Wastewater Valorization: Practice around the World at Pilot- and Full-Scale
Abstract
:1. Introduction
- (1)
- Develop wastewater initiatives as part of a basin planning framework to maximize benefits, improve efficiency and resource allocation, and engage stakeholders;
- (2)
- Build utilities of the future by shifting away from WWTPs to WRRFs, thus realizing the value of wastewater;
- (3)
- Explore and support the development of innovative financing and sustainable business models in the water sector;
- (4)
- Implement the necessary policy, institutional, and regulatory frameworks to promote the paradigm shift.
2. Water
2.1. Municipal Wastewater Reuse
2.2. Industrial Wastewater Reuse
2.3. Water Reuse Legislation
3. Biomass
3.1. Microalgae Biomass
3.2. Single Cell Protein
4. Nutrients and Fertilizers
5. Energy
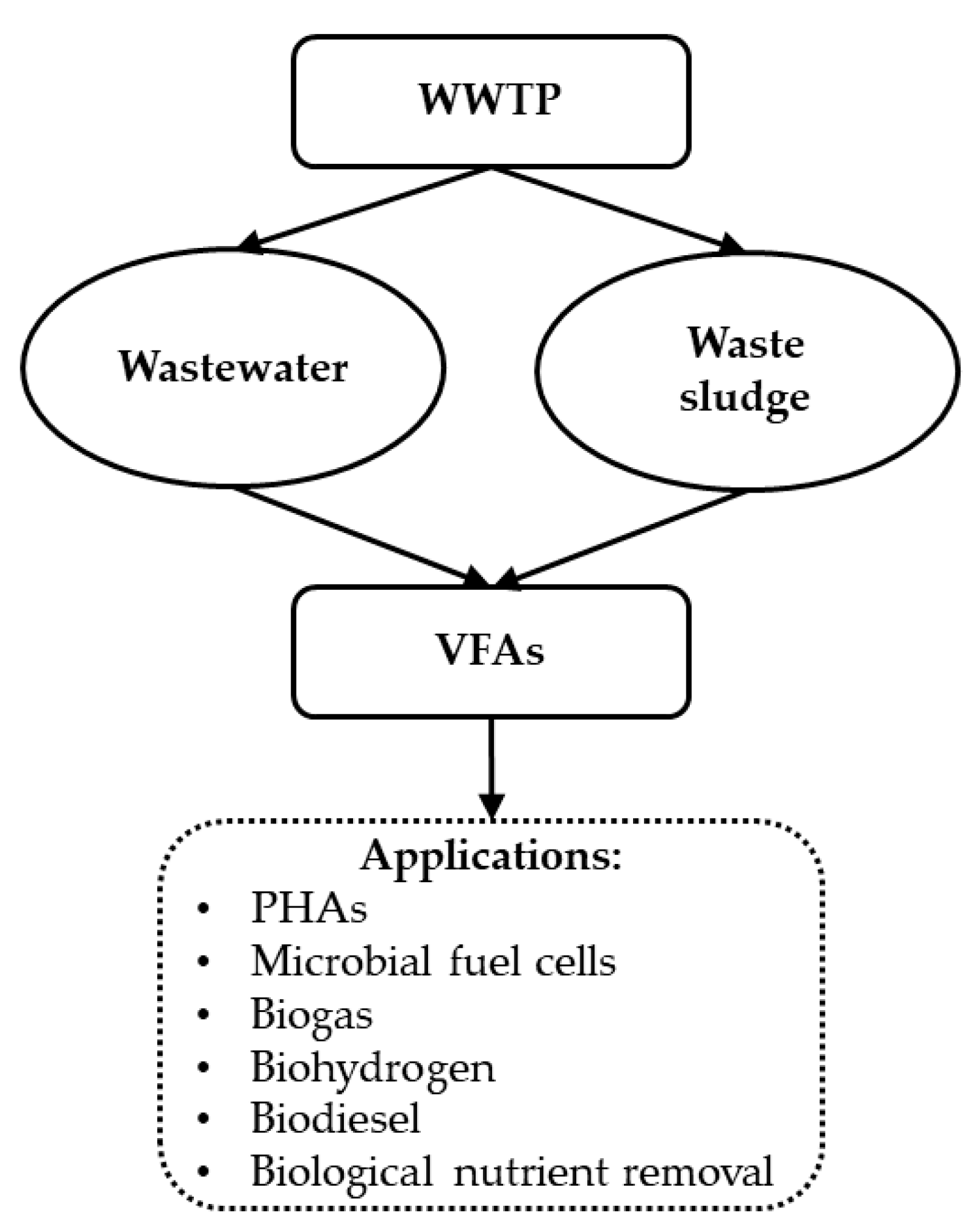
6. Volatile Fatty Acids
7. Polyhydroxyalkanoates
8. Extracellular Polymeric Substances
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- OECD (Ed.) OECD Environmental Outlook to 2050; OECD Environmental Outlook; OECD: Paris, France, 2012; ISBN 9789264122161. [Google Scholar]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants-market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef] [Green Version]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Gude, V.G. Energy and water autarky of wastewater treatment and power generation systems. Renew. Sustain. Energy Rev. 2015, 45, 52–68. [Google Scholar] [CrossRef]
- Rodriguez, D.J.; Serrano, H.A.; Delgado, A.; Nolasco, D.; Saltiel, G. From Waste to Resource—Shifting Paradigms for Smarter Wastewater Interventions in Latin America and the Caribbean; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Smith, K.M.; Fowler, G.D.; Pullket, S.; Graham, N.J.D. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, S.; Cipolletta, G.; Pastore, C.; Giosuè, C.; Akyol, Ç.; Eusebi, A.L.; Frison, N.; Tittarelli, F.; Fatone, F. Pilot scale cellulose recovery from sewage sludge and reuse in building and construction material. Waste Manag. 2019, 100, 208–218. [Google Scholar] [CrossRef]
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valorization 2021, 12, 4185–4211. [Google Scholar] [CrossRef]
- Golnaraghi Ghomi, A.; Asasian-Kolur, N.; Sharifian, S.; Golnaraghi, A. Biosorpion for sustainable recovery of precious metals from wastewater. J. Environ. Chem. Eng. 2020, 8, 103996. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW)—Managing Systems at Risk; The Food and Agriculture Organization of the United Nations and Earthscan: Rome, Italy; London, UK, 2011; ISBN 9781849713269. [Google Scholar]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated wastewater reuse for irrigation: Pros and cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef]
- European Commission. Study to support the Commission’s Impact Assessment of an EU level instrument on water reuse-Final report EU-level instruments on water reuse. In Final Report to Support the Commission’s Impact Assessment; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Lafforgue, M. Supplying water to a water-stressed city: Lessons from Windhoek. La Houille Blanche 2016, 102, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Fluence-News-Team Israel Leads World in Water Recycling. Available online: https://www.fluencecorp.com/israel-leads-world-in-water-recycling (accessed on 13 August 2021).
- Messing, A.; Sela, Y. SHAFDAN (Greater Tel Aviv Wastewater Treatment Plant): Recent upgrade and expansion. Water Pract. Technol. 2016, 11, 288–297. [Google Scholar] [CrossRef]
- Holmgren, K.E.; Li, H.; Verstraete, W.; Cornel, P. State of the Art Compendium Report on Resource Recovery from Water; IWA: London, UK, 2016. [Google Scholar]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- SSWM Wastewater Reuse in Industry. Available online: https://sswm.info/water-nutrient-cycle/water-use/hardwares/optimisation-water-use-industries/wastewater-reuse-in-industry (accessed on 13 August 2021).
