Examining Wing Length–Abundance Relationships and Pyrethroid Resistance Mutations among Aedes albopictus in a Rapidly Growing Urban Area with Implications for Mosquito Surveillance and Control
Abstract
:1. Introduction
2. Materials and Methods
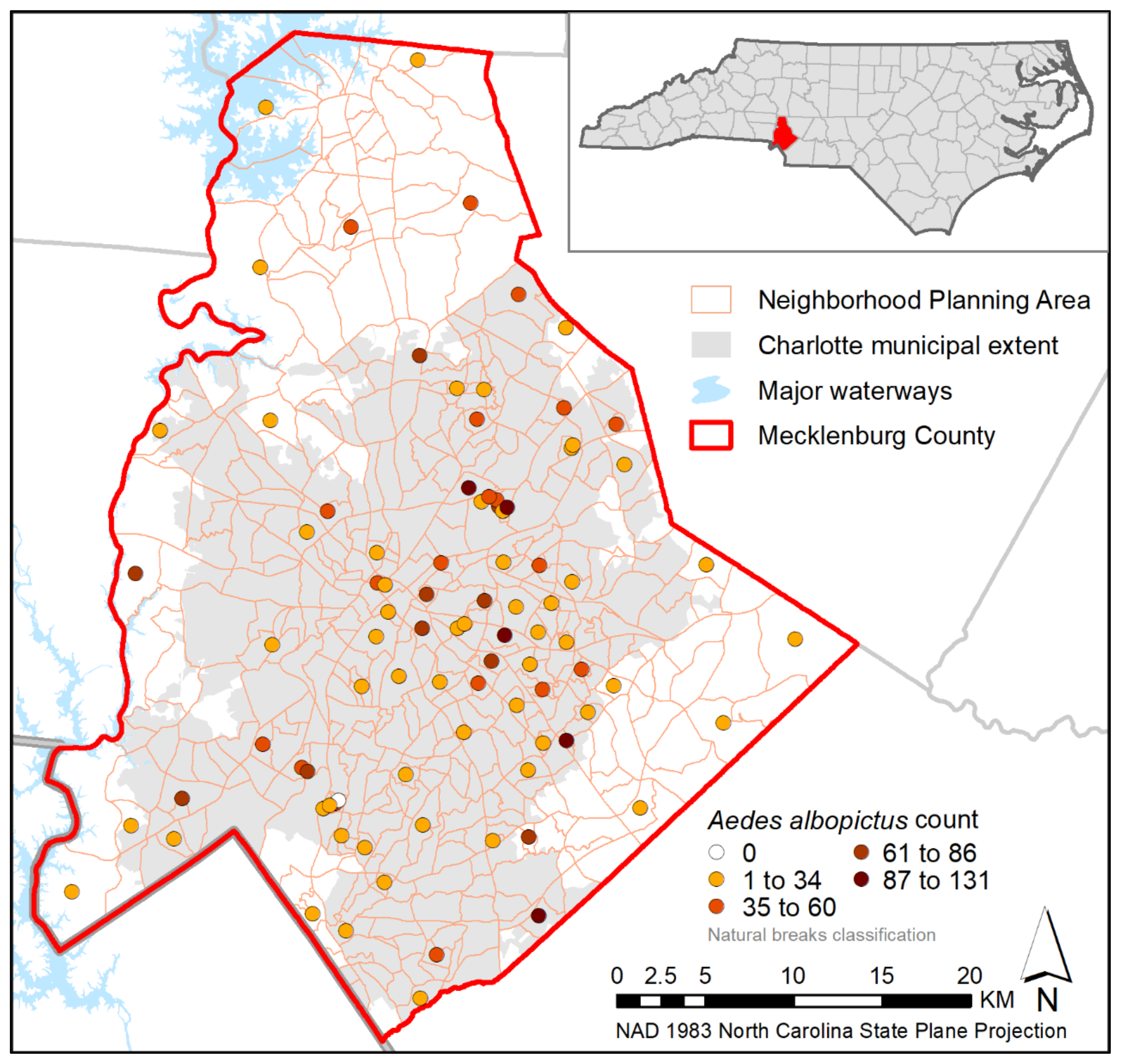
2.1. Study Site
2.2. DNA Extraction, Amplification, and Sequencing
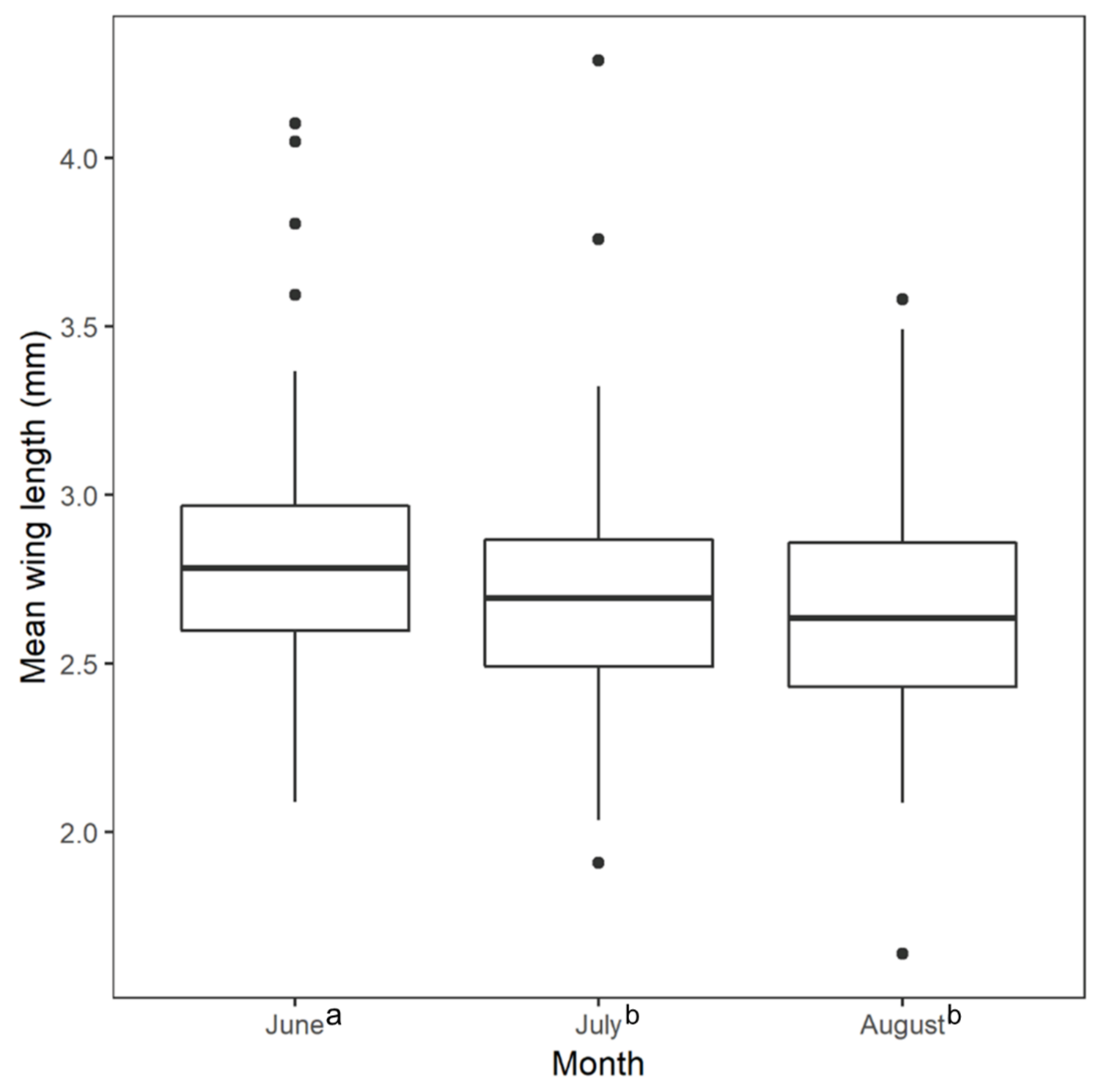
2.3. Wing Length Measurements and Statistical Tests
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| kdr domain II | Forward | 5’-GACAATGTGGATCGCTTCCC-3’ | Kasai et al., 2011 [44] |
| Reverse | 5’-GCAATCTGGCTTGTTAACTTG-3’ | ||
| kdr domain III | Forward | 5’-GAGAACTCGCCGATGAACTT-3’ | Kasai et al., 2011 [44] |
| Reverse | 5’-AACAGCAGGATCATGCTCTG-3’ | ||
| kdr domain IV | Forward | 5’-TCGAGAAGTACTTCGTGTCG-3’ | Kasai et al., 2011 [44] |
| Reverse | 5’-AACAGCAGGATCATGCTCTG-3’ |
Appendix B
| Site ID | Neighborhood Classification | kdr Domain II | kdr Domain IV |
|---|---|---|---|
| 2 | 4 | MZ964062 | |
| 3 | 1 | MZ964063 | |
| 4 | 2 | MZ964064 | |
| 5 | 3 | MZ964065 | |
| 6 | 1 | MZ964066 | |
| 7 | 2 | MZ964067 | |
| 8 | 1 | MZ964068 | |
| 9 | 1 | MZ964069 | |
| 10 | 4 | MZ964070 | |
| 11 | 2 | MZ964035 | MZ964071 |
| 12 | 3 | MZ964072 | |
| 13 | 4 | MZ964073 | |
| 14 | 5 | MZ964074 | |
| 16 | 2 | MZ964036 | MZ964075 |
| 17 | 3 | MZ964037 | MZ964076 |
| 18 | 4 | MZ964077 | |
| 19 | 5 | MZ964078 | |
| 20 | 1 | MZ964038 | MZ964079 |
| 22 | 3 | MZ964080 | |
| 23 | 5 | MZ964039 | MZ964081 |
| 24 | 5 | MZ964040 | MZ964082 |
| 25 | 1 | MZ964083 | |
| 26 | 4 | MZ964084 | |
| 27 | 3 | MZ964041 | MZ964085 |
| 28 | 2 | MZ964057 | MZ964086 |
| 29 | 4 | MZ964087 | |
| 30 | 2 | MZ964058 | MZ964088 |
| 31 | 3 | MZ964089 | |
| 33 | 3 | MZ964042 | MZ964090 |
| 34 | 4 | MZ964091 | |
| 35 | 3 | MZ964092 | |
| 37 | 1 | MZ964093 | |
| 38 | 1 | MZ964094 | |
| 40 | 4 | MZ964043 | |
| 41 | 5 | MZ964056 | MZ964095 |
| 42 | 4 | MZ964044 | MZ964096 |
| 43 | 5 | MZ964097 | |
| 44 | 3 | MZ964045 | MZ964098 |
| 45 | 4 | MZ964099 | |
| 47 | 3 | MZ964100 | |
| 48 | 3 | MZ964101 | |
