Evaluating Therapeutic Effects of ADHD Medication Objectively by Movement Quantification with a Video-Based Skeleton Analysis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Participants
2.2. Movements Recording and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of adhd: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef]
- Taylor, E.; Chadwick, O.; Heptinstall, E.; Danckaerts, M. Hyperactivity and conduct problems as risk factors for adolescent development. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, D.M.; Lynskey, M.T.; Horwood, L.J. Attentional difficulties in middle childhood and psychosocial outcomes in young adulthood. J. Child Psychol. Psychiatry Allied Discip. 1997, 38, 633–644. [Google Scholar] [CrossRef]
- Merrell, C.; Tymms, P.B. Inattention, hyperactivity and impulsiveness: Their impact on academic achievement and progress. Br. J. Educ. Psychol. 2001, 71, 43–56. [Google Scholar] [CrossRef]
- McGee, R.; Prior, M.; Willams, S.; Smart, D.; Sanson, A. The long-term significance of teacher-rated hyperactivity and reading ability in childhood: Findings from two longitudinal studies. J. Child Psychol. Psychiatry Allied Discip. 2002, 43, 1004–1017. [Google Scholar] [CrossRef]
- Willoughby, M.T. Developmental course of adhd symptomatology during the transition from childhood to adolescence: A review with recommendations. J. Child Psychol. Psychiatry Allied Discip. 2003, 44, 88–106. [Google Scholar] [CrossRef]
- Barbaresi, W.J.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Jacobsen, S.J. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: A population-based perspective. J. Dev. Behav. Pediatrics JDBP 2007, 28, 265–273. [Google Scholar] [CrossRef]
- Galéra, C.; Bouvard, M.P.; Encrenaz, G.; Messiah, A.; Fombonne, E. Hyperactivity-inattention symptoms in childhood and suicidal behaviors in adolescence: The youth gazel cohort. Acta Psychiatr. Scand. 2008, 118, 480–489. [Google Scholar] [CrossRef]
- Aldemir, R.; Demirci, E.; Bayram, A.K.; Canpolat, M.; Ozmen, S.; Per, H.; Tokmakci, M. Evaluation of two types of drug treatment with qeeg in children with adhd. Transl. Neurosci. 2018, 9, 106–116. [Google Scholar] [CrossRef]
