | 0.522 | −0.015 | −0.173 | −0.136 |
| LDL | r | 0.224 | 0.239 | 0.009 | 0.089 |
| HbA1c | r | 0.224 | 0.118 | 0.668 * | 0.672 * |
| Duration | r | 0.596 * | 0.133 | −0.794 * | −0.813 * |
| CS | r | - | −0.265 | −0.354 | −0.298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Park, C.H. Diagnosis of Muscle Fatigue Using Surface Electromyography and Analysis of Associated Factors in Type 2 Diabetic Patients with Neuropathy: A Preliminary Study. Int. J. Environ. Res. Public Health 2021, 18, 9635. https://doi.org/10.3390/ijerph18189635
Park SY, Park CH. Diagnosis of Muscle Fatigue Using Surface Electromyography and Analysis of Associated Factors in Type 2 Diabetic Patients with Neuropathy: A Preliminary Study. International Journal of Environmental Research and Public Health. 2021; 18(18):9635. https://doi.org/10.3390/ijerph18189635
Chicago/Turabian StylePark, So Young, and Chan Hyuk Park. 2021. "Diagnosis of Muscle Fatigue Using Surface Electromyography and Analysis of Associated Factors in Type 2 Diabetic Patients with Neuropathy: A Preliminary Study" International Journal of Environmental Research and Public Health 18, no. 18: 9635. https://doi.org/10.3390/ijerph18189635
APA StylePark, S. Y., & Park, C. H. (2021). Diagnosis of Muscle Fatigue Using Surface Electromyography and Analysis of Associated Factors in Type 2 Diabetic Patients with Neuropathy: A Preliminary Study. International Journal of Environmental Research and Public Health, 18(18), 9635. https://doi.org/10.3390/ijerph18189635