| 50 | 5 | MZ964046 | MZ964102 |
| 51 | 5 | MZ964047 | |
| 52 | 5 | MZ964103 | |
| 53 | 1 | MZ964048 | MZ964104 |
| 54 | 3 | MZ964049 | MZ964105 |
| 55 | 4 | MZ964106 | |
| 56 | 3 | MZ964107 | |
| 57 | 2 | MZ964050 | MZ964108 |
| 58 | 4 | MZ964109 | |
| 59 | 4 | MZ964110 | |
| 61 | 5 | MZ964051 | MZ964111 |
| 62 | 2 | MZ964052 | MZ964112 |
| 63 | 1 | MZ964113 | |
| 64 | 3 | MZ964114 | |
| 65 | 2 | MZ964115 | |
| 66 | 2 | MZ964053 | MZ964116 |
| 67 | 3 | MZ964117 | |
| 68 | 5 | MZ964118 | |
| 69 | 2 | MZ964054 | MZ964119 |
| 71 | 1 | MZ964120 | |
| 72 | 5 | MZ964121 | |
| 73 | 2 | MZ964122 | |
| 75 | 3 | MZ964123 | |
| 76 | 1 | MZ964124 | |
| 77 | 4 | MZ964125 | |
| 78 | 1 | MZ964055 | MZ964126 |
| 79 | 5 | MZ964127 | |
| 80 | 4 | MZ964059 | MZ964128 |
| 81 | 4 | MZ964129 | |
| 82 | 5 | MZ964130 | |
| 83 | 1 | MZ964131 | |
| 85 | 1 | MZ964132 | |
| 86 | 5 | MZ964133 | |
| 87 | 4 | MZ964134 | |
| 88 | 1 | MZ964060 | |
| 89 | 5 | MZ964061 | MZ964135 |
References
- Hahn, M.B.; Eisen, R.J.; Eisen, L.; Boegler, K.A.; Moore, C.G.; McAllister, J.; Savage, H.M.; Mutebi, J. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae). J. Med. Entomol. 2016, 5, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlandingham, D.L.; Higgs, S.; Huang, Y.J.S. Aedes albopictus (Diptera: Culicidae) and mosquito-borne viruses in the United States. J. Med. Entomol. 2016, 5, 1024–1028. [Google Scholar] [CrossRef]
- Rezza, G. Aedes albopictus and the reemergence of Dengue. BMC Public Health 2012, 1, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delatte, H.; Desvars, A.; Bouétard, A.; Bord, S.; Gimonneau, G.; Vourc’h, G.; Fontenille, D. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector Borne Zoonotic Dis. 2010, 3, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 7, e0005625. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Bargielowski, I.; Carrasquilla, M.C.; Nishimura, N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Peninsular Florida two decades after competitive displacements. J. Med. Entomol. 2016, 6, 1385–1390. [Google Scholar] [CrossRef]