- Aich, N.; Nama, S.; Biswal, A.; Paul, T. A review on recirculating aquaculture systems: Challenges and opportunities for sustainable aquaculture. Inno. Farm. 2020, 5, 17–24. [Google Scholar]
- Verhuelsdonk, M.; Glas, K.; Parlar, H. Economic evaluation of the reuse of brewery wastewater. J. Environ. Manag. 2021, 281, 111804. [Google Scholar] [CrossRef]
- Vergine, P.; Salerno, C.; Libutti, A.; Beneduce, L.; Gatta, G.; Berardi, G.; Pollice, A. Closing the water cycle in the agro-industrial sector by reusing treated wastewater for irrigation. J. Clean. Prod. 2017, 164, 587–596. [Google Scholar] [CrossRef]
- Fraga, F.A.; García, H.A.; Hooijmans, C.M.; Míguez, D.; Brdjanovic, D. Evaluation of a membrane bioreactor on dairy wastewater treatment and reuse in Uruguay. Int. Biodeterior. Biodegrad. 2017, 119, 552–564. [Google Scholar] [CrossRef]
- Murray, F.; Bostock, J.; Fletcher, D. Review of Recirculation Aquaculture System Technologies and Their Commercial Application. Final Report March 2014; Highlands and Islands Enterprise: Inverness, UK, 2014. [Google Scholar]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Bauer, S.; Linke, H.J.; Wagner, M. Combining industrial and urban water-reuse concepts for increasing the water resources in water-scarce regions. Water Environ. Res. 2020, 92, 1027–1041. [Google Scholar] [CrossRef]
- European Commission. EU-Level Instruments on Water Reuse—Final Report to Support the Commission’s Impact Assessment; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Water Reuse Europe Water Reuse Europe—Policy and Regulations. Available online: https://ec.europa.eu/environment/water/blueprint/pdf/EU_level_instruments_on_water-2nd-IA_support-study_AMEC.pdf (accessed on 13 August 2021).
- Dingemans, M.M.L.; Smeets, P.W.M.H.; Medema, G.; Frijns, J.; Raat, K.J.; van Wezel, A.P.; Bartholomeus, R.P. Responsible water reuse needs an interdisciplinary approach to balance risks and benefits. Water 2020, 12, 1264. [Google Scholar] [CrossRef]
- EPA. Safe. Drinking Water ACT-(Title XIV of Public Health Service ACT); EPA: Washington, DC, USA, 1944. [Google Scholar]
- EPA. Clean Water Laws, Regulations, and Executive Orders related to Section 404; EPA: Washington, DC, USA, 1972. [Google Scholar]
- EPA. Guidelines for Water Reuse. EPA/600/R-12/618; EPA: Washington, DC, USA, 2012. [Google Scholar]
- EPA. Water Reuse Action Plan. Available online: https://www.epa.gov/waterreuse/water-reuse-action-plan (accessed on 13 August 2021).
- EPA. National Water Reuse Action Plan; EPA: Washington, DC, USA, 2020.
- Christian-Smith, J.; Gleick, P.H.; Ross, N.; Allen, L.; Cohen, M.J.; Schulte, P.; Smith, C. California Farm Water Success Stories; Pacific Institute: Oakland, CA, USA, 2010. [Google Scholar]
- Kirhensteine, I.; Cherrier, V.; Jarritt, N.; Farmer, A.; de Paoli, G.; Delacamara, G.; Psomas, A. EU-Level Instruments on Water Reuse; European Union: Luxembourg, 2016; ISBN 9789279626166. [Google Scholar]
- Radcliffe, J.C.; Page, D. Water reuse and recycling in Australia—History, current situation and future perspectives. Water Cycle 2020, 1, 19–40. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Text with EEA Relevance); European Union: Luxembourg, 2008. [Google Scholar]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef] [Green Version]
- Mu, R.; Jia, Y.; Ma, G.; Liu, L.; Hao, K.; Qi, F.; Shao, Y. Advances in the use of microalgal–bacterial consortia for wastewater treatment: Community structures, interactions, economic resource reclamation, and study techniques. Water Environ. Res. 2020, 93, 1217–1230. [Google Scholar] [CrossRef]
- Mantovani, M.; Marazzi, F.; Fornaroli, R.; Bellucci, M.; Ficara, E.; Mezzanotte, V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total Environ. 2020, 710, 135583. [Google Scholar] [CrossRef]
- Pizzera, A.; Scaglione, D.; Bellucci, M.; Marazzi, F.; Mezzanotte, V.; Parati, K.; Ficara, E. Digestate treatment with algae-bacteria consortia: A field pilot-scale experimentation in a sub-optimal climate area. Bioresour. Technol. 2019, 274, 232–243. [Google Scholar] [CrossRef]
- Marazzi, F.; Ficara, E.; Fornaroli, R.; Mezzanotte, V. Factors Affecting the Growth of Microalgae on Blackwater from Biosolid Dewatering. Water Air Soil Pollut. 2017, 228, 68. [Google Scholar] [CrossRef]
- Marazzi, F.; Bellucci, M.; Rossi, S.; Fornaroli, R.; Ficara, E.; Mezzanotte, V. Outdoor pilot trial integrating a sidestream microalgae process for the treatment of centrate under non optimal climate conditions. Algal Res. 2019, 39, 101430. [Google Scholar] [CrossRef] [Green Version]
- Villegas, G.I.R.; Fiamengo, M.; Fernández, F.G.A.; Grima, E.M. Outdoor production of microalgae biomass at pilot-scale in seawater using centrate as the nutrient source. Algal Res. 2017, 25, 538–548. [Google Scholar] [CrossRef]
- Ren, H.; Tuo, J.; Addy, M.M.; Zhang, R.; Lu, Q.; Anderson, E.; Chen, P.; Ruan, R. Cultivation of Chlorella vulgaris in a pilot-scale photobioreactor using real centrate wastewater with waste glycerol for improving microalgae biomass production and wastewater nutrients removal. Bioresour. Technol. 2017, 245, 1130–1138. [Google Scholar] [CrossRef]
- Tan, X.; Chu, H.; Zhang, Y.; Yang, L.; Zhao, F.; Zhou, X. Chlorella pyrenoidosa cultivation using anaerobic digested starch processing wastewater in an airlift circulation photobioreactor. Bioresour. Technol. 2014, 170, 538–548. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Z.; Wang, X.; Yuan, Z. Cultivation of Chlorella sp. using raw dairy wastewater for nutrient removal and biodiesel production: Characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Bioresour. Technol. 2015, 192, 382–388. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Dixit, G.; Shah, E.; Patel, A. Techno-economic analysis of microalgae production with simultaneous dairy effluent treatment using a pilot-scale High Volume V-shape pond system. Renew. Energy 2020, 145, 1620–1632. [Google Scholar] [CrossRef]
- Hena, S.; Znad, H.; Heong, K.T.; Judd, S. Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef]
- Silveira, C.F.; de Assis, L.R.; Paulo, A.; Oliveira, D.S.; Calijuri, M.L. Valorization of swine wastewater in a circular economy approach: Effects of hydraulic retention time on microalgae cultivation. Sci. Total Environ. 2021, 789, 147861. [Google Scholar] [CrossRef] [PubMed]
- de Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour. Technol. 2009, 100, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, S.; Yuan, H.; Zhou, Q.; Gu, G. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007, 41, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Hülsen, T.; Carvalho, G.; Egger, F.; Cruz, H.; Vertstraete, W.; Batstone, D.J.; Pikaar, I. Production of single-cell proteins from organic matter and residual nitrogen. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–389. [Google Scholar] [CrossRef]
- Lee, J.Z.; Logan, A.; Terry, S.; Spear, J.R. Microbial response to single-cell protein production and brewery wastewater treatment. Microb. Biotechnol. 2015, 8, 65–76. [Google Scholar] [CrossRef]
- Oesterholt, F.I.H.M.; Palmen, L.; Verstraete, W.; Boere, J. Power-to-Protein: Next step towards consumable single cell proteins from waste water and renewable hydrogen. In Proceedings of the 3rd IWA Resource Recovery Conference, Venice, Italy, 8–12 September 2019. [Google Scholar]
- Villamil, J.A.; Chacon, S.; Moreno, E.; Molina, R.; Martinez, F.; Melero, J.A.; Puyol, D. Quick start-up of a novel purple photosyntehtic bacteria-based anaerobic raceway at pilot scale for resource recovery from mixed urban biowaste sources. In Proceedings of the 5th International Conference on Ecotechnologies for Wastewater Treatment, Milan, Italy, 21–25 June 2021. [Google Scholar]
