Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications
Abstract
1. Introduction
2. Public Health Concerns
3. Results
3.1. Evidence on the Carcinogenicity of NDMA, NDEA, NMBA, and the Larger Group of NMAs
3.1.1. Animal Bioassays
3.1.2. Metabolism
3.1.3. Genotoxicity
3.2. Cancer Risk Estimate
3.2.1. Extra Cancer Risk Calculation for NDMA
3.2.2. Extra Cancer Risk Calculation for NDEA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- US FDA. FDA Announces Voluntary Recall of Several Medicines Containing Valsartan Following Detection of an Impurity. Available online: https://www.fda.gov/news-events/press-announcements/fda-announces-voluntary-recall-several-medicines-containing-valsartan-following-detection-impurity (accessed on 7 May 2020).
- US FDA. Drug Recalls. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/drug-recalls (accessed on 2 July 2021).
- US FDA. FDA Alerts Health Care Professionals and Patients to a Voluntary Recall of Varenicline (Chantix) to the Warehouse Level. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-alerts-health-care-professionals-and-patients-voluntary-recall-varenicline-chantix-warehouse?utm_medium=email&utm_source=govdelivery (accessed on 2 July 2021).
- Banzi, R.; Bertele, V. Regulatory response to contaminated valsartan. BMJ 2018, 362, k3855. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. CHAMPIX (Varenicline)—Potential Risk Posed by Long-Term Exposure to Nitrosamine Impurity, N-Nitrosovarenicline, Exceeding Acceptable Intake Limit. Available online: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2021/75961a-eng.php (accessed on 12 July 2021).
- OEHHA (Office of Environmental Health Hazard Assessment). Evidence on the Carcinogenicity of N-Nitrosomethyl-n-Alkylamines; OEHHA: Oakland, CA, USA, 2014. Available online: https://oehha.ca.gov/media/downloads/proposition-65/chemicals/n-nitrosomethyl-n-alklyaminesaugust2014.pdf (accessed on 1 July 2021).
- IARC. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans. In Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs Volumes 1–42; IARC: Lyon, France, 1987; Available online: https://publications.iarc.fr/139 (accessed on 1 July 2021).
- US EPA. Integrated Risk Information System (IRIS) Chemical Assessment Summary. N-Nitrosodimethylamine; CASRN 62-75-9: Washington DC, USA, 2002. Available online: https://iris.epa.gov/static/pdfs/0045_summary.pdf (accessed on 1 July 2021).
- US EPA. Integrated Risk Information System (IRIS) Chemical Assessment Summary. N-Nitrosodiethylamine; CASRN 55-18-5: Washington DC, USA, 2003. Available online: https://iris.epa.gov/static/pdfs/0042_summary.pdf (accessed on 1 July 2021).
- NTP (National Toxicology Program). Report on Carcinogens, Fourteenth Edition; U.S. Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2016. [Google Scholar]
- Lijinsky, W.; Reuber, M.D.; Saavedra, J.E.; Singer, G.M. Carcinogenesis in F344 rats by N-nitrosomethyl-n-propylamine derivatives. J. Natl. Cancer Inst. 1983, 70, 959–963. [Google Scholar]
- Thomas, B.J.; Kovatch, R.M.; Lijinsky, W. The induction of bladder tumors in F344 rats by intravesicular administration of some nitrosamines. Jpn J. Cancer Res. 1988, 79, 309–313. [Google Scholar] [CrossRef] [PubMed]
- US FDA. M7(R1) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk; U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER): Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/media/85885/download (accessed on 1 July 2021).
- EMA (European Medicines Agency). Committee for Medicinal Products for Human Use (CHMP) Assessment Report. Referral under Article 31 of Directive 2001/83/EC Angiotensin-II-Receptor Antagonists (Sartans) Containing a Tetrazole Group. Available online: https://www.ema.europa.eu/en/documents/variation-report/angiotensin-ii-receptor-antagonists-sartans-article-31-referral-chmp-assessment-report-impact_en.pdf (accessed on 1 July 2021).
- US FDA. Control of Nitrosamine Impurities in Human Drugs. Guidance for Industry (Revision 1); Center for Drug Evaluation and Research (CDER). Available online: https://www.fda.gov/media/141720/download (accessed on 1 February 2021).
