Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management
Abstract
1. Introduction
2. Clinical Features and Relationships
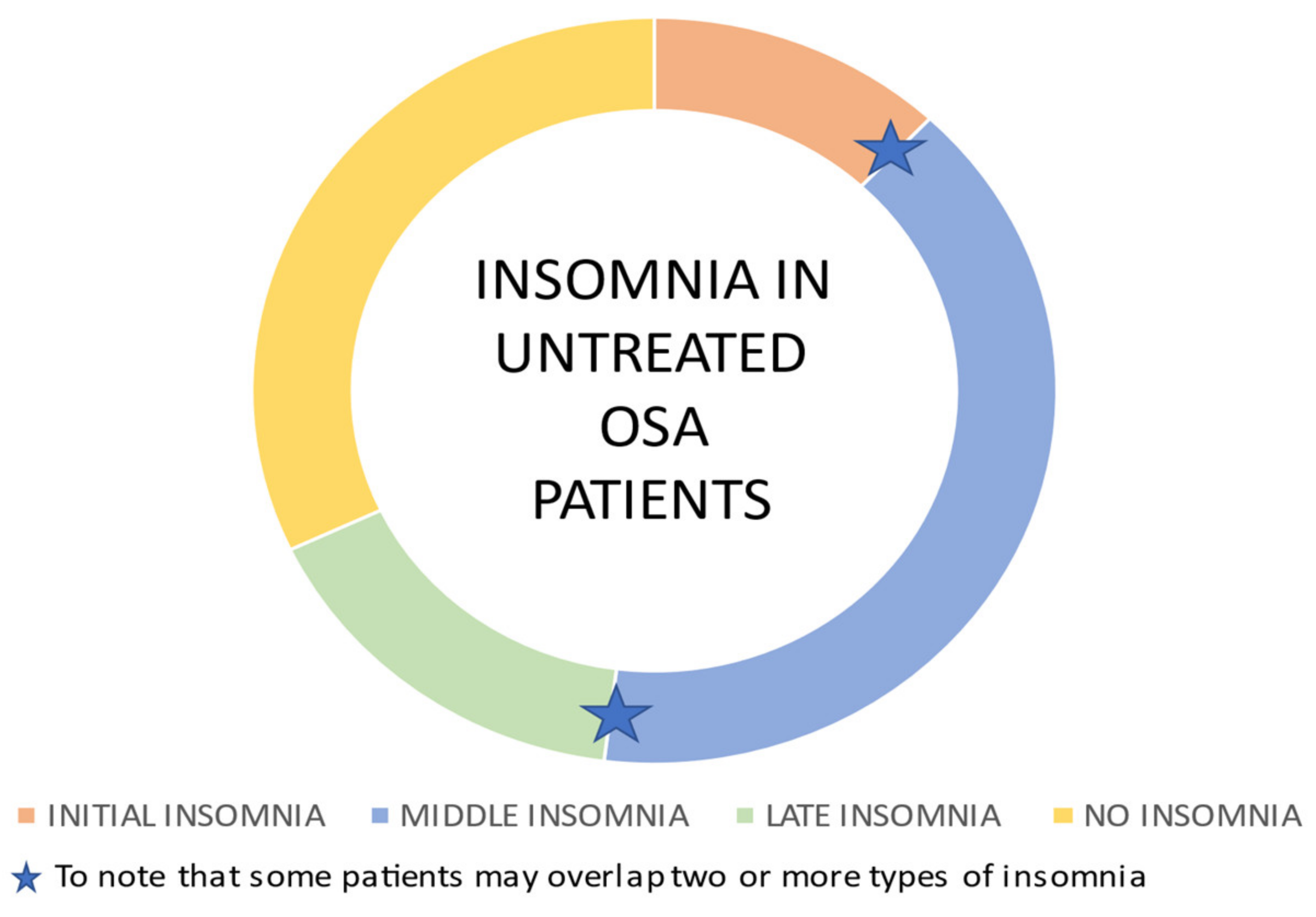
2.1. Insomnia and Sleep Apnea
2.2. Patients Phenotypes
3. Pathogenetic Mechanisms and Models
3.1. Systemic Mechanisms
3.2. Molecular Mechanism
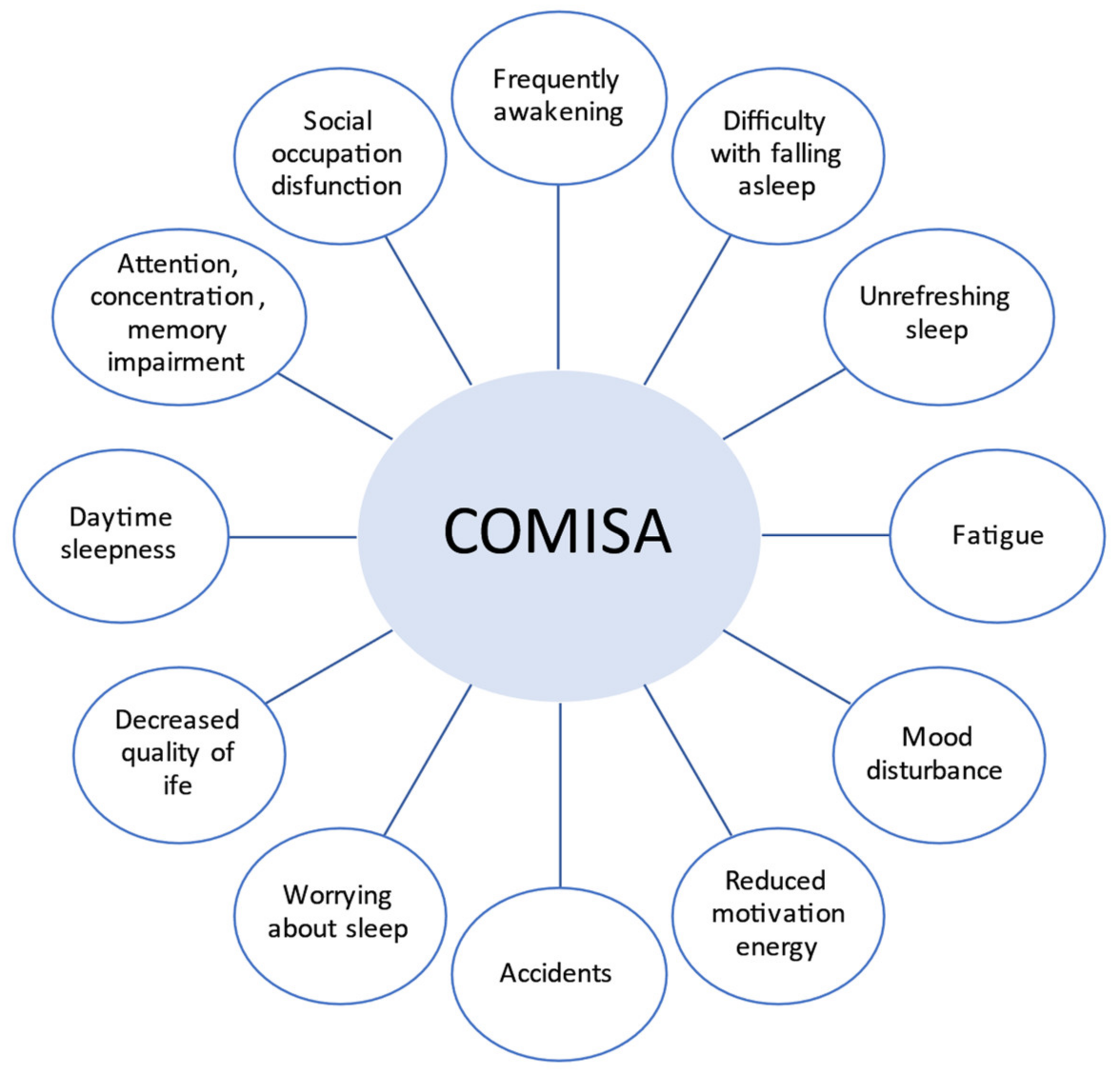
4. Diagnostic Evaluation of COMISA and Clinical Implications
5. Current Concepts of Treatment of COMISA
5.1. Positive Airway Pressure Devices
5.2. Oral Appliance and Surgery
5.3. Cognitive-Behavioral Therapy for Insomnia
5.4. Benzodiazepine Agents
5.5. Non-Benzodiazepine Agents
5.6. Anti-Depressive Agents
5.7. Sleep Hygiene Education
5.8. Melatonin and Herbal Therapies
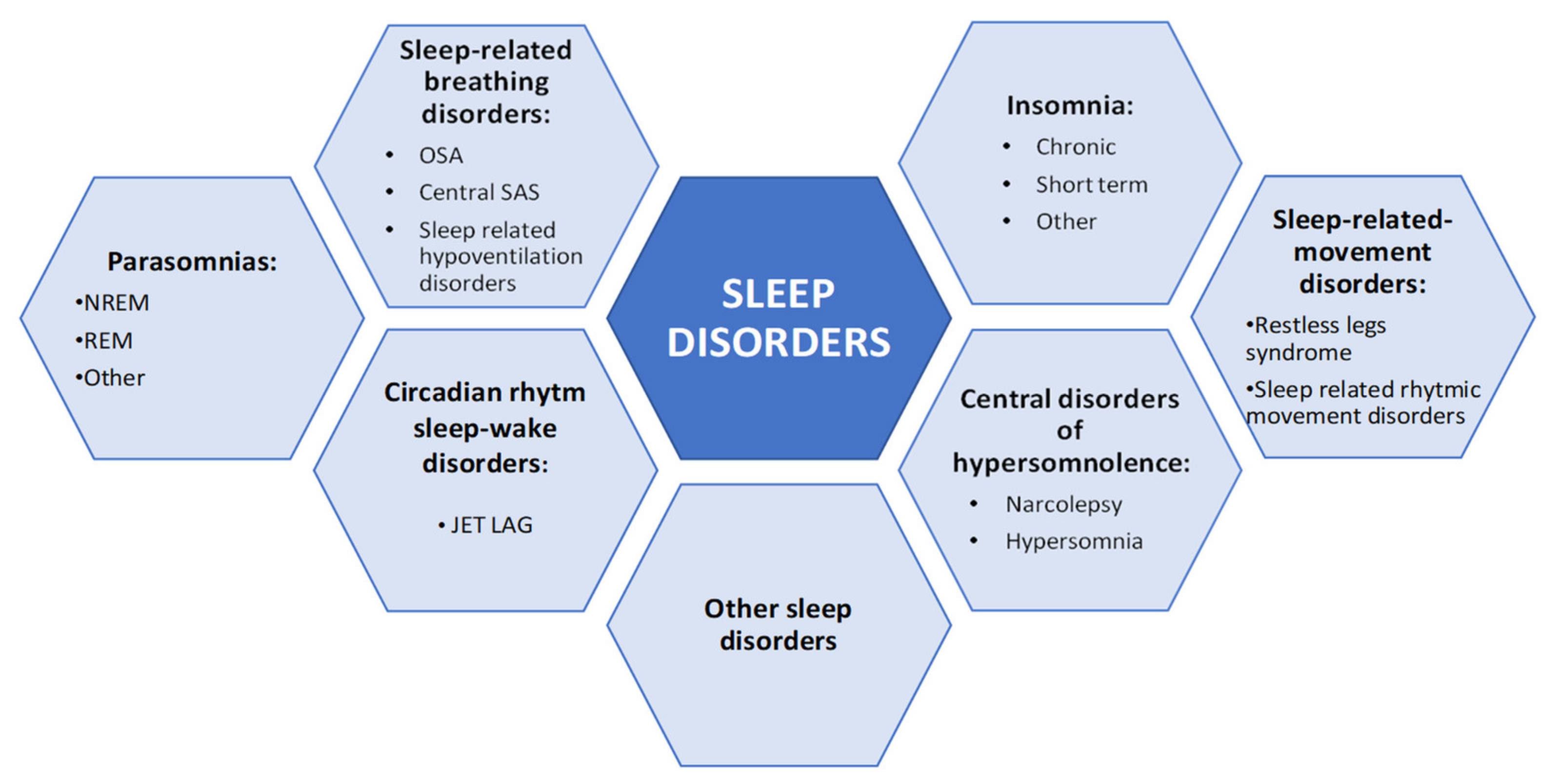
6. COMISA and Other Sleep Disorders
6.1. Sleep Related Movement Disorders
6.2. Epilepsy and Parasomnias
6.3. Central Disorders: Narcolepsy
6.4. Circadian Rhythm Disorders
7. Implications of the Findings and Future Research
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, S.; Sullivan, K.; Hopkins, W.; Douglas, J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med. 2004, 5, 449–456. [Google Scholar] [CrossRef]
- Ohayon, M. Epidemiological Overview of sleep Disorders in the General Population. Sleep Med. Res. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Collop, N.A. Insomnia and sleep-related breathing disorders. Chest 2010, 137, 1449–1463. [Google Scholar] [CrossRef]
- Bjornsdottir, E.; Janson, C.; Sigurdsson, F.; Gehrman, P.; Perlis, M.; Juliusson, S.; Arnardottir, E.S.; Kuna, S.T.; Pack, A.I.; Gislason, T. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive air way pressure treatment. Sleep 2013, 36, 1901–1909. [Google Scholar] [CrossRef]
- Nguyen, X.; Chaskalovic, J.; Rakotonanahary, D.; Fleurya, B. Insomnia symptoms and CPAP compliance in OSAS patients: A descriptive study using Data Mining methods. Sleep Med. 2010, 11, 777–784. [Google Scholar] [CrossRef]
- Williams, J.; Roth, A.; Vatthauer, K.; McCrae, C.S. Cognitive Behavioral Treatment of Insomnia. Chest 2013, 143, 554–565. [Google Scholar] [CrossRef]
- Krakov, B.; Melendrez, D.; Ferreira, E.; Clark, J.; Warner, T.D.; Sisley, B.; Sklar, D. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 2001, 120, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Tasbakan, M.S.; Gunduz, C.; Pirildar, S.; Basoglu, O.K. Quality of life in obstructive sleep apnea is related to female gender and comorbid insomnia. Sleep Breath. 2018, 22, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, K.L.; Justin Thomas, S.; Woosley, J.A.; Geyer, J.D. Co-occurring insomnia and obstructive sleep apnea. Sleep Med. 2013, 14, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M. (Ed.) The International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, CT, USA, 2014; pp. 13–18. [Google Scholar]