- Santos, A.F.; Almeida, P.V.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. From wastewater to fertilizer products: Alternative paths to mitigate phosphorus demand in European countries. Chemosphere 2021, 284, 131258. [Google Scholar] [CrossRef]
- Hülsen, T.; Hsieh, K.; Lu, Y.; Tait, S.; Batstone, D.J. Simultaneous treatment and single cell protein production from agri-industrial wastewaters using purple phototrophic bacteria or microalgae—A comparison. Bioresour. Technol. 2018, 254, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Capson-Tojo, G.; Batstone, D.J.; Grassino, M.; Vlaeminck, S.E.; Puyol, D.; Verstraete, W.; Kleerebezem, R.; Oehmen, A.; Ghimire, A.; Pikaar, I.; et al. Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotechnol. Adv. 2020, 43, 107567. [Google Scholar] [CrossRef]
- Kleemann, R.; Chenoweth, J.; Clift, R.; Morse, S.; Pearce, P.; Saroj, D. Evaluation of local and national effects of recovering phosphorus at wastewater treatment plants: Lessons learned from the UK. Resour. Conserv. Recycl. 2015, 105, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Barjenbruch, M.; Kabbe, C.; Inial, G.; Remy, C. Phosphorus recovery from municipal and fertilizer wastewater: China’s potential and perspective. J. Environ. Sci. 2017, 52, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Saliu, T.D.; Oladoja, N.A. Nutrient recovery from wastewater and reuse in agriculture: A review. Environ. Chem. Lett. 2021, 19, 2299–2316. [Google Scholar] [CrossRef]
- Crutchik, D.; Morales, N.; Vázquez-Padín, J.R.; Garrido, J.M. Enhancement of struvite pellets crystallization in a full-scale plant using an industrial grade magnesium product. Water Sci. Technol. 2017, 75, 609–618. [Google Scholar] [CrossRef]
- Brienza, C.; Sigurnjak, I.; Meier, T.; Michels, E.; Adani, F.; Schoumans, O.; Vaneeckhaute, C.; Meers, E. Techno-economic assessment at full scale of a biogas refinery plant receiving nitrogen rich feedstock and producing renewable energy and biobased fertilisers. J. Clean. Prod. 2021, 308, 127408. [Google Scholar] [CrossRef]
- Richter, L.; Wichern, M.; Grömping, M.; Robecke, U.; Haberkamp, J. Ammonium recovery from process water of digested sludge dewatering by membrane contactors. Water Pract. Technol. 2020, 15, 84–91. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [Green Version]
- USGOV. Standards for the Use or Disposal of Sewage Sludge; USGOV: Washington, DC, USA, 2018. [Google Scholar]
- Cecconet, D.; Raček, J.; Callegari, A.; Hlavínek, P. Energy recovery from wastewater: A study on heating and cooling of a multipurpose building with sewage-reclaimed heat energy. Sustainabillity 2020, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [Green Version]
- Hanum, F.; Yuan, L.C.; Kamahara, H.; Aziz, H.A. Treatment of Sewage Sludge Using Anaerobic Digestion in Malaysia: Current State and Challenges. Front. Energy Res. 2019, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Ince, O.; Gurol, Z.C.; Ozbayram, E.G.; Iglesias, M.M.; Ince, B.; Massalha, N.; Robles, Á.; Sabbah, I.; Seco, A. Anaerobic treatment of municipal wastewater. In Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environment; Lema, J.M., Suarez, S., Eds.; IWA Publishing: London, UK, 2017; ISBN 9781780407876. [Google Scholar]
- Nghiem, L.D.; Koch, K.; Bolzonella, D.; Drewes, J.E. Full scale co-digestion of wastewater sludge and food waste: Bottlenecks and possibilities. Renew. Sustain. Energy Rev. 2017, 72, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Serna-García, R.; Ruiz-Barriga, P.; Noriega-Hevia, G.; Serralta, J.; Pach, M. Maximising resource recovery from wastewater grown microalgae and primary sludge in an anaerobic membrane co-digestion pilot plant coupled to a composting process. J. Environ. Manag. 2021, 281, 111890. [Google Scholar] [CrossRef] [PubMed]
- Bolzonella, D.; Battistoni, P. Anaerobic codigestion of waste activated sludge and OFMSW: The experiences of Viareggio and Treviso plants (Italy) Anaerobic codigestion of waste activated sludge and OFMSW: The experiences of Viareggio and Treviso plants (Italy). Water Sci. Technol. 2006, 58, 203–211. [Google Scholar] [CrossRef]
- Razaviarani, V.; Buchanan, I.D.; Malik, S.; Katalambula, H. Pilot-scale anaerobic co-digestion of municipal wastewater sludge with biodiesel waste glycerin. Bioresour. Technol. 2013, 133, 206–212. [Google Scholar] [CrossRef]
- Maragkaki, A.E.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef]
- Le Hyaric, R.; Canler, J.-P.; Barillon, B.; Naquin, P.; Gourdon, R. Pilot-scale anaerobic digestion of screenings from wastewater treatment plants. Bioresour. Technol. 2010, 101, 9006–9011. [Google Scholar] [CrossRef]
- Wickham, R.; Xie, S.; Galway, B.; Bustamante, H.; Nghiem, L.D. Pilot-scale operation experience of anaerobic Co-digestion for possible full scale implementation. Int. Biodeterior. Biodegrad. 2019, 142, 137–142. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, G.; Li, J.; Li, Z.; Yu, G.; Wang, Y. A process combining hydrothermal pretreatment, anaerobic digestion and pyrolysis for sewage sludge dewatering and co-production of biogas and biochar: Pilot-scale veri fi cation. Bioresour. Technol. 2018, 254, 187–193. [Google Scholar] [CrossRef]
- Barroso Soares, R.; Ferreira Martins, M.; Franci Gonçalves, R. Experimental investigation of wastewater microalgae in a pilot-scale downdraft gasifier. Algal Res. 2020, 51, 102049. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Ieropoulos, I.A. Recent advancements in real-world microbial fuel cell applications. Curr. Opin. Electrochem. 2018, 11, 78–83. [Google Scholar] [CrossRef]
- Hiegemann, H.; Herzer, D.; Nettmann, E.; Lübken, M.; Schulte, P.; Schmelz, K.G.; Gredigk-Hoffmann, S.; Wichern, M. An integrated 45 L pilot microbial fuel cell system at a full-scale wastewater treatment plant. Bioresour. Technol. 2016, 218, 115–122. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef]
- Tota-Maharaj, K.; Paul, P. Performance of pilot-scale microbial fuel cells treating wastewater with associated bioenergy production in the Caribbean context. Int. J. Energy Environ. Eng. 2015, 6, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Qu, Y.; He, W.; Du, Y.; Liu, J.; Han, X.; Feng, Y. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour. Technol. 2015, 195, 66–72. [Google Scholar] [CrossRef]
- Liang, P.; Duan, R.; Jiang, Y.; Zhang, X.; Qiu, Y.; Huang, X. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res. 2018, 141, 1–8. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [Green Version]
- Carvalheira, M.; Duque, A.F. From Food Waste to Volatile Fatty Acids towards a Circular Economy. In Fermentation—Processes, Benefits and Risks; IntechOpen: London, UK, 2021. [Google Scholar]
- Eggeman, T.; Verser, D. Recovery of organic acids from fermentation broths. Appl. Biochem. Biotechnol. 2005, 122, 605–618. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Esteban-Gutiérrez, M.; Irizar, I.; González-Mtnez de Goñi, J.; Aymerich, E. Continuous acidogenic fermentation: Narrowing the gap between laboratory testing and industrial application. Bioresour. Technol. 2019, 282, 407–416. [Google Scholar] [CrossRef]