- Jensen, P.S. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 1999, 56, 1073–1086. [Google Scholar]
- Cornforth, C.; Sonuga-Barke, E.; Coghill, D. Stimulant drug effects on attention deficit/hyperactivity disorder: A review of the effects of age and sex of patients. Curr. Pharm. Des. 2010, 16, 2424–2433. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.M.; Kraemer, H.C.; Hinshaw, S.P.; Arnold, L.E.; Conners, C.K.; Abikoff, H.B.; Clevenger, W.; Davies, M.; Elliott, G.R.; Greenhill, L.L.; et al. Clinical relevance of the primary findings of the mta: Success rates based on severity of adhd and odd symptoms at the end of treatment. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Wolraich, M.L.; Lambert, W.; Doffing, M.A.; Bickman, L.; Simmons, T.; Worley, K. Psychometric properties of the vanderbilt adhd diagnostic parent rating scale in a referred population. J. Pediatric Psychol. 2003, 28, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Woolsey, C.; Smoldon, J.; Devney, R. Initial development of an attention-deficit/hyperactivity disorder visual analog scale for rapid assessment of medication effects. J. Am. Assoc. Nurse Pract. 2020, 32, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tateuchi, H.; Koyama, Y.; Akiyama, H.; Goto, K.; So, K.; Kuroda, Y.; Ichihashi, N. Radiographic and clinical factors associated with one-leg standing and gait in patients with mild-to-moderate secondary hip osteoarthritis. Gait Posture 2016, 49, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Tateuchi, H.; Shiratori, S.; Ichihashi, N. The effect of three-dimensional postural change on shear elastic modulus of the iliotibial band. J. Electromyogr. Kinesiol. 2016, 28, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kwon, O.Y.; Park, K.N.; Jeon, I.C.; Weon, J.H. Lower extremity strength and the range of motion in relation to squat depth. J. Hum. Kinet. 2015, 45, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Tseng, M.H.; Henderson, A.; Chow, S.M.; Yao, G. Relationship between motor proficiency, attention, impulse, and activity in children with adhd. Dev. Med. Child Neurol. 2004, 46, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.C.; Asherson, P.; Rijsdijk, F.; Kuntsi, J. Is overactivity a core feature in adhd? Familial and receiver operating characteristic curve analysis of mechanically assessed activity level. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1023–1030. [Google Scholar] [CrossRef]