- Janssen, H.C.J.P.; Venekamp, L.N.; Peeters, G.A.M.; Pijpers, A.; Pevernagie, D.A.A. Management of insomnia in sleep disorder breathing. Eur. Respir. Rev. 2019, 28, 190080. [Google Scholar] [CrossRef] [PubMed]
- Bahr, K.; Camara, R.J.A.; Gouveris, H.; Tuin, I. Current treatment of comorbid insomnia and obstructive sleep apnea with CBTI and PAP-therapy: A systematic review. Front. Neurol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Guilleminault, C.; Eldridge, F.L.; Dement, W.C. Insomnia with sleep apnea: A new syndrome. Science 1973, 181, 856–858. [Google Scholar] [CrossRef]
- Luyster, F.S.; Buysse, D.J.; Strollo, P.J. Comorbid insomnia and obstructive sleep apnea: Challenges for clinical practice and research. J. Clin. Sleep Med. 2010, 6, 196–204. [Google Scholar] [CrossRef]
- Ong, J.C.; Crawford, M.R. Insomnia and obstructive sleep apnea. Sleep Med. Clin. 2013, 8, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.; Eisenschenk, S.; Robinson, M. Severity and likelihood of chronic pain in individuals with obstructive sleep apnea and insomnia. J. Pain 2017, 18, 573. [Google Scholar] [CrossRef]
- Krell, S.B.; Kapur, V.K. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Liu, T.C.; Chung, C.H.; Chen, W.C. Association between sleep disorders (SD) and hypertension in Taiwan: A nationwide population-based retrospective cohort study. J. Hum. Hypertens. 2017, 31, 220–224. [Google Scholar] [CrossRef]
- Vozoris, N.T. Sleep apnea-plus: Prevalence, risk factors, and association with cardiovascular disease using United States population-level data. Sleep Med. 2012, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Meira e Cruz, M.; Seixas, L.; Santos, A.; Garrido, J.; Lopes, Y.; Paolombini, L.; Gozal, D.; Galtieri, R.; Sales, C. Cardiovascular and metabolic risk in patients with suspected comorbid insomnia and obstructive sleep apnea (COMISA). Sleep 2021, 44, A189–A190. [Google Scholar] [CrossRef]
- Gupta, M.A.; Knapp, K. Cardiovascular and Psychiatric Morbidity in Obstructive Sleep Apnea (OSA) with Insomnia (Sleep Apnea Plus) versus Obstructive Sleep Apnea without Insomnia: A Case-Control Study from Nationally Representative US sample. PLoS ONE 2014, 9, e90021. [Google Scholar] [CrossRef]
- Cho, Y.W.; Kim, K.T.; Moon, H.J.; Korostyshevskiy, V.R.; Motamedi, G.K.; Yang, K.I. Comorbid insomnia with obstructive sleep apnea: Clinical characteristics and risk factors. J. Clin. Sleep Med. 2018, 14, 409–417. [Google Scholar] [CrossRef]
- Lang, C.J.; Appleton, S.L.; Vakulin, A.; McEvoy, R.D.; Witter, G.A.; Martin, S.A.; Catcheside, P.G.; Antic, N.A.; Lack, L.; Adams, R.J. Comorbid OSA and insomnia increases depression prevalence and severity in men. Respirology 2017, 22, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration 2005, 72, 460–465. [Google Scholar] [CrossRef]