- Reis, M.; Albuquerque, M.; Villano, M.; Majone, M. Mixed culture processes for polyhydroxyalkanoate production from agro-industrial surplus/wastes as feedstocks. In Encyclopaedia Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 669–684. ISBN 9780080885049. [Google Scholar]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Lin, L.; Li, X. Yan Acidogenic fermentation of iron-enhanced primary sedimentation sludge under different pH conditions for production of volatile fatty acids. Chemosphere 2018, 194, 692–700. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Biochemical methane potential test for pulp and paper mill sludge with different food/microorganisms ratios and its kinetics. Int. Biodeterior. Biodegrad. 2017, 117, 197–204. [Google Scholar] [CrossRef]
- Da Ros, C.; Conca, V.; Eusebi, A.L.; Frison, N.; Fatone, F. Sieving of municipal wastewater and recovery of bio-based volatile fatty acids at pilot-scale. Water Res. 2020, 174, 115633. [Google Scholar] [CrossRef]
- Longo, S.; Katsou, E.; Malamis, S.; Frison, N.; Renzi, D.; Fatone, F. Recovery of volatile fatty acids from fermentation of sewage sludge in municipal wastewater treatment plants. Bioresour. Technol. 2015, 175, 436–444. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Tyagi, R.D. Value-added bio-products from sewage sludge. In Current Developments in Biotechnology and Bioengineering: Solid Waste Management; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780444636751. [Google Scholar]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Cinelli, P.; Mallegni, N.; Gigante, V.; Montanari, A.; Seggiani, M.; Coltelli, M.B.; Bronco, S.; Lazzeri, A. Biocomposites based on polyhydroxyalkanoates and natural fibres from renewable byproducts. Appl. Food Biotechnol. 2019, 6, 35–43. [Google Scholar] [CrossRef]
- Tomé, L.C.; Pinto, R.J.B.; Trovatti, E.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Transparent bionanocomposites with improved properties prepared from acetylated bacterial cellulose and poly(lactic acid) through a simple approach. Green Chem. 2011, 13, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Duque, A.F.; Oliveira, C.S.S.; Carmo, I.T.D.; Gouveia, A.R.; Pardelha, F.; Ramos, A.M.; Reis, M.A.M. Response of a three-stage process for PHA production by mixed microbial cultures to feedstock shift: Impact on polymer composition. New Biotechnol. 2014, 31, 276–288. [Google Scholar] [CrossRef]
- Gouveia, A.R.; Freitas, E.B.; Galinha, C.F.; Carvalho, G.; Duque, A.F.; Reis, M.A.M. Dynamic change of pH in acidogenic fermentation of cheese whey towards polyhydroxyalkanoates production: Impact on performance and microbial population. New Biotechnol. 2017, 37, 108–116. [Google Scholar] [CrossRef]
- Ribau Teixeira, M.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A.M. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. New Biotechnol. 2020, 57, 4–10. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Carvalho, G.; Pedras, I.; Karst, S.M.; Oliveira, C.S.S.; Duque, A.F.; Nielsen, P.H.; Reis, M.A.M. Functional redundancy ensures performance robustness in 3-stage PHA-producing mixed cultures under variable feed operation. New Biotechnol. 2018, 40, 207–217. [Google Scholar] [CrossRef]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, M.; Aguiar, F.R.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess. Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Pakalapati, H.; Chang, C.-K.; Show, P.L.; Arumugasamy, S.K.; Lan, J.C.-W. Development of polyhydroxyalkanoates production from waste feedstocks and applications. J. Biosci. Bioeng. 2018, 126, 282–292. [Google Scholar] [CrossRef]
- Estévez-Alonso, Á.; Pei, R.; van Loosdrecht, M.C.M.; Kleerebezem, R.; Werker, A. Scaling-up microbial community-based polyhydroxyalkanoate production: Status and challenges. Bioresour. Technol. 2021, 327, 124790. [Google Scholar] [CrossRef]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Veolia PHA. Pilot- and full-Scale Production from Domestic Wastewater. Available online: http://www.veolia.com (accessed on 13 August 2021).
- Chakravarty, P.; Mhaisalkar, V.; Chakrabarti, T. Study on poly-hydroxyalkanoate (PHA) production in pilot scale continuous mode wastewater treatment system. Bioresour. Technol. 2010, 101, 2896–2899. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Tamis, J.; Mulders, M.; Dijkman, H.; Rozendal, R.; van Loosdrecht, M.C.M.; Kleerebezem, R. Pilot-Scale Polyhydroxyalkanoate Production from Paper Mill Wastewater: Process Characteristics and Identification of Bottlenecks for Full-Scale Implementation. J. Environ. Eng. 2018, 144, 4018107. [Google Scholar] [CrossRef]
- Kinyua, M.N.; Miller, M.W.; Wett, B.; Murthy, S.; Chandran, K.; Bott, C.B. Polyhydroxyalkanoates, triacylglycerides and glycogen in a high rate activated sludge A-stage system. Chem. Eng. J. 2017, 316, 350–360. [Google Scholar] [CrossRef]
- Ntaikou, I.; Valencia Peroni, C.; Kourmentza, C.; Ilieva, V.I.; Morelli, A.; Chiellini, E.; Lyberatos, G. Microbial bio-based plastics from olive-mill wastewater: Generation and properties of polyhydroxyalkanoates from mixed cultures in a two-stage pilot scale system. J. Biotechnol. 2014, 188, 138–147. [Google Scholar] [CrossRef]
- Jia, Q.; Xiong, H.; Wang, H.; Shi, H.; Sheng, X.; Sun, R.; Chen, G. Production of polyhydroxyalkanoates (PHA) by bacterial consortium from excess sludge fermentation liquid at laboratory and pilot scales. Bioresour. Technol. 2014, 171, 159–167. [Google Scholar] [CrossRef]
- Tamis, J.; Lužkov, K.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Enrichment of Plasticicumulans acidivorans at pilot-scale for PHA production on industrial wastewater. J. Biotechnol. 2014, 192, 161–169. [Google Scholar] [CrossRef]
- Conca, V.; da Ros, C.; Valentino, F.; Eusebi, A.L.; Frison, N.; Fatone, F. Long-term validation of polyhydroxyalkanoates production potential from the sidestream of municipal wastewater treatment plant at pilot scale. Chem. Eng. J. 2020, 390, 124627. [Google Scholar] [CrossRef]
- Valentino, F.; Moretto, G.; Lorini, L.; Bolzonella, D.; Pavan, P.; Majone, M. Pilot-Scale Polyhydroxyalkanoate Production from Combined Treatment of Organic Fraction of Municipal Solid Waste and Sewage Sludge. Ind. Eng. Chem. Res. 2019, 58, 12149–12158. [Google Scholar] [CrossRef]
- Werker, A.; Bengtsson, S.; Korving, L.; Hjort, M.; Anterrieu, S.; Alexandersson, T.; Johansson, P.; Karlsson, A.; Karabegovic, L.; Magnusson, P.; et al. Consistent production of high quality PHA using activated sludge harvested from full scale municipal wastewater treatment—PHARIO. Water Sci. Technol. 2018, 78, 2256–2269. [Google Scholar] [CrossRef]
- European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment; European Union: Luxembourg, 2019. [Google Scholar]
- Laspidou, C.S.; Rittmann, B.E. A unified theory for extracellular polymeric substances, soluble microbial products, and active and inert biomass. Water Res. 2002, 36, 2711–2720. [Google Scholar] [CrossRef]
- Lotti, T.; Carretti, E.; Berti, D.; Martina, M.R.; Lubello, C.; Malpei, F. Extraction, recovery and characterization of structural extracellular polymeric substances from anammox granular sludge. J. Environ. Manag. 2019, 236, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. What are bacterial extracellular polymeric substances? In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. ISBN 978-3-642-60147-7. [Google Scholar]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Irvin, R.T.; Cheng, K.J. The bacterial glycocalyx in nature and disease. Annu. Rev. Microbiol. 1981, 35, 299–324. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Jahn, A.; Palmgren, R. Conceptual model for production and composition of exopolymers in biofilms. Water Sci. Technol. 1997, 36, 11–19. [Google Scholar] [CrossRef]