- Garcia Murillo, L.; Cortese, S.; Anderson, D.; Di Martino, A.; Castellanos, F.X. Locomotor activity measures in the diagnosis of attention deficit hyperactivity disorder: Meta-analyses and new findings. J. Neurosci. Methods 2015, 252, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, H.; Qin, L.; Li, D.; Zhang, X.; Johnstone, S.J. Aiding the diagnosis of ad/hd in childhood: Using actigraphy and a continuous performance test to objectively quantify symptoms. Res. Dev. Disabil. 2016, 59, 35–42. [Google Scholar] [CrossRef]
- Martin-Martinez, D.; Casaseca-de-la-Higuera, P.; Alberola-Lopez, S.; Andres-de-Llano, J.; Lopez-Villalobos, J.A.; Ardura-Fernandez, J.; Alberola-Lopez, C. Nonlinear analysis of actigraphic signals for the assessment of the attention-deficit/hyperactivity disorder (adhd). Med. Eng. Phys. 2012, 34, 1317–1329. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Armando, M.; Mazzone, L.; Ciliberto, M.; Sciannamea, M.; Figueroa, C.; Janiri, L.; Quested, D.; Vicari, S. The use of actigraphy in the monitoring of methylphenidate versus placebo in adhd: A meta-analysis. Atten. Deficit Hyperact. Disord. 2014, 6, 49–58. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Licchelli, S.; Ciabattini, M.; Menghini, D.; Armando, M.; Alfieri, P.; Mazzone, L.; Pontrelli, G.; Livadiotti, S.; Foti, F.; et al. The use of actigraphy in the monitoring of sleep and activity in adhd: A meta-analysis. Sleep Med. Rev. 2016, 26, 9–20. [Google Scholar] [CrossRef]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.E.; Sheikh, Y. Openpose: Realtime multi-person 2d pose estimation using part affinity fields. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 43, 172–186. [Google Scholar] [CrossRef] [Green Version]
- Hovorka, M.; Jung, C.; Langenau, T.; Lütge, P.; Podest, P.; Schrenk, V.; Tiefengraber, B.; Stifter, M.J.J.J. Opening Opencv, Junior Journal. 2014; 1–7. [Google Scholar]