- Sivertsen, B.; Bjornsdòttir, E.; Øverland, S.; Bjorvtan, B.; Salo, P. The joint contribution of insomnia and obstructive sleep apnea on sickness absence. J. Sleep Res. 2013, 22, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, E.; Keenan, T.B.; Eysteinsdottir, B.; Arnardottir, E.S.; Janson, C.; Gislason, T.; Sigurdsson, J.F.; Kuna, S.T.; Pack, A.I.; Benediktsdottir, B. Quality of life among untreated sleep apnea patients compared to the general population and changes after treatment with positive air way pressure. J. Sleep Res. 2015, 24, 328–338. [Google Scholar] [CrossRef]
- Saaresranta, T.; Hedner, J.; Bonsignore, M.R.; Riha, R.L.; McNicholas, W.T.; Penzel, T.; Anttalainen, U.; Kvamme, J.A.; Pretl, M.; Sliwinski, P. Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS ONE 2016, 11, e0163439. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Kim, K.T.; Moon, H.J.; Yang, K.I. Is cormobid insomnia in obstructive sleep apnea linked to heart disease? Sleep Med. 2017, 40 (Suppl. 1), 259. [Google Scholar] [CrossRef]
- Subramanian, S.; Guntupalli, B.; Murugan, T.; Bopparaiu, S.; Chanamolu, S.; Casturi, L.; Surani, S. Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep Breath. 2011, 15, 711–715. [Google Scholar] [CrossRef]
- Shepertycky, M.R.; Banno, K.; Kryger, M.H. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 2005, 28, 309–314. [Google Scholar] [PubMed]
- Stelzer, G.; Garcia, E.; Schorr, F.; Barea, L.M.; Barros, H.T. Prevalence of chronic insomnia in patients with obstructive sleep apnea. Braz. J. Psychiatry 2020. [Google Scholar] [CrossRef]
- Hagen, C.; Patel, A.; McCall, W.V. Prevalence of insomnia symptoms in sleep laboratory patients with and without sleep apnea. Psychiatry Res. 2009, 170, 276–277. [Google Scholar] [CrossRef]
- Beneto, A.; Gomez-Siurana, E.; Rubio-Sanchez, P. Comorbidity between sleep apnea and insomnia. Sleep Med. Rev. 2009, 13, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, K.L.; Riedel, B.W.; Lester, K.W.; Aguillard, R.N. Occult sleep apnea in a recruited sample of older adults with insomnia. J. Consult. Clin. Psychol. 1999, 67, 405–410. [Google Scholar] [CrossRef] [PubMed]
- McCall, W.V.; Kimball, J.; Boggs, N.; Lasater, B.; D’Agostino, R.B., Jr.; Rosenquist, P.B. Prevalence and prediction of primary sleep disorders in a clinical trial of depressed patients with insomnia. J. Clin. Sleep Med. 2009, 5, 454–458. [Google Scholar] [CrossRef]
- Cronlein, T.; Geisler, P.; Langguth, B.; Eichhammer, P.; Jara, C.; Pieh, C.; Zulley, J.; Hajak, G. Polysomnography reveals unexpectedly high rates of organic sleep disorders in patients with prediagnosed primary insomnia. Sleep Breath. 2012, 16, 1097–1103. [Google Scholar] [CrossRef]