- Dignac, M.F.; Urbain, V.; Rybacki, D.; Bruchet, A.; Snidaro, D.; Scribe, P. Chemical description of extracellular polymers: Implication on activated sludge floc structure. In Proceedings of the Water Science and Technology, Vancouver, BC, Canada, 21–26 June 1998; Volume 38, pp. 45–53. [Google Scholar]
- Badireddy, A.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Role of extracellular polymeric substances in bioflocculation of activated sludge microorganisms under glucose-controlled conditions. Water Res. 2010, 44, 4505–4516. [Google Scholar] [CrossRef]
- Adav, S.S.; Lee, D.-J. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J. Hazard. Mater. 2008, 154, 1120–1126. [Google Scholar] [CrossRef]
- Guibaud, G.; Comte, S.; Bordas, F.; Dupuy, S.; Baudu, M. Comparison of the complexation potential of extracellular polymeric substances (EPS), extracted from activated sludges and produced by pure bacteria strains, for cadmium, lead and nickel. Chemosphere 2005, 59, 629–638. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater. 2008, 151, 185–193. [Google Scholar] [CrossRef]
- Pronk, M.; Giesen, A.; Thompson, A.; Robertson, S.; Van Loosdrecht, M. Aerobic granular biomass technology: Advancements in design, applications and further developments. Water Pract. Technol. 2017, 12, 987–996. [Google Scholar] [CrossRef]
- Lin, Y.; de Kreuk, M.; van Loosdrecht, M.C.M.; Adin, A. Characterization of alginate-like exopolysaccharides isolated from aerobic granular sludge in pilot-plant. Water Res. 2010, 44, 3355–3364. [Google Scholar] [CrossRef]
- Flez, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of Structural Extracellular Polymeric Substances from Aerobic Granular Sludge. JoVE 2016, 115, e54534. [Google Scholar] [CrossRef] [Green Version]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) resulting from activated sludge according to their type: Soluble or bound. Process. Biochem. 2006, 41, 815–823. [Google Scholar] [CrossRef]
- Guo, H.; Felz, S.; Lin, Y.; van Lier, J.B.; de Kreuk, M. Structural extracellular polymeric substances determine the difference in digestibility between waste activated sludge and aerobic granules. Water Res. 2020, 181, 115924. [Google Scholar] [CrossRef]
- Kaumera. Available online: https://kaumera.com/english/ (accessed on 13 August 2021).
- Luiz de Sousa Rollemberg, S.; Queiroz de Oliveira, L.; Nascimento de Barros, A.; Igor Milen Firmino, P.; Bezerra dos Santos, A. Pilot-scale aerobic granular sludge in the treatment of municipal wastewater: Optimizations in the start-up, methodology of sludge discharge, and evaluation of resource recovery. Bioresour. Technol. 2020, 311, 123467. [Google Scholar] [CrossRef]
- Van Leeuwen, K.; De Vries, E.; Roest, K.; Koop, S. The Energy & Raw Materials Factory: Role and Potential Contribution to the Circular Economy of the Netherlands. Environ. Manage. 2018, 61, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.S.; Amorim, C.L.; Ramos, M.A.; Mesquita, D.P.; Inocêncio, P.; Ferreira, E.C.; van Loosdrecht, M.; Castro, P.M.L. Variability in the composition of extracellular polymeric substances from a full-scale aerobic granular sludge reactor treating urban wastewater. J. Environ. Chem. Eng. 2020, 8, 104156. [Google Scholar] [CrossRef]
- Boleij, M.; Pabst, M.; Neu, T.R.; van Loosdrecht, M.C.M.; Lin, Y. Identification of Glycoproteins Isolated from Extracellular Polymeric Substances of Full-Scale Anammox Granular Sludge. Environ. Sci. Technol. 2018, 52, 13127–13135. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yan, Y.; Zhao, Y.; Shi, Q.; Wang, Y. Characterization of stratified EPS and their role in the initial adhesion of anammox consortia. Water Res. 2020, 169, 115223. [Google Scholar] [CrossRef]
- Pronk, M.; de Kreuk, M.K.; de Bruin, B.; Kamminga, P.; Kleerebezem, R.; van Loosdrecht, M.C.M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sharma, P.K.; van Loosdrecht, M.C.M. The chemical and mechanical differences between alginate-like exopolysaccharides isolated from aerobic flocculent sludge and aerobic granular sludge. Water Res. 2013, 47, 57–65. [Google Scholar] [CrossRef]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.M.; Lin, Y.M. Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef]
- Felz, S. Structural Extracellular Polymeric Substances from Aerobic Granular Sludge. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2019. [Google Scholar]
- Cetin, E.; Karakas, E.; Dulekgurgen, E.; Ovez, S.; Kolukirik, M.; Yilmaz, G. Effects of high-concentration influent suspended solids on aerobic granulation in pilot-scale sequencing batch reactors treating real domestic wastewater. Water Res. 2018, 131, 74–89. [Google Scholar] [CrossRef]
- Long, B.; Yang, C.-Z.; Pu, W.-H.; Yang, J.-K.; Jiang, G.-S.; Dan, J.-F.; Li, C.-Y.; Liu, F.-B. Rapid cultivation of aerobic granular sludge in a pilot scale sequencing batch reactor. Bioresour. Technol. 2014, 166, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.J.; Duan, C.S.; Yu, C.; Song, Y.X.; Chai, L.Y.; Xiao, R.; Wei, Z.; Min, X.B. Removal of nitrogen from wastewaters by anaerobic ammonium oxidation (ANAMMOX) using granules in upflow reactors. Environ. Chem. Lett. 2017, 15, 311–328. [Google Scholar] [CrossRef]
- Karakas, I.; Sam, S.B.; Cetin, E.; Dulekgurgen, E.; Yilmaz, G. Resource recovery from an aerobic granular sludge process treating domestic wastewater. J. Water Process. Eng. 2020, 34, 101148. [Google Scholar] [CrossRef]
- Lin, Y.M.; Nierop, K.G.J.; Girbal-Neuhauser, E.; Adriaanse, M.; van Loosdrecht, M.C.M. Sustainable polysaccharide-based biomaterial recovered from waste aerobic granular sludge as a surface coating material. Sustain. Mater. Technol. 2015, 4, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Lotti, T.; Carretti, E.; Berti, D.; Montis, C.; Del Buffa, S.; Lubello, C.; Feng, C.; Malpei, F. Hydrogels formed by anammox extracellular polymeric substances: Structural and mechanical insights. Sci. Rep. 2019, 9, 11633. [Google Scholar] [CrossRef]
- Zlopasa, J.; Koenders, E.A.B.; Picken, S.J. Using bio-based polymers for curing cement-based materials. In Proceedings of the 1st Ageing of Materials & Structures Conference, Delft, The Netherlands, 26–28 May 2014. [Google Scholar]
- Guibaud, G.; Bhatia, D.; D’Abzac, P.; Bourven, I.; Bordas, F.; van Hullebusch, E.D.; Lens, P.N.L. Cd(II) and Pb(II) sorption by extracellular polymeric substances (EPS) extracted from anaerobic granular biofilms: Evidence of a pH sorption-edge. J. Taiwan Inst. Chem. Eng. 2012, 43, 444–449. [Google Scholar] [CrossRef]
- Li, N.; Wei, D.; Wang, S.; Hu, L.; Xu, W.; Du, B.; Wei, Q. Comparative study of the role of extracellular polymeric substances in biosorption of Ni(II) onto aerobic/anaerobic granular sludge. J. Colloid Interface Sci. 2017, 490, 754–761. [Google Scholar] [CrossRef]
- Suh, J.H.; Kim, D.S. Comparison of different sorbents (inorganic and biological) for the removal of Pb2+ from aqueous solutions. J. Chem. Technol. Biotechnol. 2000, 75, 279–284. [Google Scholar] [CrossRef]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame retardant property of flax fabrics coated by extracellular polymeric substances recovered from both activated sludge and aerobic granular sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D.; Gk, Q. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [Green Version]
- ROYAL HASKONINGDHV Kaumera Nereda® Gum. Available online: https://global.royalhaskoningdhv.com/services/a-z-services/kaumera (accessed on 13 August 2021).