- Delatte, H.; Paupy, C.; Dehecq, J.S.; Thiria, J.; Failloux, A.B.; Fontenille, D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: Biology and control. Parasite 2008, 1, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 4, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 9602, 1840–1846. [Google Scholar] [CrossRef]
- Lindh, E.; Argentini, C.; Remoli, M.E.; Fortuna, C.; Faggioni, G.; Benedetti, E. The Italian 2017 outbreak chikungunya virus belongs to an emerging Aedes albopictus-adapted virus cluster introduced from the Indian Subcontinent. Open Forum Infect. Dis. 2018, 1, ofy321. [Google Scholar]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001, 5, 807. [Google Scholar] [CrossRef] [PubMed]
- Byrd, B.D.; Williams, C.J.; Staples, J.E.; Burkhalter, K.L.; Savage, H.M.; Doyle, M.S. Notes from the Field: Spatially associated coincident and noncoincident cases of La Crosse Encephalitis-North Carolina, 2002–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 39, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microb. Infect. 2009, 14–15, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; White, A.V.; Byrd, B.D.; Reiskind, M.H.; Doyle, M.S. Evaluation of insecticide resistance in Aedes albopictus (Diptera: Culicidae) in North Carolina, 2017. J. Med. Entomol. 2018, 3, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 7, e101992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.; Ramirez, D.; Thomas, C.; Connelly, C.R. Baseline susceptibility status of Florida populations of Aedes aegypti (Diptera: Culicidae) and Aedes albopictus. J. Med. Entomol. 2020, 5, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Dowling, Z.; Ladeau, S.L.; Armbruster, P.; Biehler, D.; Leisnham, P.T. Socioeconomic status affects mosquito (Diptera: Culicidae) larval habitat type availability and infestation level. J. Med. Entomol. 2013, 4, 764–772. [Google Scholar] [CrossRef]
