Enhanced Denitrification of Integrated Sewage Treatment System by Supplementing Denitrifying Carbon Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Release Experiments
2.2.2. Denitrification Performance of Carbon Source
Selection of Optimal Carbon Source Dosage
Determination of Denitrification Performance
2.2.3. Enhanced Denitrification Effect of ISTY by Supplementing Carbon Sources
2.2.4. Economic Benefit Evaluation of Adding Carbon Source in Denitrification Stage of ISTY
2.3. Characterization and Analytical Methods
2.4. Data Analysis Method
3. Results and Discussion
3.1. Release Experiments
3.1.1. Carbon Source Element Content Analysis
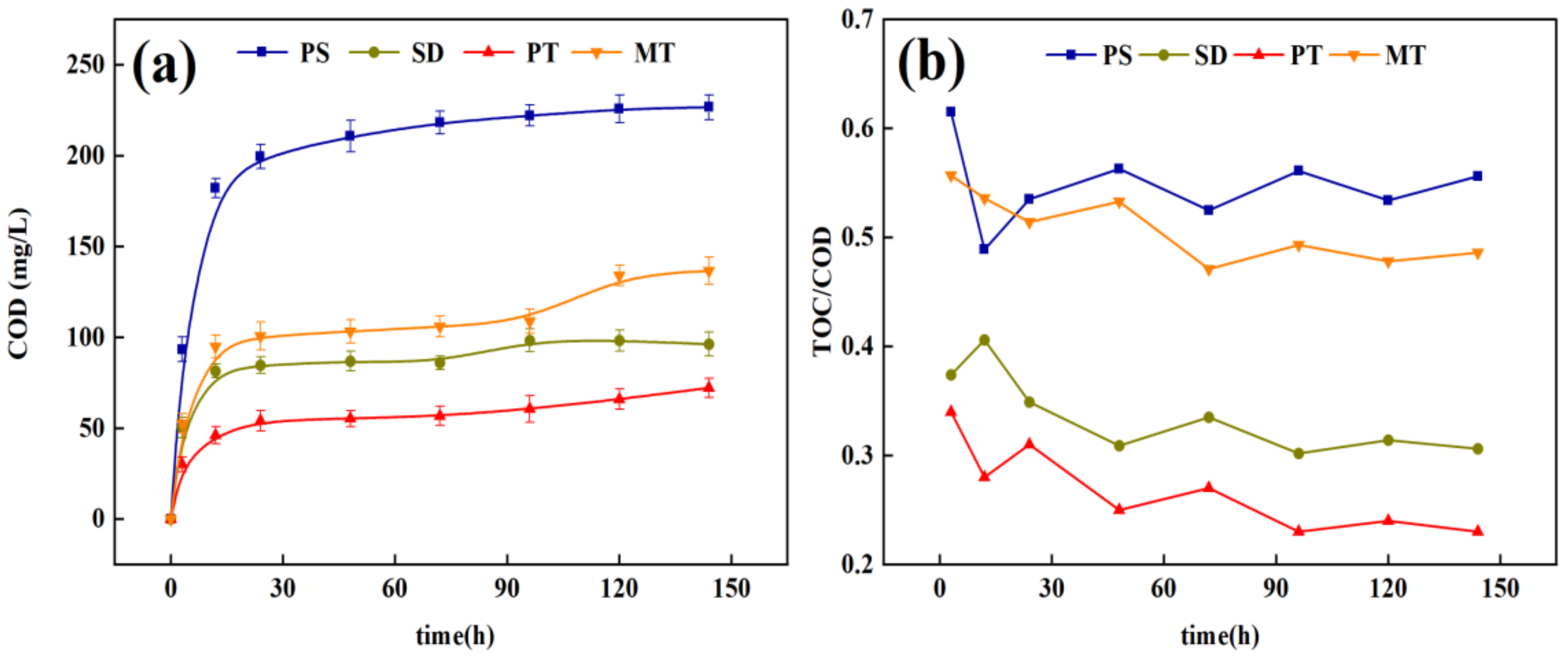
3.1.2. Carbon Release Performance of Carbon Source
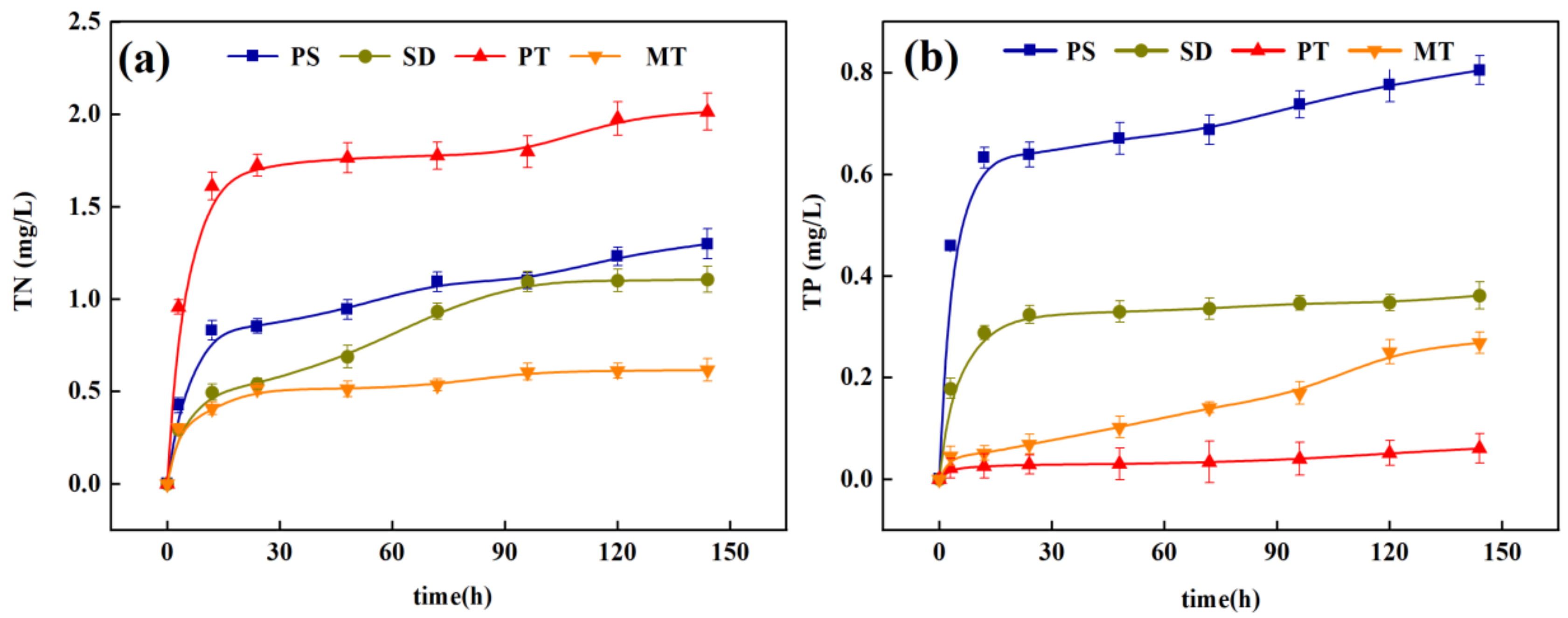
3.1.3. Release of Nitrogen and Phosphorus from Carbon Sources
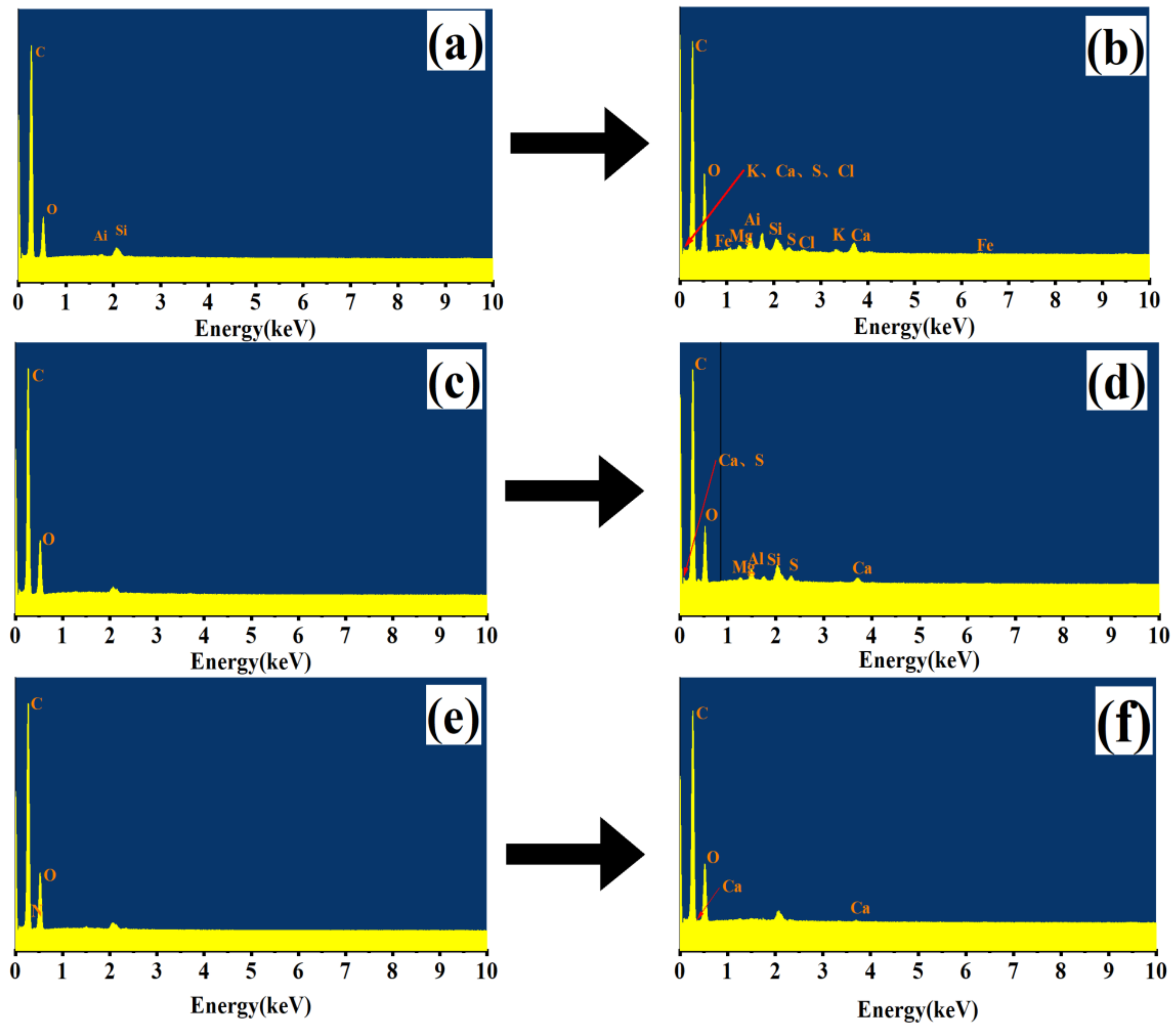
3.1.4. FT IR Characteristics of Carbon Source before and after Carbon Release
3.2. Denitrification Performance of Carbon Source
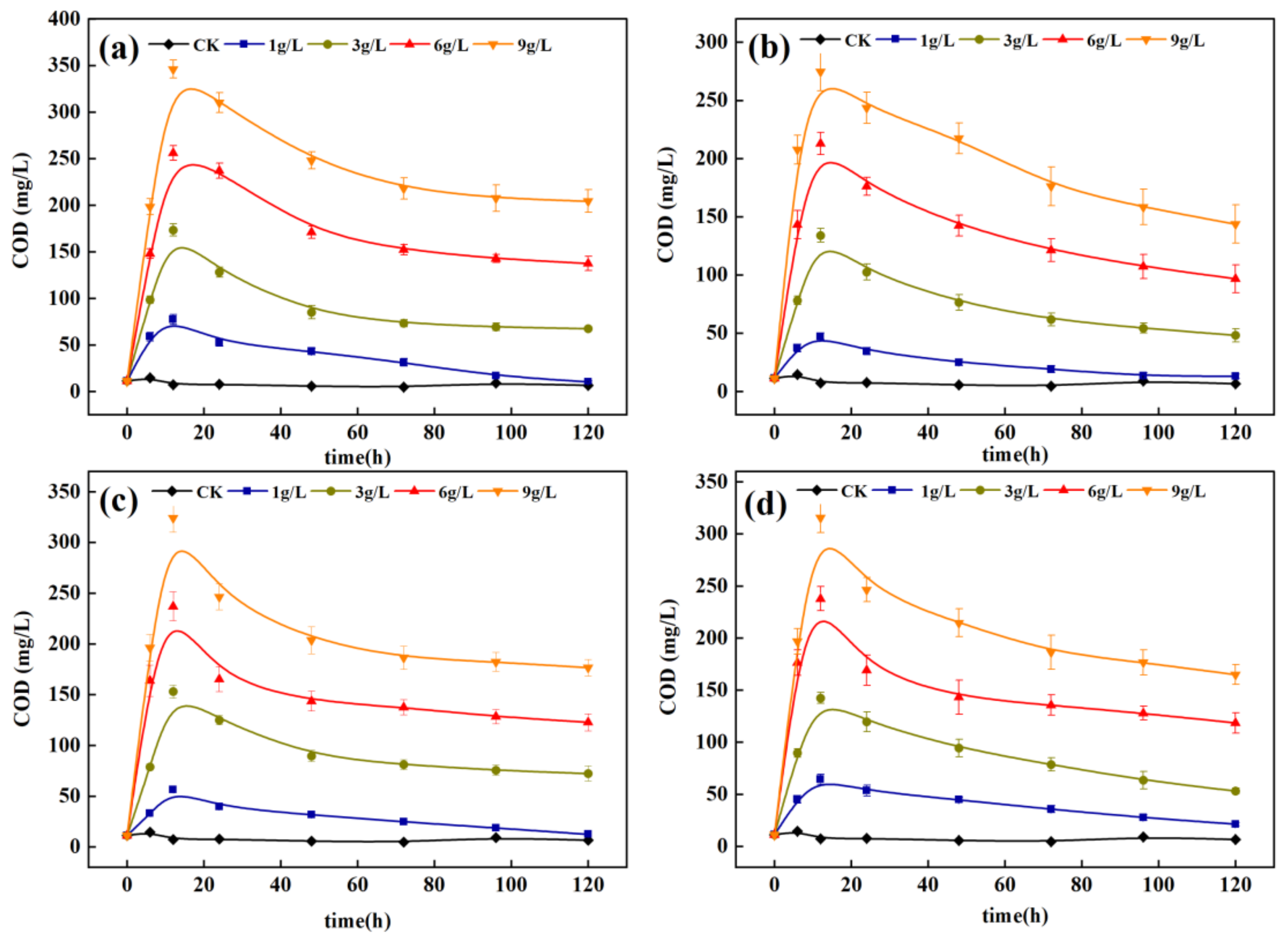
3.2.1. Screening of Carbon Source Dosage
3.2.2. Denitrification Performance
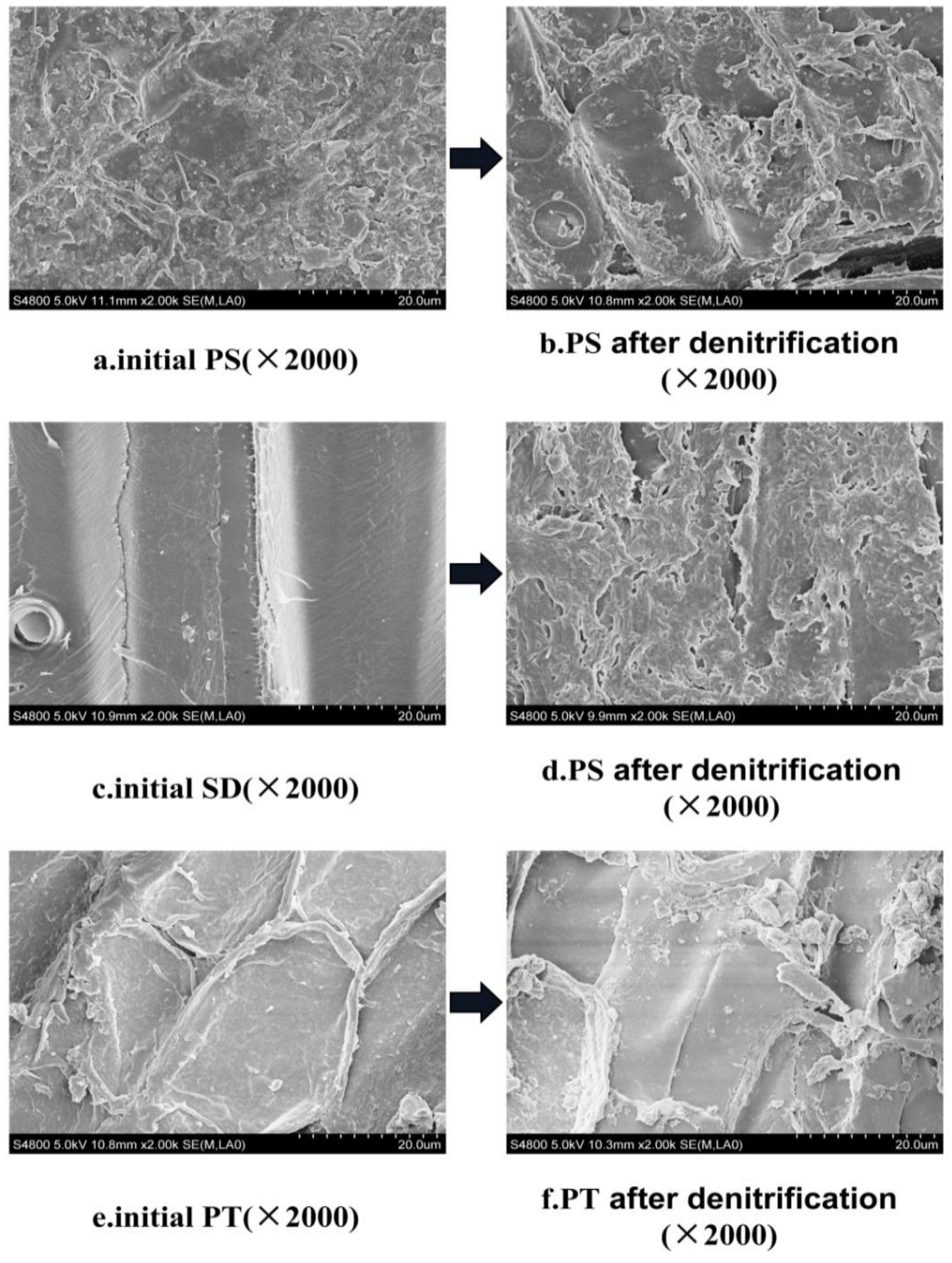
3.2.3. Characteristics of Surface Structure Change of Carbon Source
3.3. Enhanced Denitrification Effect of ISTY by Supplementing Carbon Sources
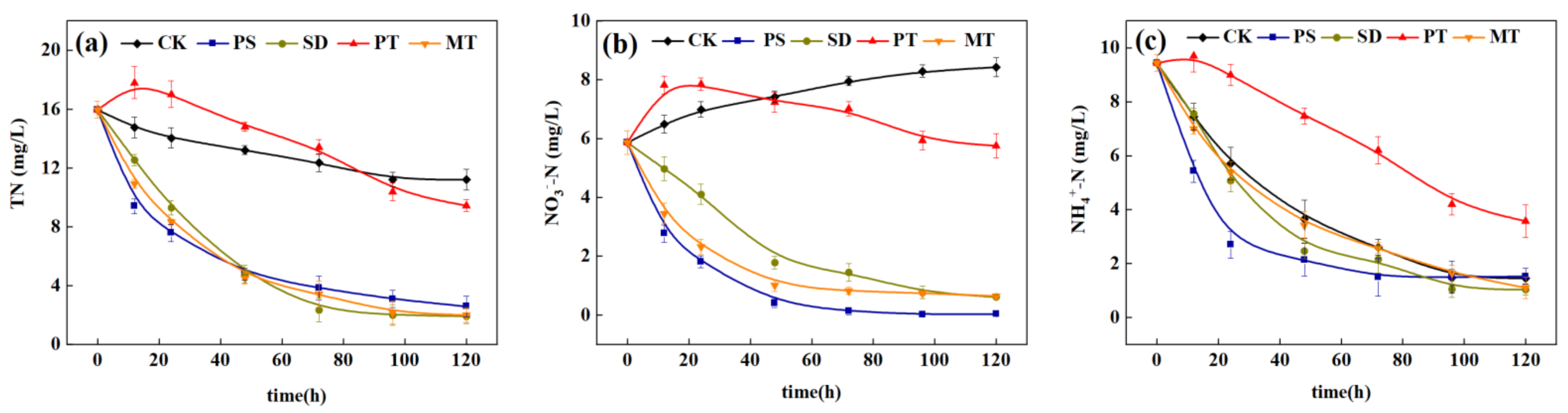
3.3.1. Effect of Carbon Source Supplement on Denitrification Enhancement of ISTY