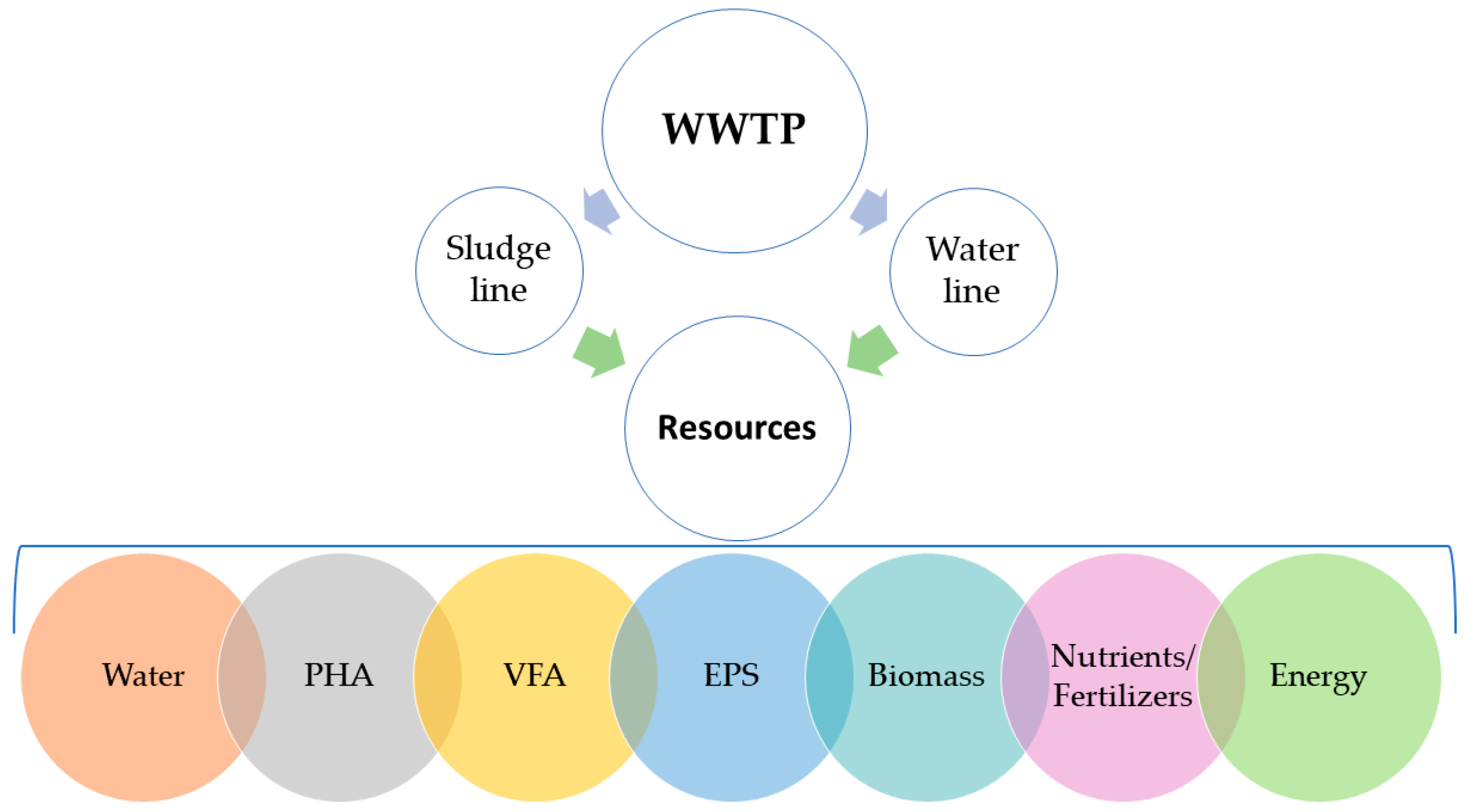

| City (Country) | Population 2016 | Wastewater On-Site Sanitation/Sewer Service Coverage | Wastewater Treatment | Treated Wastewater Reused | Energy Recovered | Fertilizer Recovery | Potential to Reduce Emissions from Improved Wastewater Management |
|---|---|---|---|---|---|---|---|
| Aqaba (Jordan) | 194,000 | 10/90% | 100% 45 M. L/d | 69% | 100% | NO | 81,000 ton CO2 eq/year |
| Bangkok (Thailand) | 5.6 M | 60/40% | 100% 1.3 B L/d | 5% | 62% | YES | 638,000 ton CO2 eq/year |
| Beijing (China) | 21.7 M | 5/95% | 88% 4.4 B L/d | 15% | 45% | YES | 1,044,000 ton CO2 eq/year |
| Chennai (India) | 8.5 M | 100% | 70% 769 M L/d | 49% | 77% | NO | 235,000 ton CO2 eq/year |
| Durban (South African) | 3.7 M | 84/16% | 100% 108 M L/d | 44% | 8% | YES | 438,000 ton CO2 eq/year |
| Kampala (Uganda) | 1.5 M | 60/40% | 100% 87 M L/d | 100% | 227,000 kWh/y | YES | 114,000 ton CO2 eq/year |
| Lima (Peru) | 10 M | 17/83% | 15% 240 M L/d | 5% | Low | NO | 652,000 ton CO2 eq/year |
| Industrial Sector | Wastewater Source | Treatment | Reuse of the Treated Water | Reference |
|---|---|---|---|---|
| Aquaculture | Fish tanks | Mechanical filtration, biological filtration, oxygenation, and sterilization (ozone or UV) | Refeed the fish tanks | [19] |
| Brewery (beer) | Effluent from anaerobic digester | Flotation, membrane bioreactor, ultrafiltration, and reverse osmosis | Drinking water production | [20] |
| Agro-food (horticulture sector) | Mixture of water streams from vegetable processing, cleaning activities, and toilets | Oil removal, activated sludge process (with pre-denitrification scheme), sand filtration, membrane ultrafiltration, and UV sterilization | Irrigation of the own company fields | [21] |
| Dairy | Mixture of the powder (82%) and the butter (18%) effluent streams | Grease removal pond, anaerobic pond, and membrane bioreactor (ultrafiltration) with anoxic and aerobic zones. | Irrigation | [22] |
| Science and Specifications | Technology Development |
|---|---|
| Develop a list of constituents of concern and acceptable levels (or ranges) in potable water reuse. | Develop consistent approval processes and standards for new treatment technologies. |
| Develop guidelines for reviewing and permitting fit-for-purpose reuse applications. | Research science and technology gaps for onsite urban and stormwater reuse. |
| Research fit-for-purpose specifications and data gaps for oil and gas produced wastewater. | Research management and use for brine from reuse projects. |
| Develop a plan to manage and regulate high salt loads and disposal options from reuse water. |
| Stream Source | Type of Reactor | Biomass Productivity | Reference |
|---|---|---|---|
| Centrate resulting from sludge dewatering of urban WWTP in northern Italy | Raceway pond (Working volume of 1200 L) | 5.5 ± 7.4 g TSS/m2/d | [42] |
| Centrate resulting from the solid fraction of piggery wastewater, energy crops, and agricultural waste co-digestion | Raceway pond (Working volume of 880 L) | 8.2 g TSS/m2/d | [43] |
| Centrate resulting from sludge dewatering from a municipal Bresso WWTP, northern Italy | Column reactor (Working volume of 85 L) | 50 mg TSS/L/d | [44] |
| Centrate resulting from sludge dewatering from a municipal Bresso WWTP, northern Italy | Bubble column reactor (Working volume of 82 L) | 40 ± 62 mg TSS/L/d | [45] |
| Centrate resulting from sludge dewatering (20%) plus seawater | Tubular photobioreactors (Working volume of 340 L) | 0.60 g biomass/L/d (at a dilution rate of 0.3/d) | [46] |
| Centrate resulting from sludge dewatering from a municipal WWTP plus crude glycerol (1 g/L) | Photobioreactor | 460 mg TVS/L/d | [47] |
| Anaerobic digested starch processing wastewater | Airlift photobioreactor (Working volume of 890 L, 1.80 m length × 0.62/0.30 m breadth × 1.10 m height). | 0.37 g/L/d | [48] |
| Raw dairy wastewater (25%) | Photobioreactors (Working volume of 40 L, 272 cm diameter × 1720 cm height) | 110 mg/L/d | [49] |
| Dairy effluent | High-Volume V-shaped pond (working volume of 3 m3) | 171 g/m2/d | [50] |
| Dairy farm wastewater | Single loop raceway (2.5 m × 0.7 m × 0.7 m and mixed by paddle wheel at 20 rpm) | 0.38 ± 0.09 g/L/d | [51] |
| Swine wastewater (after grit removal and Canadian-type anaerobic digestion) | Raceway (Working volume of 15 L) | Up to 300 mg VSS/L | [52] |
| Swine manure (after pre-treatment to reduce the total suspended solid content by 70%) diluted 20- and 10-fold with tap water | Raceway (Working volume of 464 L with a surface of 1.54 m2 (2.3 m long × 0.70 m wide × 0.30 m deep) | Up to 21.3 and 27.7 g/m2/d (respectively) | [53] |
| Waste Stream | Process | Culture | Productivity | Reference |
|---|---|---|---|---|
| Acidified stream from brewery industry | Aerobic with SRT < 8 days and nutrient addition | Enrichment of Alpha- and Beta-proteobacteria | >55% crude protein content | [56] |
| Mixture composed of CO2 and NH3 from sludge treatment plant and H2 and O2 from water electrolysis | Autotrophic by hydrogen using bacteria | H2-oxidizing bacteria | 49–75% crude protein content 1 kg SCP/d | [57] |
| Domestic wastewater mixed with organic fraction of municipal solid waste | Anaerobic raceway | Purple phototrophic bacteria | n.r. | [58] |
| Product | Process | Stream | Recovery | Reference |