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine contamination in pharmaceuticals: Threat, impact, and control. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Bharate, S.S. Critical Analysis of Drug Product Recalls due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.P.; Johnson, G.E.; Snodin, D.J. Tolerability of risk: A commentary on the nitrosamine contamination issue. J. Pharm. Sci. 2021, 110, 2311–2328. [Google Scholar] [CrossRef]
- US FDA. General Advice Letter. Available online: https://www.fda.gov/media/122643/download (accessed on 17 April 2020).
- US FDA. FDA Requests Removal of All Ranitidine Products (Zantac) from the Market. Available online: https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market?utm_campaign=040120_PR_FDA%20Requests%20Removal%20of%20Ranitidine%20Products%20%28Zantac%29%20from%20the%20Market&utm_medium=email&utm_source=Eloqua (accessed on 17 April 2020).
- Erskine, D.; Wood, D. What is the significance of nitrosamine contamination in medicines? Drug Ther. Bull. 2021, 59, 39–42. [Google Scholar] [CrossRef]
- OEHHA. Risk-Specific Intake Levels for the Proposition 65 Carcinogen: N-Nitrosodimethylamine; OEHHA: Oakland, CA, USA, 1988. [Google Scholar]
- OEHHA. Risk-Specific Intake Levels for the Proposition 65 Carcinogen: N-Nitrosodiethylamine; OEHHA: Oakland, CA, USA, 1988. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Estimated Hypertension Prevalence, Treatment, and Control among U.S. Adults. Available online: https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html (accessed on 15 June 2021).
- Kane, S.P. Angiotensin 2 Receptor Blocker, ClinCalc DrugStats Database, Version 21.1. Available online: https://clincalc.com/DrugStats/EPC/Angiotensin2ReceptorBlocker (accessed on 15 June 2021).
- Kane, S.P. Histamine-2 Receptor Antagonist, ClinCalc DrugStats Database, Version 21.1. Available online: https://clincalc.com/DrugStats/EPC/Histamine2ReceptorAntagonist (accessed on 15 June 2021).
- NIH MedlinePlus Magazine. Heartburn: What You Need to Know. Available online: https://magazine.medlineplus.gov/article/heartburn-what-you-need-to-know (accessed on 15 June 2021).
- Kane, S.P. Metformin Hydrochloride, ClinCalc DrugStats Database, Version 21.1. Available online: https://clincalc.com/DrugStats/Drugs/MetforminHydrochloride (accessed on 15 June 2021).
- Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020. [Google Scholar]
- Le, S.; Lee, G.C. Emerging Trends in Metformin Prescribing in the United States from 2000 to 2015. Clin. Drug Investig. 2019, 39, 757–763. [Google Scholar] [CrossRef]
- McHale, C.M.; Osborne, G.; Morello-Frosch, R.; Salmon, A.G.; Sandy, M.S.; Solomon, G.; Zhang, L.; Smith, M.T.; Zeise, L. Assessing health risks from multiple environmental stressors: Moving from G × E to I × E. Mutat. Res. Rev. Mutat. Res. 2018, 775, 11–20. [Google Scholar] [CrossRef]
- US FDA. What to Know and Do about Possible Nitrosamines in Your Medication. Available online: https://www.fda.gov/consumers/consumer-updates/what-know-and-do-about-possible-nitrosamines-your-medication (accessed on 6 July 2021).
- US FDA. FDA Updates Table of Interim Limits for Nitrosamine Impurities in ARBs. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan (accessed on 1 July 2021).
- IARC. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some N-Nitroso Compounds; IARC: Lyon, France, 1978; Available online: https://publications.iarc.fr/35 (accessed on 1 July 2021).