3.3.2. Effects of Carbon Source Supplementation on Denitrifying Microorganisms
3.3.3. Economic Benefit Analysis of Adding Carbon Source in Denitrification Stage of ISTY
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Zhang, S.; Huang, D.; Zhang, L.; Zhang, J. A pilot-scale deep bed denitrification filter for secondary effluent treatment using sodium acetate as external carbon. Water Environ. Res. 2019, 91, 491–499. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, Y.; Guo, Y.; Zhang, R.; Pan, Y.; Zhou, T. A novel additional carbon source derived from rotten fruits: Application for the denitrification from mature landfill leachate and evaluation the economic benefits. Bioresour. Technol. 2021, 334, 125244. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, A.; Fang, D.; Zhang, Y.; Shen, Q.; Xu, X.; Ji, F. Denitrification performance and microbial diversity using starch-polycaprolactone blends as external solid carbon source and biofilm carriers for advanced treatment. Chemosphere 2020, 255, 126901. [Google Scholar] [CrossRef] [PubMed]
- Hagman, M.; Nielsen, J.; Nielsen, P.; Jansen, J. Mixed carbon sources for nitrate reduction in activated sludge-identification of bacteria and process activity studies. Water Res. 2008, 42, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, B.; Li, X.; Zhang, Q.; Wu, L.; He, Y.; Peng, Y. Achieving partial nitrification in a continuous post-denitrification reactor treating low C/N sewage. Chem. Eng. J. 2018, 335, 330–337. [Google Scholar] [CrossRef]