- LaDeau, S.; Leisnham, P.; Biehler, D.; Bodner, D. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: Understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int. J. Environ. Res. Public Health 2013, 4, 1505–1526. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, A.; Delmelle, E.; Rapp, T.; Chen, S.; Chen, G.; Dulin, M. A novel sampling method to measure socioeconomic drivers of Aedes albopictus distribution in Mecklenburg County, North Carolina. Int. J. Environ. Res. Public Health 2018, 10, 2179. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Whiteman, A.; Li, A.; Rapp, T.; Delmelle, E.; Chen, G.; Brown, C.L.; Robinson, P.; Coffman, M.J.; Janies, D.; et al. An operational machine learning approach to predict mosquito abundance based on socioeconomic and landscape patterns. Landsc. Ecol. 2019, 6, 1295–1311. [Google Scholar] [CrossRef]
- Manica, M.; Filipponi, F.; D’Alessandro, A.; Screti, A.; Neteler, M.; Rosà, R.; Solimini, A.; Della Torre, A.; Caputo, B. Spatial and temporal hot spots of Aedes albopictus abundance inside and outside a south European metropolitan area. PLoS Negl. Trop. Dis. 2016, 6, pe0004758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, K.S.; Mormann, K.; Juliano, S.A. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2005, 4, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, P.; Hutchinson, R.A. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae). J. Med. Entomol. 2002, 4, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackmore, M.S.; Lord, C.C. The relationship between size and fecundity in Aedes albopictus. J. Vector Ecol. 2000, 2, 212–217. [Google Scholar]
- Alto, B.W.; Reiskind, M.H.; Lounibos, L.P. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am. J. Trop. Med. Hyg. 2008, 79, 688–695. [Google Scholar] [CrossRef] [Green Version]
- Paulson, S.L.; Hawley, W.A. Effect of body size on the vector competence of field and laboratory populations of Aedes triseriatus for La Crosse virus. J. Am. Mosq. Control. Assoc. 1991, 2, 170–175. [Google Scholar]
- American Community Survey 5-Year Estimate. 2017. Available online: https://data.census.gov/cedsci/table?q=&t=Income%20and%20Poverty&g=0500000US37119&y=2017&tid=ACSST5Y2017.S1701 (accessed on 22 August 2021).