- Gooneratne, N.S.; Gehrman, P.R.; Nkwuo, J.E.; Bellamy, S.L.; Schutte-Rodin, S.; Dinges, D.F.; Pack, A.I. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch. Intern. Med. 2006, 166, 1732–1738. [Google Scholar] [CrossRef]
- Bhaskar, S.; Hemavathy, D.; Prasad, S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J. Fam. Med. Prim. Care 2016, 5, 780–784. [Google Scholar] [CrossRef]
- Ong, J.C.; Gress, J.L.; San Pedro-Salcedo, M.G.; Manber, P. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J. Psychosom. Res. 2009, 67, 135–141. [Google Scholar] [CrossRef]
- Kinugawa, K.; Doulazmi, M.; Sebban, C.; Schumm, S.; Mariani, J.; Nguyen-Michel, V.-H. Sleep apnea in elderly adults with chronic insomnia. J. Am. Geriatr. Soc. 2012, 60, 2366–2368. [Google Scholar] [CrossRef]
- Castillo, J.; Goparaju, B.; Bianchi, M.T. Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography. J. Psychosom. Res. 2014, 76, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Lei, F.; Xhou, J.; Wing, Y.K.; Sanford, L.D.; Tang, X. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 45, 1–17. [Google Scholar] [CrossRef]
- Sweetman, A.M.; Lack, L.C.; Catcheside, P.G.; Antic, N.A.; Chai-Coetzer, C.L.; Smith, S.S.; Douglas, J.A.; McEvoy, R.D. Developing a succesful tratment for co-morbid insomnia and sleep apnoea. Sleep Med. Rev. 2017, 33, 28–38. [Google Scholar] [CrossRef]
- Kingshott, R.N.; Engleman, H.N.; Deary, I.J.; Douglas, N.J. Does arousal frequency predict daytime function? Eur. Respir. J. 1998, 12, 1264–1270. [Google Scholar] [CrossRef]
- Caetano Mota, P.; Morals Cardoso, S.; Drummond, M.; Santos, A.C.; Almelda, J.; Winck, J.C. Prevalence of new-onset insomnia in patients with obstructive sleep apnoea syndrome treated with nocturnal ventilatory support. Rev. Port. Pneumol. 2012, 18, 15–21. [Google Scholar] [CrossRef]
- Fogel, R.B.; Trinder, J.; White, D.P.; Malhotra, A.; Raneri, J.; Schory, K.; Kleverlaan, D.; Pierce, R.J. The effect of sleep onset on upper air way muscle activity in patients with sleep apnea versus controls. J. Physiol. 2005, 564, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Pieh, C.; Brandmayr, G. Metabolomics in sleep, insomnia and sleep apnea. Int. J. Mol. Sci. 2020, 21, 7244. [Google Scholar] [CrossRef] [PubMed]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative stress and inflammation biomarkers expression in obstructive sleep apnea patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Xu, H.; Qian, Y.; Zou, J.; Yi, H.; Guan, J.; Yin, S. Association between upper-airway surgery and ameliorative risk markers of endothelial function in obstructive sleep apnea. Sci. Rep. 2019, 9, 20157. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dlenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guidelines for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Lack, L.C.; Hunter, M.; Gradisar, H.M.; Harris, J.K. Is the treatment of insomnia impaired when OSA is also present? Sleep 2011, 34, 0508. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowduri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guidline for Diagnostic Testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.S.; Dos santos, J.M. Insomnia as an expression of obstructive sleep apnea syndrome—the effect of treatment with nocturnal ventilatory support. Rev. Port. Pneumol. 2015, 21, 203–208. [Google Scholar] [CrossRef]
- Mysliwiec, V.; Martin, J.L.; Ulmer, C.S.; Chorduhuri, S.; Brock, M.S.; Spevak, C.; Sall, J. The management of chronic insomnia disorder and obstructive sleep apnea: Sinopsi of the 2019 US department of veterans affairs and US department of defense clinical practice guidelines. Ann. Intern. Med. 2020, 172, 325. [Google Scholar] [CrossRef]
- Guilleminault, C.; Davis, K.; Huynh, N.T. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep 2008, 31, 1527–1533. [Google Scholar] [CrossRef]
- Morin, C.M.; Bootzin, R.R.; Buysse, D.J.; Edinger, J.D.; Espie, C.A.; Lichstein, K.L. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep 2006, 29, 1398–1414. [Google Scholar] [CrossRef]
- Jacobs, G.D.; Pace-Schott, E.F.; Stickgold, R.; Otto, M.W. Cognitive behavior therapy and pharmacotherapy for insomnia: A randomized controlled trial and direct comparison. Arch. Intern. Med. 2004, 164, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Omvik, S.; Pallesen, S.; Bjorvatn, B.; Havik, O.E.; Kvale, G.; Høstmark Nielsen, G.; Nordus, I.H. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: A randomized controlled trial. JAMA 2006, 295, 2851–2858. [Google Scholar] [CrossRef]
- Morin, C.M.; Colecchi, C.; Stone, J.; Sood, R.; Brink, D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. JAMA 1999, 281, 991–999. [Google Scholar] [CrossRef]
- National Institutes of Health. NIH State-of-the-Science Conference statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci. Statements 2005, 22, 1–30. [Google Scholar]
- Heck, T.; Zolezzi, M. Obstructive sleep apnea: Management considerations in psychiatric patients. Neuropsychiatr. Dis. Treat. 2015, 11, 2691–2698. [Google Scholar] [PubMed]
- Dolly, F.R.; Block, A.J. Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am. J. Med. 1982, 73, 239–243. [Google Scholar] [CrossRef]
- Camacho, M.E.; Morin, C.M. The effect of temazepam on respiration in elderly insomniacs with mild sleep apnea. Sleep 1995, 18, 644–645. [Google Scholar] [CrossRef]
- Hanly, P.; Powles, P. Hypnotics should never be used in patients with sleep apnea. J. Psichosom. Res. 1993, 37, 59–65. [Google Scholar]