- Wilens, T.; Pelham, W.; Stein, M.; Conners, C.K.; Abikoff, H.; Atkins, M.; August, G.; Greenhill, L.; McBurnett, K.; Palumbo, D.; et al. Adhd treatment with once-daily oros methylphenidate: Interim 12-month results from a long-term open-label study. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Vance, A. A current treatment approach for attention deficit hyperactivity disorder. Aust. Prescr. 2008, 31, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Box, G.E.P.; Jenkins, G.M.; Reinsel, G.C. Time Series Analysis; Forecasting and Control, 3rd ed.; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Janet, B.W.; Fi Spitzer, R.L.; Gibbon, M.; Skodol, A.E.; Williams. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- Association, A.P. Diagnostic and statistical manual of mental disorders. BMC Med. 2013, 17, 133–137. [Google Scholar]
- Costa, D.S.; de Paula, J.J.; Malloy-Diniz, L.F.; Romano-Silva, M.A.; Miranda, D.M. Parent snap-iv rating of attention-deficit/hyperactivity disorder: Accuracy in a clinical sample of adhd, validity, and reliability in a brazilian sample. J. Pediatr. (Rio. J.) 2019, 95, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.L.; Guo, B.; Valentine, A.Z.; Groom, M.J.; Daley, D.; Sayal, K.; Hollis, C. The validity of the snap-iv in children displaying adhd symptoms. Assessment 2020, 27, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Gau, S.S.; Shang, C.Y.; Liu, S.K.; Lin, C.H.; Swanson, J.M.; Liu, Y.C.; Tu, C.L. Psychometric properties of the chinese version of the swanson, nolan, and pelham, version iv scale-parent form. Int. J. Methods Psychiatr. Res. 2008, 17, 35–44. [Google Scholar] [CrossRef]
- Sato, K.; Nagashima, Y.; Mano, T.; Iwata, A.; Toda, T. Quantifying normal and parkinsonian gait features from home movies: Practical application of a deep learning-based 2d pose estimator. PLoS ONE 2019, 14, e0223549. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Gabriel, P.; Alasfour, A.; Gong, C.; Doyle, W.K.; Devinsky, O.; Friedman, D.; Dugan, P.; Melloni, L.; Thesen, T.; et al. Patient-specific pose estimation in clinical environments. IEEE J. Transl. Eng. Health Med. 2018, 6, 2101111. [Google Scholar] [CrossRef]