- Hua, G.; Salo, M.; Schmit, C.; Hay, C. Nitrate and phosphate removal from agricultural subsurface drainage using laboratory woodchip bioreactors and recycled steel byproduct filters. Water Res. 2016, 102, 180–189. [Google Scholar] [CrossRef]
- Liang, X.; Lin, L.; Ye, Y.; Gu, J.; Wang, Z.; Xu, L.; Jin, Y.; Ru, Q.; Tian, G. Nutrient removal efficiency in a rice-straw denitrifying bioreactor. Bioresour. Technol. 2015, 198, 746–754. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Q.; Song, H.; Gu, T.; Xia, M. Selection and application of agricultural wastes as solid carbon sources and biofilm carriers in MBR. J. Hazard. Mater. 2015, 283, 186–192. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, L.; Wei, C.; Wu, W.; Zhao, X.; Lu, T. Enhanced nitrogen removal using solid carbon source in constructed wetland with limited aeration. Bioresour. Technol. 2018, 248, 98–103. [Google Scholar] [CrossRef]
- Ling, Y.; Yan, G.; Wang, H.; Dong, W.; Wang, H.; Chang, Y.; Chang, M.; Li, C. Release Mechanism, Secondary Pollutants and Denitrification Performance Comparison of Six Kinds of Agricultural Wastes as Solid Carbon Sources for Nitrate Removal. Int. J. Environ. Res. Public Health 2021, 18, 1232. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Q.; Yu, P.; Zhang, L.; Ye, L.; Zhang, X.; Ren, H. Denitrification using excess activated sludge as carbon source: Performance and the microbial community dynamics. Bioresour. Technol. 2017, 238, 624–632. [Google Scholar] [CrossRef]