- US EPA. Integrated Risk Information System (IRIS) Chemical Assessment Summary. N-Nitroso-N-methylethylamine; CASRN 10595-95-6: Washington, DC, USA, 2003. [Google Scholar]
- Zhu, Y.; Wang, P.P.; Zhao, J.; Green, R.; Sun, Z.; Roebothan, B.; Squires, J.; Buehler, S.; Dicks, E.; Zhao, J.; et al. Dietary N-nitroso compounds and risk of colorectal cancer: A case-control study in Newfoundland and Labrador and Ontario, Canada. Br. J. Nutr. 2014, 111, 1109–1117. [Google Scholar] [CrossRef]
- Zheng, J.; Stuff, J.; Tang, H.; Hassan, M.M.; Daniel, C.R.; Li, D. Dietary N-nitroso compounds and risk of pancreatic cancer: Results from a large case-control study. Carcinogenesis 2019, 40, 254–262. [Google Scholar] [CrossRef]
- Loh, Y.H.; Jakszyn, P.; Luben, R.N.; Mulligan, A.A.; Mitrou, P.N.; Khaw, K.T. N-Nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am. J. Clin. Nutr. 2011, 93, 1053–1061. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef] [PubMed]
- Liteplo, R.G.; Meek, M.E.; Windle, W. Concise International Chemical Assessment Document 38. N-Nitrosodimethylamine; International Programme on Chemical Safety (IPCS); World Health Organization: Geneva, Switzerland, 2002; Available online: https://www.who.int/ipcs/publications/cicad/en/cicad38.pdf (accessed on 1 July 2021).
- Magee, P.N. Metabolism of nitrosamines: An overview. In Microsomes, Drug Oxidations and Chemical Carcinogenesis; Coon, M.J., Conney, A.H., Estabrook, R.W., Gelboin, H.V., Gillette, J.R., O’Brien, P.J., Eds.; Academic Press: Cambridge, MA, USA, 1980; pp. 1081–1092. [Google Scholar]
- Michejda, C.J.; Kroeger-Koepke, M.B.; Koepke, S.R.; Sieh, D.H. Activation of Nitrosamines to Biological Alkylating Agents. In N-Nitroso Compounds; ACS Symposium Series; American Chemical Society: Washington DC, USA, 1981; Volume 174, pp. 3–20. [Google Scholar]
- Haggerty, H.G.; Holsapple, M.P. Role of metabolism in dimethylnitrosamine-induced immunosuppression: A review. Toxicology 1990, 63, 1–23. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man; IARC: Lyon, France, 1972; Available online: https://publications.iarc.fr/19 (accessed on 1 July 2021).
- Singer, G.M.; Lijinsky, W.; Buettner, L.; McClusky, G.A. Relationship of rat urinary metabolites of N-nitrosomethyl-N-alkylamine to bladder carcinogenesis. Cancer Res. 1981, 41, 4942–4946. [Google Scholar]
- Okada, M.; Suzuki, E.M.; Mochizuki, M. Possible important role of urinary N-methyl-N(3-carboxypropyl)nitrosamine in the induction of bladder tumors in rats by N-methyl-N-dodecylnitrosamine. Gan 1976, 67, 771–772. [Google Scholar] [PubMed]
- Huang, Q.; Wang, S.; Chen, S.; Babcook, D.; Park, S.; Gelboin, H.; Mirvish, S. Hydroxylation and dealkylation of methyl-n-butylnitrosamine and role of certain cytochrome P-450 isozymes in these reactions. Cancer Lett. 1993, 69, 107–116. [Google Scholar] [CrossRef]
- CCRIS. Chemical Carcinogenesis Research Information System (CCRIS): N-Nitroso-N-Methyl-4-Aminobutyric acid (NMBA). 2018. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/363899073 (accessed on 1 July 2021).
- Kawaguchi, S.; Nakamura, T.; Tsuda, S.; Murashige, R.; Sasaki, Y. Detection of in vitro genotoxicity of pro-mutagens using the comet assay under human and rat liver S9 fractions. MOJ Toxicol. 2018, 4, 255–261. [Google Scholar]
- OEHHA. Public Health Goals for Chemicals in Drinking Water: N-Nitrosodimethylamine; OEHHA: Oakland, CA, USA, 2006. Available online: https://oehha.ca.gov/media/downloads/water/chemicals/phg/122206ndmaphg.pdf (accessed on 1 July 2021).