|---|---|---|---|---|
| Struvite | Crystallization | Reject water from sludge anaerobic digester (1.1–2.2 mmol PO4−3/L and 70 mmol NH4+/L) | 77% of the Phosphorous Pellets of 0.5–5.0 mm | [65] |
| Ammonium sulphate | Vacuum stripping using gypsum and scrubbing | Reject water from sludge anaerobic digester (4.4 g NH4+-N/kg digestate) | 57% of Ammonium | [66] |
| Ammonium sulphate | Pre-treatment with caustic soda, lamella clarification, filtration, and three-stage membrane contactor with sulphuric acid addition | Reject water from sludge anaerobic digester (1000 mg NH4+-N/L) | 96% of Nitrogen removal efficiency and 4.1% of Nitrogen in the (NH4)2SO4 produced | [67] |
| Feedstock | Scale | Type of Reactor | Biogas Productivity | Reference |
|---|---|---|---|---|
| Microalgae biomass plus primary sludge waste | Pilot-scale | Anaerobic membrane bioreactor | 370 mL CH4/g VS influent | [77] |
| Waste activated sludge plus organic fraction of municipal solid waste | Full-scale | Pre-thickener plus a digester | up to 0.43 m3/kg TVS/d | [78] |
| Sewage sludge plus crude glycerol | Pilot-scale | Continuous stirred tank reactor | 0.87 LCH4/g VS | [79] |
| Sewage sludge plus agro-industrial by-product (olive mill wastewater, crude glycerol, or cheese whey) (95/5, v/v). | Pilot-scale | Anaerobic digester | 34.8 ± 3.2, 185.7 ± 15.3 and 45.9 ± 3.6 L/d, respectively | [80] |
| Screenings generated from the operations of pre-treatment of municipal wastewater | Pilot-scale | Mechanical stirring cylindrical digester (working volume of 50 L) | 653 Nl/kg VS per week | [81] |
| Sewage sludge plus different beverage wastes (namely beer, soft drinks, fruit juice, or wine) | Pilot-scale | Anaerobic conical stainless steel reactor | Up to 237 L CH4/kg COD added | [82] |
| Municipal wastewater | Pilot-scale | Anaerobic membrane bioreactor | 0.09–0.29 L CH4/g COD | [75] |
| Filtrate resulting of sludge dewatering | Pilot-scale | Upflow anaerobic sludge blanket reactor | 260 mL/g COD | [83] |
| Feedstock | Type of Reactor | Power Density Produced | Net Energy Recovered | Reference |
|---|---|---|---|---|
| Effluent of the primary clarifier of a WWTP | Four single-chamber membraneless MFCs (total volume of 45 L) | Up to 82 ± 18 mW/m2 | Up to 0.025 ± 0.013 kWh/m3 | [86] |
| Synthetic wastewater with variable influent COD concentrations (200–800 mg/L) | Five stacked MFC units(total volume of 72 L) | Up to 50.9 and 42.1 W/m3 in fed-batch and continuous, respectively | n.r. | [87] |
| Domestic wastewater from a WWTP | Single-chamber MFC unit | Up to 175.9 mW/m2 | n.r. | [88] |
| Brewery wastewater | Five stacked MFC units(total volume of 90 L) | Up to 181 ± 21 mW/m2 | 0.097 kWh/m3 | [89] |
| Municipal wastewater from a WWTP | 50 stacked MFC units (total volume of 1000 L) | Up to 3.64 W/m2 (~60 W/m3) | 0.033 ± 0.005 kWh/m3 | [90] |
| Waste Stream | Scale | Operating Conditions | VFA Production | VFA Composition (%) | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | Others | |||||
| Primary sludge | Pilot scale | Sequencing batch fermentation reactor—2.6 m3 pH 6; HRT 6 days, 37 °C | 154 ± 24 mg COD/g VS 1.23 Kg COD/m3reactor d | 30 | 45 | - | [101] | |
| Sequencing batch fermentation reactor—2.6 m3 pH 6; HRT 14 days, 37 °C | 137 ± 33 mg COD/g VS 0.44 Kg COD/m3reactor d | 25 | 53 | - | ||||
| Sequencing batch fermentation reactor—2.6 m3 pH 9; HRT 6 days, 37 °C | 322 ± 56 mg COD/g VS 2.57 Kg COD/m3reactor d | 29 | 51 | - | ||||
| Sewage sludge | Pilot scale | Stirred reactor coupled to a membrane separation system pH 5.7; HRT 5 days; SRT 14 days; 35 °C | 206.5 mg COD/g TVS 3389 ± 1320 mg COD/L | 31 | 28 | 23 | [102] | |
| Stirred reactor coupled to a membrane separation system pH 10; HRT 6 days; SRT 14 days; 35 °C | 315.6 mg COD/g TVS 7453 ± 1092 mg COD/L | 40 | 24 | 17 | ||||
| Stirred reactor coupled to a membrane separation system pH 7; HRT 5 days; SRT 14 days; 35 °C | 227.9 mg COD/g TVS 5596 ± 448 mg COD/L | 42 | 30 | 15 | ||||
| Stirred reactor coupled to a membrane separation system pH 7; HRT 5 days; SRT 6 days; 35 °C | 248.6 mg COD/g TVS 3738 ± 411 mg COD/L | 42 | 30 | 16 | ||||
| Stirred reactor coupled to a membrane separation system pH 10; HRT 5 days; SRT 5 days; 35 °C | 325.0 mg COD/g TVS 3184 ± 219 mg COD/L | 50 | 16 | 10 | ||||
| Sewage sludge | Full scale | Stirred tank reactor—30 m3 pH 10–11; 35 °C | 261.32 mg COD/g VSS | 58 | 7 | - | 35 | [103] |
| Waste Stream | Operational Conditions | PHA Production | Polymer Produced | Reference |
|---|---|---|---|---|
| Milk and ice-cream wastewater | HRT = SRT 43.56 h; pH 7.1 | 0.25 Kg PHA/Kg COD degraded | PHA | [124] |
| Municipal wastewater and waste activated sludge | V reactor = 550 L; OLR = 3.0 g COD/L/d;HRT = 3 h; | 0.25–0.38 g COD-PHA/g COD substrate0.27–0.38 g PHA/g VSS | Poly-(3HB-co-3HV) | [125] |
| Paper mill wastewater | V reactor = 200 L pH 6.6 T = 30 ± 2 °C | 0.70–0.80 g PHA/g VSS | PHA | [126] |
| Municipal wastewater | V reactor = 511 L SRT = 0.28–0.56 days HRT = 30–60 min DO = 0.5–1.5 mg/L | 26.3–51.4 mg COD-PHA/g VSS | PHB, PHV | [127] |
| Olive mill wastewater (OMW) | V reactor = 30 L T = 27 ± 2 °C | 24.60 ± 0.21 g PHA/100 g VSS7.58 g PHA/L initial OMW | P3HB, P3HO or 3-HB-co-3-HO | [128] |
| Excess sludge fermentation liquid | V reactor = 70 L T = 30 °C DO > 80%pH not controlled | 0.17 g PHA/g COD 6.497 mg PHA/L/h | PHA | [129] |