- Boswell, M.A.; Uhlrich, S.D.; Kidziński, Ł.; Thomas, K.; Kolesar, J.A.; Gold, G.E.; Beaupre, G.S.; Delp, S.L. A neural network to predict the knee adduction moment in patients with osteoarthritis using anatomical landmarks obtainable from 2d video analysis. Osteoarthr. Cartil. 2021, 29, 346–356. [Google Scholar] [CrossRef]
- Grunwald, J.; Schlarb, A.A. Relationship between subtypes and symptoms of adhd, insomnia, and nightmares in connection with quality of life in children. Neuropsychiatr. Dis. Treat. 2017, 13, 2341–2350. [Google Scholar] [CrossRef] [Green Version]
- AlZaben, F.N.; Sehlo, M.G.; Alghamdi, W.A.; Tayeb, H.O.; Khalifa, D.A.; Mira, A.T.; Alshuaibi, A.M.; Alguthmi, M.A.; Derham, A.A.; Koenig, H.G. Prevalence of attention deficit hyperactivity disorder and comorbid psychiatric and behavioral problems among primary school students in western saudi arabia. Saudi Med. J. 2018, 39, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Migliarese, G.; Venturi, V.; Rossi, F.; Torriero, S.; Vigano, V.; Cerveri, G.; Mencacci, C. Adhd in adults: Clinical subtypes and associated characteristics. Riv. Psichiatr. 2019, 54, 84–89. [Google Scholar]
- Sempere-Tortosa, M.; Fernández-Carrasco, F.; Mora-Lizán, F.; Rizo-Maestre, C. Objective analysis of movement in subjects with adhd. Multidisciplinary control tool for students in the classroom. Int. J. Environ. Res. Public Health 2020, 17, 5620. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Tortosa, M.; Fernández-Carrasco, F.; Navarro-Soria, I.; Rizo-Maestre, C. Movement patterns in students diagnosed with adhd, objective measurement in a natural learning environment. Int. J. Environ. Res. Public Health 2021, 18, 3870. [Google Scholar] [CrossRef]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ (Clin. Res. Ed.) 2015, 351, h5203. [Google Scholar] [CrossRef] [Green Version]
- Storebø, O.J.; Ramstad, E.; Krogh, H.B.; Nilausen, T.D.; Skoog, M.; Holmskov, M.; Rosendal, S.; Groth, C.; Magnusson, F.L.; Moreira-Maia, C.R.; et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (adhd). Cochrane Database Syst. Rev. 2015, 11, 1–692, Cd009885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storebø, O.J.; Gluud, C. Methylphenidate for adhd rejected from the who essential medicines list due to uncertainties in benefit-harm profile. BMJ Evid.-Based Med. 2021, 26, 172–175. [Google Scholar] [CrossRef] [PubMed]