- Yamazaki, H.; Mori, Y.; Toyoshi, K.; Mori, H.; Sugie, S.; Yoshimi, N.; Konishi, Y. Genotoxicity of carcinogenic N-nitrosopropylamine derivatives in the hepatocyte primary culture/DNA-repair test. Mutat. Res. 1985, 144, 197–202. [Google Scholar] [CrossRef]
- Morita, T.; Hamada, S.; Masumura, K.; Wakata, A.; Maniwa, J.; Takasawa, H.; Yasunaga, K.; Hashizume, T.; Honma, M. Evaluation of the sensitivity and specificity of in vivo erythrocyte micronucleus and transgenic rodent gene mutation tests to detect rodent carcinogens. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 802, 1–29. [Google Scholar] [CrossRef]
- Mehta, R.D.; von Borstel, R.C. Genetic activity in yeast assays of reputed nonmutagenic, carcinogenic N-nitroso compounds and methapyrilene hydrochloride. IARC Sci. Publ. 1984, 57, 721–729. [Google Scholar]
- Lijinsky, W.; Andrews, A.W. The superiority of hamster liver microsomal fraction for activating nitrosamines to mutagens in Salmonella typhimurium. Mutat. Res. 1983, 111, 135–144. [Google Scholar] [CrossRef]
- IRIS (Integrated Risk Information System). N-Nitroso-N-Methylethylamine; CASRN 10595-95-6: Washington DC, USA, 1987. [Google Scholar]
- Bonde, P.; Gao, D.; Chen, L.; Duncan, M.; Miyashita, T.; Montgomery, E.; Harmon, J.W.; Wei, C. Selective decrease in the DNA base excision repair pathway in squamous cell cancer of the esophagus. J. Thorac. Cardiovasc. Surg. 2007, 133, 74–81.e73. [Google Scholar] [CrossRef][Green Version]
- Mirvish, S.S.; Huang, Q.; Williamson, J.; Chen, S.-C.; Gelboin, H.V. Use of monoclonal antibodies to cytochrome P450s to indicate the critical dealkylation and the P450s involved in methyl-n-amylnitrosamine mutagenicity in the presence of induced rat liver microsomes. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1995, 331, 161–170. [Google Scholar] [CrossRef]
- US FDA. Laboratory Analysis of Valsartan Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-valsartan-products (accessed on 1 July 2021).
- US FDA. Laboratory Analysis of Ranitidine and Nizatidine Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-ranitidine (accessed on 1 July 2021).
- US FDA. Laboratory Analysis of Metformin Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-metformin (accessed on 1 July 2021).
- US FDA. DIOVAN® (Valsartan) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021283s50lbl.pdf (accessed on 28 May 2020).
- Pottegard, A.; Kristensen, K.B.; Ernst, M.T.; Johansen, N.B.; Quartarolo, P.; Hallas, J. Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study. BMJ 2018, 362, k3851. [Google Scholar] [CrossRef]
- OEHHA. Final Statement of Reasons 22 California Code of Regulations. 1989. Available online: https://oehha.ca.gov/media/downloads/crnr/1270512711benzidinebcefsornov1989_0.pdf (accessed on 1 July 2021).
- Peto, R.; Gray, R.; Brantom, P.; Grasso, P. Nitrosamine carcinogenesis in 5120 rodents: Chronic administration of sixteen different concentrations of NDEA, NDMA, NPYR and NPIP in the water of 4440 inbred rats, with parallel studies on NDEA alone of the effect of age of starting (3, 6 or 20 weeks) and of species (rats, mice or hamsters). IARC Sci. Publ. 1984, 57, 627–665. [Google Scholar]
- US FDA. Laboratory Analysis of Rifampin/Rifapentine Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-analysis-rifampinrifapentine-products (accessed on 21 June 2021).