| Candy bar factory wastewater | V reactor = 200 L T = 30 ± 2 °C | 0.76 g PHA/g VSS 0.30 ± 0.04 g COD-PHA/g COD | PHB, PHV | [130] |
| Municipal wastewater—anaerobic reject water | V reactor = 1 m3 | 0.40–0.44 g PHA/g VSS 0.58–0.61 g COD-PHA/g COD-VFA 224–234 mg PHA/L/h | PHB, PHV | [131] |
| Combined organic fraction of municipal solid waste and sewage sludge | V reactor = 50–70 L T = 22–25 °C pH = 8.0–9.0 | 0.43–0.46 g PHA/g VSS 0.44–0.50 g COD-PHA/g COD-VFA 0.29–0.36 g PHA/L/h | PHB, PHV | [132] |
| Activated sludge harvested from full scale municipal wastewater treatment—PHARIO | V reactor = 500 L T = 25 °C | 0.41 g PHA/g VSS 0.40–0.45 g COD-PHA/g COD-substrate consumed | PHB, PHV | [133] |
| Technology and Waste Stream | Scale | Operational Conditions | Extraction Method | Characteristics of Polymers | Yield | Reference |
|---|---|---|---|---|---|---|
| AGS—industrial diary wastewater | Full-scale SBR | Nereda® process; n.r. | Heating method (Na2CO3, T = 80 °C) | Kaumera® gum; n.r. | 225 mg VSS sEPS/g VSS AGS | [154] |
| AGS—municipal wastewater | Full-scale SBR | Nereda® process; COD tot,in = 585 mg/L; TSS,in = 195 mg/L; NH4-N,in = 55 mg/L; PO4-P,in = 6.3 mg/L; TSS,react = 8–10 g/L. | Heating method (Na2CO3, T = 80 °C) | 69 ± 9% PolyGG blocks; 2 ± 1% PolyMM blocks; 15 ± 2% PolyMG blocks. Sodium alginate equivalent: 486 ± 22 mg Alginate/g VSS sEPS; Protein: <100 mg BSA/g VSS sEPS. | 160 ± 4 mg VSS sEPS/g VSS AGS | [160] |
| AGS—municipal wastewater | Full-scale SBR | Nereda® process; n.r. | Heating method (Na2CO3, T = 80 °C) | Polysaccharides: 138 mg Glucose/g VSS sEPS; Proteins: 381 mg BSA/g VSS sEPS;Uronic acid: 72 mg galact. acid/g VSS sEPS; Phenolic compound: 286 mg humic acid/g VSS sEPS. | 282 mg VSS sEPS/g VSS AGS | [149,161,162] |
| AGS—mixed domestic, pluvial and industrial wastewater | Full-scale SBR | Nereda® process; n.r. | Heating method (Na2CO3, T = 80 °C) | Polysaccharides: ≈ 10–15 mg Glucose/g VSS sEPS; Proteins: ≈ 60–80 mg BSA/g VSS sEPS; Humic substances: 317 mg humic acid/g VSS sEPS. | 122–149 mg VSS sEPS/g VSS AGS | [155] |
| AGS—municipal wastewater | Pilot-scale SBR | COD tot,in = 461 mg/L; BOD5,in = 148 mg/L; TN,in = 43 mg/L; TP,in = 5 mg/L VER = 60%; SRT = 12–15 days. | Heating method (Na2CO3, T = 80 °C) | Polysaccharides: 136 mg Glucose/g VSS; Proteins: 514 mg BSA/g VSS. | 219 mg VSS sEPS/g VSS AGS | [153] |
| AGS—wastewater from university campus | Pilot-scale SBR | VER = 60%; SRT= 10 d; COD tot,in = 1200 mg/L; TN,in = 52 mg/L; TP,in =12 mg/L. | Cation Exchange Resin (CER). | Polysaccharides: 224–252 mg Glucose/g VSS; Proteins: ≈11 mg BSA/g VSS. | n.r. | [163] |
| AGS—synthetic wastewater | Pilot-scale SBR | VER = 60%; COD tot,in = 8 g/L; TN,in = 450 mg/L; TP,in = 90 mg/L. | Thermal extraction (T = 80 °C) | Polysaccharides: 92 mg Glucose/g VSS; Proteins: 144 mg BSA/g VSS. | n.r. | [164] |
| Anammox | Full-scale | Two stage partial nitritation-anammox. | Alkaline extraction (NaOH) | Polysaccharides: 4–287 mg Glucose/g VSS; Proteins: 17–307 mg BSA/g VSS. | up to 380 mg VSS sEPS/g VSS AMX | [136,138,156,165] |
| Application of EPS | Features | Reference |
|---|---|---|
| Coating material | The functional groups present in EPS provide abundant binding sites, both hydrophilic and hydrophobic functional groups, conferring improved waterproof capacity to surfaces. | [167,168] |
| Curing of cement | The hydrophilic properties of the EPS improve the curing of cement, reducing moisture loss from the surface of cement-based materials | [166] |
| Bioadsorbent | The physicochemical interactions between the adsorbates and functional groups of EPS, promote the adsorption of metals or other compounds | [170,171] |
| Flame retardant | sEPS can be extinguished bio-based flame retardant materials for flax fabrics due to effective char formation. | [173] |
| Bioflocculation | Some functional groups present on the EPS contribute to the flocculation abilities of these biopolymers | [174] |
| Soil conditioning | The water-binding capacity makes EPS applicable in the agronomic sector, as these biopolymers are able to retain water in soil and to reduce the leaching of fertilizers. | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque, A.F.; Campo, R.; Val del Rio, A.; Amorim, C.L. Wastewater Valorization: Practice around the World at Pilot- and Full-Scale. Int. J. Environ. Res. Public Health 2021, 18, 9466. https://doi.org/10.3390/ijerph18189466
Duque AF, Campo R, Val del Rio A, Amorim CL. Wastewater Valorization: Practice around the World at Pilot- and Full-Scale. International Journal of Environmental Research and Public Health. 2021; 18(18):9466. https://doi.org/10.3390/ijerph18189466
Chicago/Turabian StyleDuque, Anouk F., Riccardo Campo, Angeles Val del Rio, and Catarina L. Amorim. 2021. "Wastewater Valorization: Practice around the World at Pilot- and Full-Scale" International Journal of Environmental Research and Public Health 18, no. 18: 9466. https://doi.org/10.3390/ijerph18189466
APA StyleDuque, A. F., Campo, R., Val del Rio, A., & Amorim, C. L. (2021). Wastewater Valorization: Practice around the World at Pilot- and Full-Scale. International Journal of Environmental Research and Public Health, 18(18), 9466. https://doi.org/10.3390/ijerph18189466