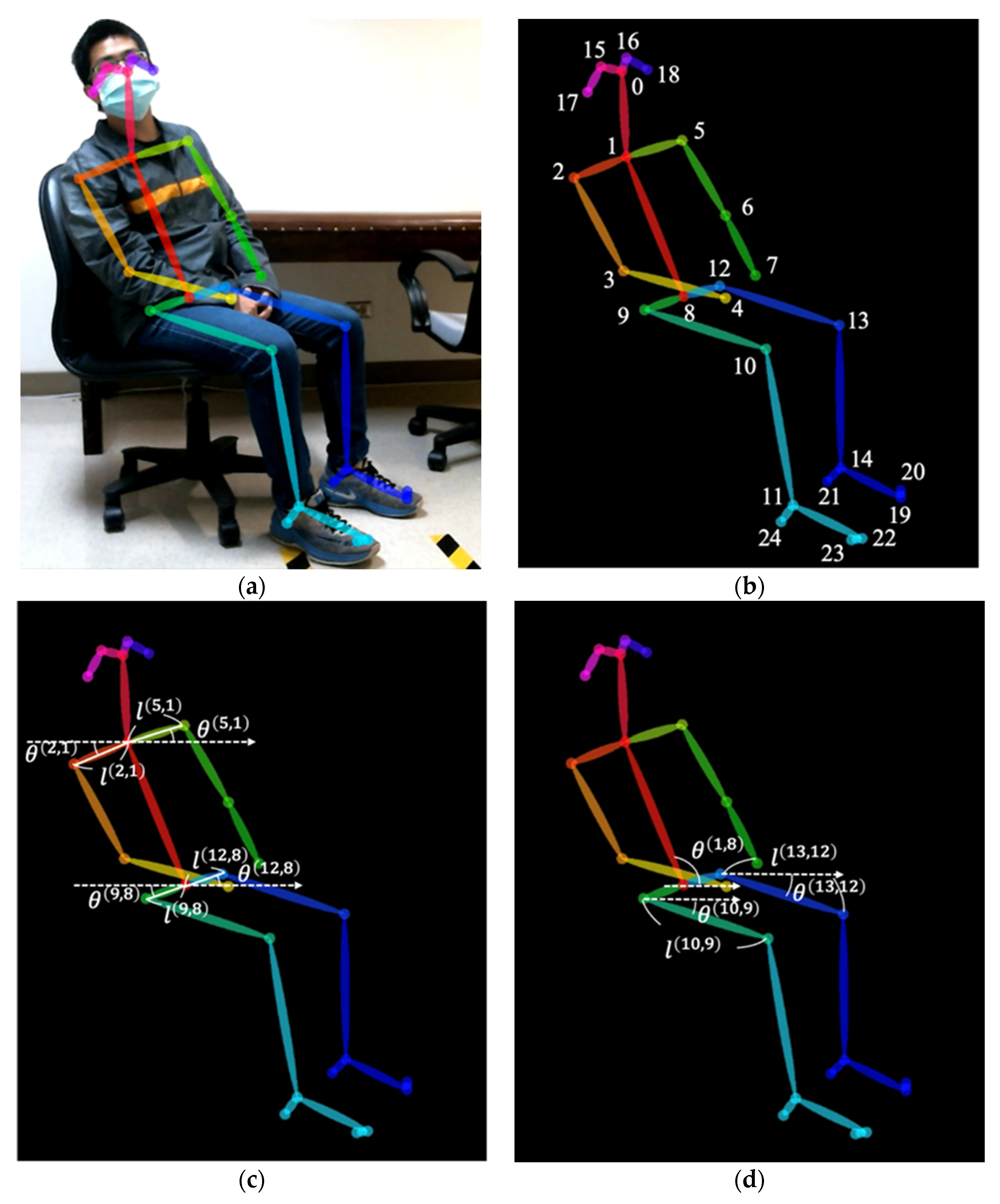

| Parameters | Before Treatment | After Treatment | p Value |
|---|---|---|---|
| Inattentiveness (P) | 15.79 ± 5.36 | 13.71 ± 5.08 | 0.3307 |
| Hyperactivity (P) | 14.86 ± 5.24 | 11.29 ± 5.42 | 0.0322 * |
| Oppositional (P) | 11.57 ± 5.33 | 11.36 ± 6.12 | 0.8944 |
| Inattentiveness (T) | 15.14 ± 4.52 | 15.71 ± 5.36 | 0.7249 |
| Hyperactivity (T) | 13.14 ± 7.64 | 9.21 ± 6.99 | 0.0476 * |
| Oppositional (T) | 9.43 ± 5.27 | 6.71 ± 5.53 | 0.0492 * |
| Parameters | Before Treatment | After Treatment | p Value |
|---|---|---|---|
| Left Shoulder angle | 0.94 ± 0.03 | 0.94 ± 0.03 | 0.3990 |
| Left Shoulder length | 0.98 ± 0.15 | 0.97 ± 0.02 | 0.0311 * |
| Right Shoulder angle | 0.94 ± 0.03 | 0.95 ± 0.03 | 0.4068 |
| Right Shoulder length | 0.98 ± 0.01 | 0.96 ± 0.05 | 0.0441 * |
| Left hip angle | 0.92 ± 0.03 | 0.91 ± 0.05 | 0.6704 |
| Left hip length | 0.96 ± 0.03 | 0.92 ± 0.06 | 0.0082 ** |
| Right hip angle | 0.93 ± 0.04 | 0.91 ± 0.07 | 0.3884 |
| Right hip length | 0.97 ± 0.02 | 0.95 ± 0.03 | 0.0005 *** |
| Right thigh angle | 0.98 ± 0.03 | 0.98 ± 0.02 | 0.7906 |
| Right thigh length | 0.98 ± 0.02 | 0.97 ± 0.03 | 0.1706 |
| Trunk angle | 0.99 ± 0.02 | 0.97 ± 0.03 | 0.1177 |
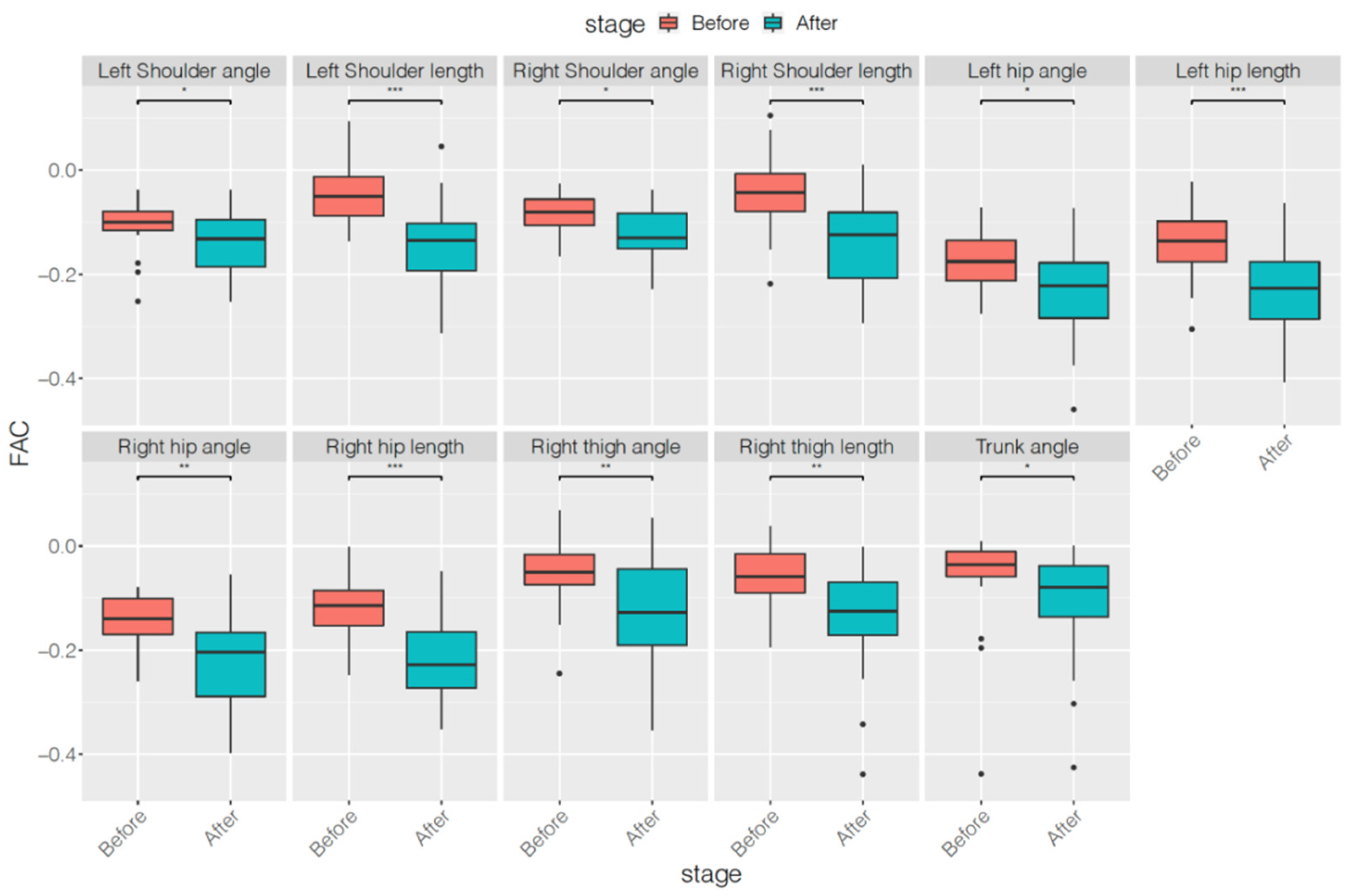
| Parameters | Before Treatment | After Treatment | p Value |
|---|---|---|---|
| Left Shoulder angle | −0.11 ± 0.05 | −0.14 ± 0.06 | 0.0387 * |
| Left Shoulder length | −0.05 ± 0.06 | −0.14 ± 0.08 | <0.0001 *** |
| Right Shoulder angle | −0.08 ± 0.04 | −0.12 ± 0.06 | 0.0191 * |
| Right Shoulder length | −0.05 ± 0.07 | −0.13 ± 0.09 | <0.0001 *** |
| Left hip angle | −0.18 ± 0.06 | −0.23 ± 0.09 | 0.0112 * |
| Left hip length | −0.14 ± 0.07 | −0.23 ± 0.08 | 0.0002 *** |
| Right hip angle | −0.15 ± 0.05 | −0.21 ± 0.10 | 0.0010 ** |
| Right hip length | −0.12 ± 0.06 | −0.21 ± 0.09 | <0.0001 *** |
| Right thigh angle | −0.05 ± 0.07 | −0.12 ± 0.10 | 0.0017 ** |
| Right thigh length | −0.06 ± 0.06 | −0.14 ± 0.10 | 0.0043 ** |
| Trunk angle | −0.06 ± 0.09 | −0.11 ± 0.11 | 0.0470 * |
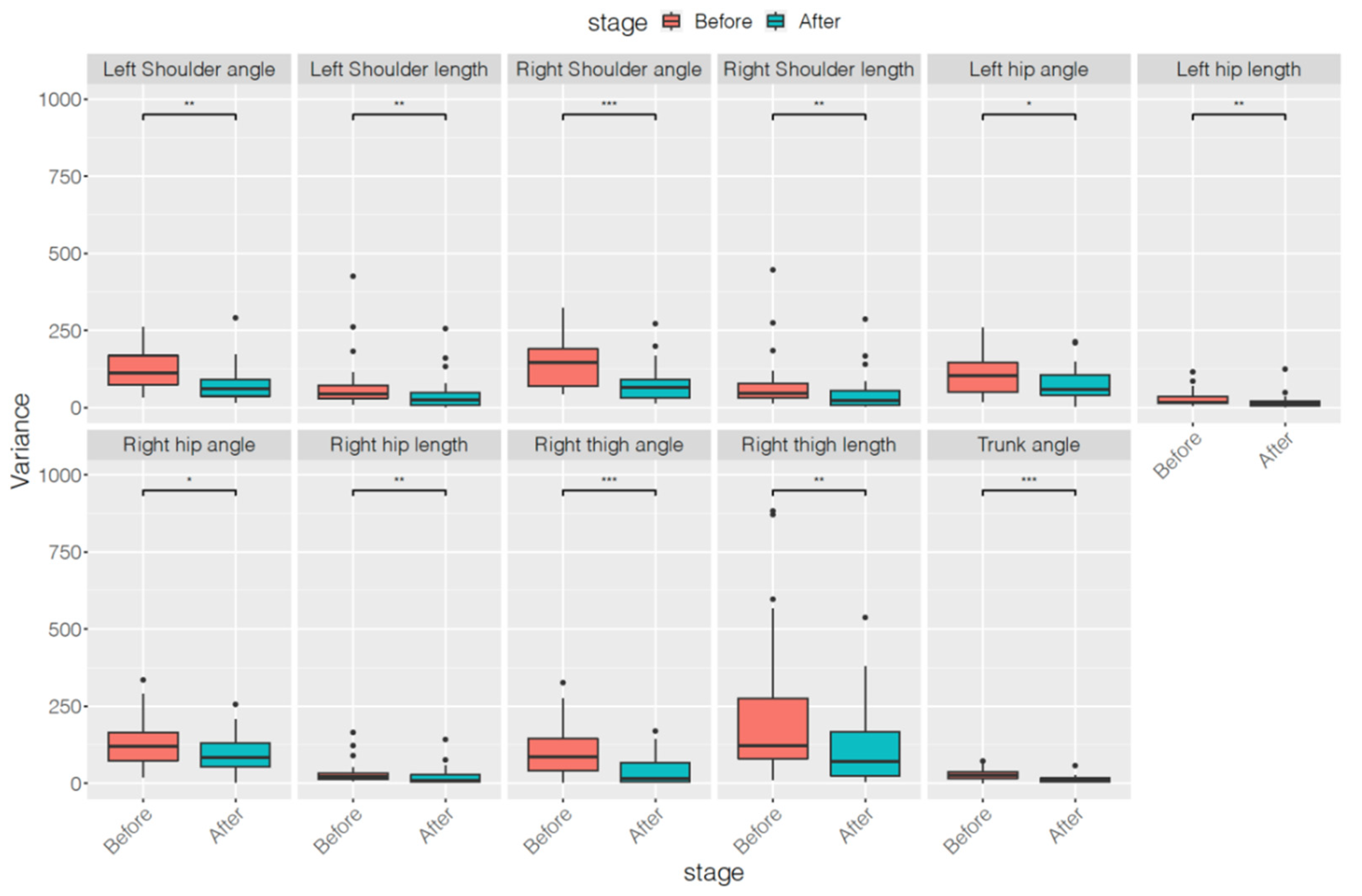
| Parameters | Before Treatment | After Treatment | p Value |