- Li, L.; Yan, G.; Wang, H.; Chu, Z.; Li, Z.; Ling, Y.; Wu, T. Denitrification and microbial community in MBBR using A. donax as carbon source and biofilm carriers for reverse osmosis concentrate treatment. J. Environ. Sci. 2019, 84, 133–143. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Jiang, X.; Zhou, P.; Zhang, J.; Zhang, Z. Treatment of artificial secondary effluent for effective nitrogen removal using a combination of corncob carbon source and bamboo charcoal filter. Int. Biodeterior. Biodegrad. 2016, 115, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, H.; Dong, W.; Chang, Y.; Yan, G.; Chu, Z.; Ling, Y.; Wang, Z.; Fan, T.; Li, C. Nitrogen removal and microbial community for the treatment of rural domestic sewage with low C/N ratio by A/O biofilter with Arundo donax as carbon source and filter media. J. Water Process. Eng. 2020, 37, 101509. [Google Scholar] [CrossRef]
- Xiong, R.; Yu, X.; Zhang, Y.; Peng, Z.; Yu, L.; Cheng, L.; Li, T. Comparison of agricultural wastes and synthetic macromolecules as solid carbon source in treating low carbon nitrogen wastewater. Sci. Total Environ. 2020, 739, 139885. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.; Peppas, N. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Vavilin, V.; Rytov, S. Nitrate denitrification with nitrite or nitrous oxide as intermediate products: Stoichiometry, kinetics and dynamics of stable isotope signatures. Chemosphere 2015, 134, 417–426. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Chao, H.; Lee, M.; Shin, J.; Kim, E.; Kim, Y. Lignin fractionation from waste wood using organosolv treatment combined with membrane filtration. Membr. Water Treat. 2020, 11, 25–29. [Google Scholar]
- Zou, W.; Yu, L.; Liu, X.; Chen, L.; Zhang, X.; Qiao, D.; Zhang, R. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 2012, 87, 1583–1588. [Google Scholar] [CrossRef]
- Le, P.; Nguyen, D.; Aburto-Medina, A.; Linklater, D.; Crawford, R.; MacLaughlin, S.; Ivanova, E. Nanoscale Surface Roughness Influences Candida albicans Biofilm Formation. ACS Appl. Bio Mater. 2020, 3, 8581–8591. [Google Scholar] [CrossRef]
- Kulikova, N.; Filippova, O.; Volikov, A.; Perminova, I. Slow nitrogen release from humic substances modified with aminoorganosilanes. J. Soils Sediments 2018, 18, 1400–1408. [Google Scholar] [CrossRef]
- He, X.; Sun, Q.; Xu, T.; Dai, M.; Wei, D. Removal of nitrogen by heterotrophic nitrification–aerobic denitrification of a novel halotolerant bacterium Pseudomonas mendocina TJPU04. Bioprocess Biosyst. Eng. 2019, 42, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Yang, K.; Wang, H.; Zhou, J.; Zhang, H. Aerobic denitrification by Pseudomonas stutzeri C3 incapable of heterotrophic nitrification. Bioprocess Biosyst. Eng. 2015, 38, 407–409. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.; Peng, F.; Zhang, A.; Xie, X.; Wang, Z.; Wang, X.; Lian, J.; Ni, L.; Cui, Y.; et al. Spatiotemporal distribution and interaction of denitrifying functional genes in a novel DAS-NUA biofilter used for groundwater nitrate treatment. Sci. Total Environ. 2020, 712, 136595. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lv, Y.; Liu, Y.; Ren, R. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp. S1. Bioresour. Technol. 2016, 220, 142–150. [Google Scholar] [CrossRef]
- Kirchman, D. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002, 39, 91–100. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Rusanowska, P.; Zielińska, M.; Bernat, K. Structure of nitrogen-converting communities induced by hydraulic retention time and COD/N ratio in constantly aerated granular sludge reactors treating digester supernatant. Bioresour. Technol. 2014, 154, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, X.; Wen, X.; Xia, Y. Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis. Bioresour. Technol. 2012, 117, 72–79. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, F.; Yao, S.; Liu, Y.; Chen, J. Application of Pseudomonas flava WD-3 for sewage treatment in constructed wetland in winter. Environ. Technol. 2015, 36, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S. Biodegradation of polychlorinated biphenyls (PCBs), Aroclor 1260, in wastewater by isolate MD2 (Pseudomonas aeruginosa) from wastewater from Notwane sewage treatment plant in Gaborone, Botswana. J. Bioremed. Biodegred. 2014, 5, 1. [Google Scholar]