- Berry, R.B.; Kouchi, K.; Bower, J.; Prosise, G.; Light, R.W. Triazolam in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151, 450–454. [Google Scholar] [CrossRef]
- Cirignotta, F.; Mondini, S.; Zucconi, M.; Gerardi, R.; Farolfi, A.; Lugaresi, E. Zolpidem polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol. Biochem. Behav. 1988, 29, 807–809. [Google Scholar] [CrossRef]
- Berry, R.B.; Patel, P.B. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep 2006, 29, 1052–1056. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nigam, G.; Camacho, M.; Riaz, M. The effect of nonbenzodiazepines sedative hypnotics on apnea-hypopnea index: A meta-analysis. Ann. Thorac. Med. 2019, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kryger, M.; Wang-Weygand, S.; Roth, T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007, 11, 159–164. [Google Scholar] [CrossRef]
- Carley, D.W.; Olopada, C.; Ruigt, G.S.; Radulovacki, M. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep 2007, 30, 35–41. [Google Scholar] [CrossRef]
- Smales, E.T.; Edwards, B.A.; Deyoung, P.N.; McSharry, D.G.; Wellman, A.; Velasquez, A.; Owens, R.; Orr, J.E.; Malhotra, A. Trazodone effects on Obstructive Sleep Apnea and Non-REM arousal threshold. Ann. Am. Thorac. Soc. 2015, 12, 758–764. [Google Scholar] [CrossRef]
- Yi, X.Y.; Ni, S.F.; Ghadami, M.R.; Meng, H.-Q.; Chen, M.-Y.; Kuang, L.; Zhang, Y.-Q.; Zhang, L.; Zhou, X.-Y. Trazodone for the treatment of insomnia: A meta-analysis of randomized placebo controller trials. Sleep Med. 2018, 45, 25–32. [Google Scholar] [CrossRef]
- Chung, K.F.; Lee, C.T.; Yeung, W.F.; Chan, M.S.; Chung, E.W.-Y.; Lin, W.-L. Sleep hygiene education as a treatment of insomnia: A systematic review and metaanalysis. Fam. Pract. 2018, 35, 366–375. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, H.S.; Min, J.Y.; Hwang, K.J.; Kim, S.W. Sleep hygiene-related conditions in patients with mild to moderate obstructive sleep apnea. Auris Nasus Larynx 2019, 46, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773. [Google Scholar]
- Leach, M.J.; Page, A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, J.H.; Duffy, J.F. Circadian Rhythm Sleep Disorders. J. Clin. Outcomes Manag. 2013, 20, 513–528. [Google Scholar]
- Edinger, J.D. Cognitive and behavioral anomalies among insomnia patients with mixed restless legs and periodic limb movement disorder. Behav. Sleep Med. 2003, 1, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Joo, E.Y.; Lee, Y.J.; Hong, S.B. Suicidal ideation and insomnia symptoms in subjects with obstructive sleep apnea syndrome. Sleep Med. 2015, 16, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, F.; Geisler, P.; Wetter, T.C.; Cronlein, T. Sleep apnea syndrome comorbid with and without restless legs syndrome: Differences in insomnia specific symptoms. Sleep Breath. 2020, 24, 1167–1172. [Google Scholar] [CrossRef]