|---|---|---|---|
| Left Shoulder angle | 118.83 ± 60.41 | 75.38 ± 61.48 | 0.0042 ** |
| Left Shoulder length | 76.63 ± 92.18 | 43.32 ± 59.61 | 0.0043 ** |
| Right Shoulder angle | 141.41 ± 68.36 | 79.30 ± 63.18 | 0.0009 *** |
| Right Shoulder length | 80.77 ± 95.89 | 48.93 ± 65.17 | 0.0072 ** |
| Left hip angle | 109.00 ± 65.89 | 75.56 ± 55.56 | 0.0220 * |
| Left hip length | 28.70 ± 27.01 | 18.41 ± 25.06 | 0.0039 ** |
| Right hip angle | 134.14 ± 81.47 | 97.67 ± 65.26 | 0.0310 * |
| Right hip length | 34.34 ± 38.00 | 22.62 ± 31.20 | 0.0096 ** |
| Right thigh angle | 110.61 ± 89.58 | 40.80 ± 47.95 | <0.0001 *** |
| Right thigh length | 295.68 ± 366.08 | 136.11 ± 151.64 | 0.0039 ** |
| Trunk angle | 28.80 ± 18.54 | 12.57 ± 11.64 | 0.0003 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, C.-S.; Chiu, Y.-H.; Chiang, C.-T.; Wu, R.-C.; Lin, Y.-T.; Yang, R.-C.; Lin, L.-C. Evaluating Therapeutic Effects of ADHD Medication Objectively by Movement Quantification with a Video-Based Skeleton Analysis. Int. J. Environ. Res. Public Health 2021, 18, 9363. https://doi.org/10.3390/ijerph18179363
Ouyang C-S, Chiu Y-H, Chiang C-T, Wu R-C, Lin Y-T, Yang R-C, Lin L-C. Evaluating Therapeutic Effects of ADHD Medication Objectively by Movement Quantification with a Video-Based Skeleton Analysis. International Journal of Environmental Research and Public Health. 2021; 18(17):9363. https://doi.org/10.3390/ijerph18179363
Chicago/Turabian StyleOuyang, Chen-Sen, Yi-Hung Chiu, Ching-Tai Chiang, Rong-Ching Wu, Ying-Tong Lin, Rei-Cheng Yang, and Lung-Chang Lin. 2021. "Evaluating Therapeutic Effects of ADHD Medication Objectively by Movement Quantification with a Video-Based Skeleton Analysis" International Journal of Environmental Research and Public Health 18, no. 17: 9363. https://doi.org/10.3390/ijerph18179363
APA StyleOuyang, C.-S., Chiu, Y.-H., Chiang, C.-T., Wu, R.-C., Lin, Y.-T., Yang, R.-C., & Lin, L.-C. (2021). Evaluating Therapeutic Effects of ADHD Medication Objectively by Movement Quantification with a Video-Based Skeleton Analysis. International Journal of Environmental Research and Public Health, 18(17), 9363. https://doi.org/10.3390/ijerph18179363