- Su, J.; Xue, L.; Huang, T.; Wang, Z.; Wang, J. Kinetic analysis of denitrification coupled with Cd(II) removal by Cupriavidus sp. CC1 and its removal mechanism. Res. Microbiol. 2019, 170, 214–221. [Google Scholar] [CrossRef] [PubMed]





| Dispensing Agent | Dosage (g/L) | Trace Element Liquid Composition | Concentration (g/L) | Water Quality Index | Concentration (g/L) |
|---|---|---|---|---|---|
| KNO3 | 0.06 | FeCl3 | 0.8 | NO3−-N | 19.17 ± 0.96 |
| KH2PO4 | 0.02 | KI | 0.18 | NH4+-N | 0.54 ± 0.07 |
| MgSO4 | 0.003 | CuSO4·5H2O | 0.03 | TN | 20.14 ± 0.74 |
| NaCl | 0.03 | ZnCl2 | 0.06 | TP | 4.73 ± 0.13 |
| trace element | 2 mg/L | CoCl2·7H2O | 0.15 | COD | 11.20 ± 1.21 |
| Water Quality Index | TN | NO3−-N | NH4+-N | TP | COD |
|---|---|---|---|---|---|
| Concentration (g/L) | 19.6 ± 0.67 | 5.9 ± 0.42 | 9.4 ± 0.36 | 4.6 ± 0.14 | 69.6 ± 5.13 |
| Material | w% | |||
|---|---|---|---|---|
| C | N | P | S | |
| PS | 44.18 ± 1.22 | 1.20 ± 0.07 | 0.18 ± 0.02 | 0.30 ± 0.05 |
| SD | 48.92 ± 0.86 | 0.22 ± 0.03 | 0.17 ± 0.04 | 0.16 ± 0.04 |
| PT | 50.04 ± 1.52 | 2.27 ± 0.08 | 0.34 ± 0.06 | 0.39 ± 0.08 |
| Material | First-Order | Ritger-Peppas | ||||
|---|---|---|---|---|---|---|
| Fitting Equation | R2 | k | Fitting Equation | R2 | N | |
| PS | ] | 0.99 | 0.16 | 0.94 | 0.16 | |