- Bjornsdottir, E.; Janson, C.; Gislason, T.; Sigurdsson, J.; Pack, A.I.; Gehrman, P.; Benediktsdottir, B. Insomnia in untreated sleep apnea patients compared to control. J. Sleep Res. 2012, 21, 131–138. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yu, C.C. Different diagnostic criteria for periodic leg movements in patients with obstructive sleep apnea after continuous positive air way pressure titration. Neuropsychiatry Dis. Treat. 2019, 15, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Comorbid insomnia: Current directions and future challenges. Am. J. Manag. Care 2009, 1, S6–S13. [Google Scholar]
- Manni, R.; Terzaghi, M. Comorbidity between epilepsy and sleep disorders. Epilepsy Res. 2010, 90, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Manni, R.; Terzaghi, M.; Repetto, A. The FLEP scale in diagnosing nocturnal frontal lobe epilepsy, NREM and REM parasomnias: Data from a tertiary sleep and epilepsy unit. Epilepsia 2008, 49, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Grigg-Damberger, M.; Ralls, F. Primary sleep disorders and paroxysmal nocturnal non epileptic events in adults with epilepsy from the perspective of sleep specialists. J. Clin. Neurophysiol. 2011, 28, 120–140. [Google Scholar] [CrossRef]
- Sansa, G.; Iranzo, A.; Santamaria, J. Obstructive sleep apnea in narcolepsy. Sleep Med. 2010, 11, 93–95. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Li, X.; Wang, T.; Xu, Z.; Xu, X.; Gao, H.; Chen, G. Efficacy and safety of solriamfetol for excessive sleepiness in narcolepsy and obstructive sleep apnea: Findings from randomized controlled trials. Sleep Med. 2021, 79, 40–47. [Google Scholar] [CrossRef]
- Schweitzer, P.K.; Rosenberg, R.; Zammit, G.K.; Gotfried, M.; Chen, D.; Carter, L.P.; Wang, H.; Lu, Y.; Black, J.; Malhotra, A.; et al. Solriamfetol for Excessive Sleepiness in Obstructive Sleep Apnea (TONES 3). A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1421–1431. [Google Scholar] [CrossRef]
- Powell, J.; Piszczatoski, C.; Garland, S. Solriamfetol for Excessive Sleepiness in Narcolepsy and Obstructive Sleep Apnea. Ann. Pharmacother. 2020, 54, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Baladi, M.; Bron, M. Clinically relevant effects of solriamfetol on excessive daytime sleepiness: A posthoc analysis of the magnitude of change in clinical trials in adults with narcolepsy or obstructive sleep apnea. J. Clin. Sleep Med. 2021, 17, 711–717. [Google Scholar] [CrossRef]
- Schweitzer, P.K.; Mayer, G.; Rosenberg, R.; Malhotra, A.; Zammit, G.K.; Gotfried, M.; Chandler, P.; Baladi, M.; Strohl, K.P. Randomized Controlled Trial of Solriamfetol for Excessive Daytime Sleepiness in OSA An Analysis of Subgroups Adherent or Nonadherent to OSA Treatment. Chest 2021, 160, 307–318. [Google Scholar] [CrossRef]