| Nitrosamine | Structure | Cancer Classification 1 |
|---|---|---|
| N-Nitrosodimethylamine (NDMA, NMA-C1) | 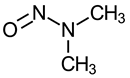 | IARC 2A (1987) US EPA B2 (1986) NTP RoC RA (1981) P65 (1987) |
| N-Nitrosodiethylamine (NDEA) | 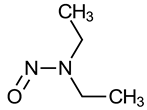 | IARC 2A (1987) US EPA B2 (1986) NTP RoC RA (1981) P65 (1987) |
| N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) | 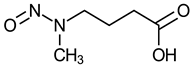 | Not evaluated |
| N-Nitrosomethylethylamine (NMA-C2) N-Nitrosomethyl-n-propylamine (NMA-C3) N-Nitrosomethyl-n-butylamine (NMA-C4) N-Nitrosomethyl-n-pentylamine (NMA-C5) N-Nitrosomethyl-n-hexylamine (NMA-C6) N-Nitrosomethyl-n-heptylamine (NMA-C7) N-Nitrosomethyl-n-octylamine (NMA-C8) N-Nitrosomethyl-n-nonylamine (NMA-C9) N-Nitrosomethyl-n-decylamine (NMA-C10) N-Nitrosomethyl-n-undecylamine (NMA-C11) N-Nitrosomethyl-n-dodecylamine (NMA-C12) N-Nitrosomethyl-n-tetradecylamine (NMA-C14) | 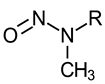 R: alkyl group (where C2 indicates a 2 carbon alkyl group, C3 indicates a 3 carbon alkyl group, and so on) | NMA-C2: IARC 2B (1987) US EPA B2 (1988) P65 (1989) NMA-C3 through NMA-C12, and NMA-C14: P65 (2014) |
| Tumor Site | Nasal Cavity | Tongue | Oropharynx | Lung | Esophagus | Forestomach | Liver | Kidney | Bladder | Others | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | R (r) | H (r) | M (r) | R (r) | H | M (r) | R (r) | H | M | R (r) | H (r) | M | R (r) | H (i) | M (r) | R (r) | H (r) | M (r) | R (r) 1 | H (r) | M | R (r) | H | M | R (r) | H (r) | M | R | H | M |
| NDMA 2 | X | X | X | X | X | X | X | X | X | X | X | X3 | X3 | X3 | ||||||||||||||||
| NDEA 2 | X | X | X | X | X4 | X | X | X | X | X | X | X | X | X | X | X | X | X5 | X5 | X5 | ||||||||||
| NMBA 6 | X | X | ||||||||||||||||||||||||||||
| NMA-C2 7 | X | X* | X | X | X | X* | X8 | |||||||||||||||||||||||
| NMA-C3 9 | X* | X* | X* | X | X | X* | X* | X* | X | X* | X* | X* | X10 | X*,10 | X*,10 | |||||||||||||||
| NMA-C4 9 | X | X* | X | X | X* | X* | X | X* | X* | |||||||||||||||||||||
| NMA-C5 9 | X* | X* | X* | X | X* | X* | X* | X | X* | X | X* | X11 | X | X*,12 | ||||||||||||||||
| NMA-C6 9 | X | X | X | X*,13 | X* | X*,13 | X | X* | X*,12 | X* | X | |||||||||||||||||||
| NMA-C7 9 | X | X* | X | X*,13 | X* | X*,13 | X* | X*,12 | X* | |||||||||||||||||||||
| NMA-C8 9 | X* | X | X* | X* | X* | X* | X* | X* | X | X*,14 | ||||||||||||||||||||
| NMA-C9 9 | X | X* | X* | |||||||||||||||||||||||||||
| NMA-C10 9 | X | X* | X | X* | ||||||||||||||||||||||||||
| NMA-C11 9 | X | X | X | X | ||||||||||||||||||||||||||
| NMA-C12 9 | X | X* | X* | X | X* | X* | X* | X*,15 | ||||||||||||||||||||||
| NMA-C14 9 | X | X | X* | |||||||||||||||||||||||||||
| Chemical | Mutagenicity 2 | DNA Damage and/or Nucleic Acid or Protein Binding 3 | Chromosomal Effects 3 |
|---|---|---|---|
| NDMA | +Rodent mutation assays (in vivo and in vitro); +SLRL mutation assay in Drosophila; +Salmonella and E. coli; +S. cerevisiae | +DNA breaks in human cells (in vitro); +DNA breaks in rodent tissues (in vivo); +UDS in human and rodent cells (in vitro); +DNA adducts in several human cells and tissues (in vitro) and in rodent tissues (in vivo) | +MN in rodent cells and tissues (in vivo and in vitro); +CAs in rodent cells (in vitro); +SCE in rodent tissues (in vivo) |