| SD | ] | 0.97 | 0.25 | 0.96 | 0.13 | |
| PT | 0.91 | 0.16 | 0.98 | 0.19 | ||
| MT | 0.90 | 0.17 | 0.95 | 0.19 | ||
| Material | NO3−-N | TN | ||
|---|---|---|---|---|
| PS | R2 = 0.99 | ] | R2 = 0.97 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Chen, P.; Zheng, X.; Cheng, W.; Wang, Q.; Wei, X. Enhanced Denitrification of Integrated Sewage Treatment System by Supplementing Denitrifying Carbon Source. Int. J. Environ. Res. Public Health 2021, 18, 9569. https://doi.org/10.3390/ijerph18189569
Chen D, Chen P, Zheng X, Cheng W, Wang Q, Wei X. Enhanced Denitrification of Integrated Sewage Treatment System by Supplementing Denitrifying Carbon Source. International Journal of Environmental Research and Public Health. 2021; 18(18):9569. https://doi.org/10.3390/ijerph18189569
Chicago/Turabian StyleChen, Dongkai, Peizhen Chen, Xiangqun Zheng, Weimin Cheng, Qiang Wang, and Xiaocheng Wei. 2021. "Enhanced Denitrification of Integrated Sewage Treatment System by Supplementing Denitrifying Carbon Source" International Journal of Environmental Research and Public Health 18, no. 18: 9569. https://doi.org/10.3390/ijerph18189569
APA StyleChen, D., Chen, P., Zheng, X., Cheng, W., Wang, Q., & Wei, X. (2021). Enhanced Denitrification of Integrated Sewage Treatment System by Supplementing Denitrifying Carbon Source. International Journal of Environmental Research and Public Health, 18(18), 9569. https://doi.org/10.3390/ijerph18189569