- Rosenberg, R.; Thorpy, M.J.; Dauvilliers, Y.; Schweitzer, P.K.; Zammit, G.; Gotfried, M.; Bujanover, S.; Scheckner, B.; Malotra, A. Incidence and duration of common early-onset adverse events in randomized controlled trials of solriamfetol for treatment of excessive daytime sleepiness in obstructive sleep apnea and narcolepsy. J. Clin. Sleep Med. 2021. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Pochetti, P.; Azzolina, D.; Ragnoli, B.; Tillio, P.A.; Cantaluppi, V. Malerba M Interrelationship among Obstructive Sleep Apnea, Renal Function and Survival: A Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 4922. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.; Catchesidem, P.G.; Antic, N.A.; Smith, S.; Chai-Coetzer, C.L.; Douglas, J.; O’grady, A.; Dunn, N.; Robinson, J. Cognitive and behavioral therapy for insomnia increases the use of continuous positive air way pressure therapy in obstructive sleep apnea participants with co-morbid insomnia: A randomized clinical trial. Sleep 2019, 42, zsz178. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C.; Crawford, M.R.; Wallace, D.M. Sleep Apnea and Insomnia: Emerging Evidence for Effective Clinical Management. Chest 2020, 159, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
| A Chronic Insomnia Disorder Diagnosis Would Apply Only When: |
| The insomnia symptoms show some independence in their onset or variation over time from the other symptoms of the co-occurring sleep disorder. When insomnia symptoms persist despite marked symptom improvement of the co-occurring sleep disorder following adequate treatment. A chronic insomnia disorder diagnosis would not apply when effective treatment of the coincident sleep disorder resolves the insomnia symptoms. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. Int. J. Environ. Res. Public Health 2021, 18, 9248. https://doi.org/10.3390/ijerph18179248
Ragnoli B, Pochetti P, Raie A, Malerba M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. International Journal of Environmental Research and Public Health. 2021; 18(17):9248. https://doi.org/10.3390/ijerph18179248
Chicago/Turabian StyleRagnoli, Beatrice, Patrizia Pochetti, Alberto Raie, and Mario Malerba. 2021. "Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management" International Journal of Environmental Research and Public Health 18, no. 17: 9248. https://doi.org/10.3390/ijerph18179248
APA StyleRagnoli, B., Pochetti, P., Raie, A., & Malerba, M. (2021). Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. International Journal of Environmental Research and Public Health, 18(17), 9248. https://doi.org/10.3390/ijerph18179248