- Chetty, R.; Hendren, N.; Kline, P.; Saez, E. Where is the land of opportunity? The geography of intergenerational mobility in the United States. Q. J. Econ. 2014, 4, 1553–1623. [Google Scholar] [CrossRef] [Green Version]
- Garo, L.; Allen-Handy, A.; Lewis, C.W. Race, poverty, and violence exposure: A critical spatial analysis of African American trauma vulnerability and educational outcomes in Charlotte, North Carolina. J. Negro Educ. 2018, 3, 246–269. [Google Scholar] [CrossRef]
- Potter, M. Identification Key to the Genera of Adult Mosquitoes for the World. Walter Reed Biosystematics Unit. 2017. Available online: http://www.wrbu.org/mqID/keysMQZoogeo.html (accessed on 1 September 2018).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 6, 1547–1549. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 7, 36–42. [Google Scholar]
- O’Neil-Dunne, J.; MacFaden, S.; Royar, A. A versatile, production-oriented approach to high-resolution tree-canopy mapping in urban and suburban landscapes using GEOBIA and data fusion. Remote Sens. 2014, 12, 12837–12865. [Google Scholar] [CrossRef] [Green Version]
- Landau, K.I.; Van Leeuwen, W.J.D. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J. Vector Ecol. 2012, 2, 407–418. [Google Scholar] [CrossRef]
- Murdock, C.C.; Evans, M.V.; McClanahan, T.D.; Miazgowicz, K.L.; Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl. Trop. Dis. 2017, 5, e0005640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 1, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, K.L.; Richards, S.L.; Anderson, A.L.; Balanay, J.A.G. Current status of mosquito control programs in North Carolina: The need for cost-effectiveness analysis. J. Environ. Health 2014, 8, 8–15. [Google Scholar]
- Richards, S.L.; Balanay, J.A.G.; Byrd, B.D.; Reiskind, M.H.; Styers, D.M. Regional survey of mosquito control knowledge and usage in North Carolina. J. Am. Mosq. Control. Assoc. 2017, 4, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.K.; Bradley, C.; Apperson, C.S.; Gould, F. An experimental field study of delayed density dependence in natural populations of Aedes albopictus. PLoS ONE 2012, 4, e35959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Climate Data. Available online: https://www.usclimatedata.com/climate/charlotte/north-carolina/united-states/usnc0121 (accessed on 22 August 2021).
- Alto, B.W.; Juliano, S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): Implications for range expansion. J. Med. Entomol. 2001, 5, 646–656. [Google Scholar] [CrossRef] [Green Version]
- Reiskind, M.H.; Zarrabi, A.A. Water surface area and depth determine oviposition choice in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2012, 1, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Ng, L.C.; Lam-Phua, S.G.; Tang, C.S.; Itokawa, K.; Komagata, O.; Kobayash, M.; Tomita, T. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus. Jpn. J. Infect. Dis. 2011, 64, 217–221. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundis, S.J.; Hamerlinck, G.; Stone, E.K.; Whiteman, A.; Delmelle, E.; Rapp, T.; Dulin, M.; Ryan, S.J. Examining Wing Length–Abundance Relationships and Pyrethroid Resistance Mutations among Aedes albopictus in a Rapidly Growing Urban Area with Implications for Mosquito Surveillance and Control. Int. J. Environ. Res. Public Health 2021, 18, 9443. https://doi.org/10.3390/ijerph18189443
Mundis SJ, Hamerlinck G, Stone EK, Whiteman A, Delmelle E, Rapp T, Dulin M, Ryan SJ. Examining Wing Length–Abundance Relationships and Pyrethroid Resistance Mutations among Aedes albopictus in a Rapidly Growing Urban Area with Implications for Mosquito Surveillance and Control. International Journal of Environmental Research and Public Health. 2021; 18(18):9443. https://doi.org/10.3390/ijerph18189443
Chicago/Turabian StyleMundis, Stephanie J., Gabriela Hamerlinck, Emily K. Stone, Ari Whiteman, Eric Delmelle, Tyler Rapp, Michael Dulin, and Sadie J. Ryan. 2021. "Examining Wing Length–Abundance Relationships and Pyrethroid Resistance Mutations among Aedes albopictus in a Rapidly Growing Urban Area with Implications for Mosquito Surveillance and Control" International Journal of Environmental Research and Public Health 18, no. 18: 9443. https://doi.org/10.3390/ijerph18189443
APA StyleMundis, S. J., Hamerlinck, G., Stone, E. K., Whiteman, A., Delmelle, E., Rapp, T., Dulin, M., & Ryan, S. J. (2021). Examining Wing Length–Abundance Relationships and Pyrethroid Resistance Mutations among Aedes albopictus in a Rapidly Growing Urban Area with Implications for Mosquito Surveillance and Control. International Journal of Environmental Research and Public Health, 18(18), 9443. https://doi.org/10.3390/ijerph18189443