| NDEA | +Rodent mutation assays (in vivo and in vitro); +SLRL mutation assay in Drosophila; +Salmonella and E. coli; +S. cerevisiae and Neurospora crassa | +DNA breaks in human cells (in vitro); +UDS in rat cells (in vitro); +DNA adducts in several human cells and tissues (in vitro); +DNA, RNA adducts in rodent tissues (in vivo) | +CAs and +SCE in CHL cells (in vitro) |
| NMBA | +Yeast; +Salmonella | NT | NT |
| NMA-C2 | +CHL mutation assay; +Salmonella | +DNA breaks in human cells (in vitro); +DNA adducts in rodent tissues (in vivo) | NT |
| NMA-C3 | +CHL mutation assay; +Salmonella and E. coli | +DNA adducts in rat tissues (in vivo) | NT |
| NMA-C4 | +Salmonella and E. coli | +DNA, RNA, protein adducts in rat tissues (in vivo) | NT |
| NMA-C5 | +Salmonella | +DNA adducts in rat cells and tissues (in vivo and in vitro); +8-oxodG in rat tissues (in vivo) | NT |
| NMA-C6–NMA-C12 | +Salmonella | +DNA adducts in rat tissues (in vivo) | NT |
| NMA-C14 | NT | NT | NT |
| Drug Class and Major Indication | Active Pharmaceutical Ingredient (Dose per tablet) | NDMA (μg/tablet) | NDEA (μg/tablet) |
|---|---|---|---|
| Angiotensin II receptor blockers (ARBs): hypertension and related heart conditions | Valsartan (160 mg) * | 0.45 | 1.31 |
| Valsartan (320 mg) * | <LOD–20.19 | <LOD–1.22 | |
| Histamine-2 blockers: heartburn and gastroesophageal reflux disease (GERD) | Ranitidine (75 mg) ** | 0.01–0.04 | NR |
| Ranitidine (150 mg) ** | 0.01–0.33 | NR | |
| Ranitidine (300 mg) ** | 0.01–0.86 | NR | |
| Nizatidine (150 mg) | 0.01–0.02 | NR | |
| Nizatidine (300 mg) | 0.01–0.03 | NR | |
| Antihyperglycemic: type 2 diabetes | Metformin, extended release (500 mg) * | <LOD–0.19 | NR |
| Metformin, extended release (750 mg) * | 0.01–0.08 | NR | |
| Metformin, extended release (1000 mg) * | <LOD–0.01 | NR | |
| Metformin, immediate release (500 mg) | <LOD | NR | |
| Metformin, immediate release (850 mg) | <LOD–0.01 | NR | |
| Metformin, immediate release (1000 mg) | <LOD | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Ricker, K.; Tsai, F.C.; Hsieh, C.J.; Osborne, G.; Sun, M.; Marder, M.E.; Elmore, S.; Schmitz, R.; Sandy, M.S. Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications. Int. J. Environ. Res. Public Health 2021, 18, 9465. https://doi.org/10.3390/ijerph18189465
Li K, Ricker K, Tsai FC, Hsieh CJ, Osborne G, Sun M, Marder ME, Elmore S, Schmitz R, Sandy MS. Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications. International Journal of Environmental Research and Public Health. 2021; 18(18):9465. https://doi.org/10.3390/ijerph18189465
Chicago/Turabian StyleLi, Kate, Karin Ricker, Feng C. Tsai, ChingYi J. Hsieh, Gwendolyn Osborne, Meng Sun, M. Elizabeth Marder, Sarah Elmore, Rose Schmitz, and Martha S. Sandy. 2021. "Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications" International Journal of Environmental Research and Public Health 18, no. 18: 9465. https://doi.org/10.3390/ijerph18189465
APA StyleLi, K., Ricker, K., Tsai, F. C., Hsieh, C. J., Osborne, G., Sun, M., Marder, M. E., Elmore, S., Schmitz, R., & Sandy, M. S. (2021). Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications. International Journal of Environmental Research and Public Health, 18(18), 9465. https://doi.org/10.3390/ijerph18189465






