Cognitive Remediation in Virtual Environments for Patients with Schizophrenia and Major Depressive Disorder: A Feasibility Study
Abstract
1. Introduction
1.1. Cognitive Remediation
1.2. Computerized Cognitive Remediation
1.3. Virtual Environments
1.4. AIMS
- To compare the effect of the VE program and standard treatment on cognitive outcome.
- To investigate the feasibility of the VE program.
- To evaluate the participants’ progress in the VE program.
2. Materials and Methods
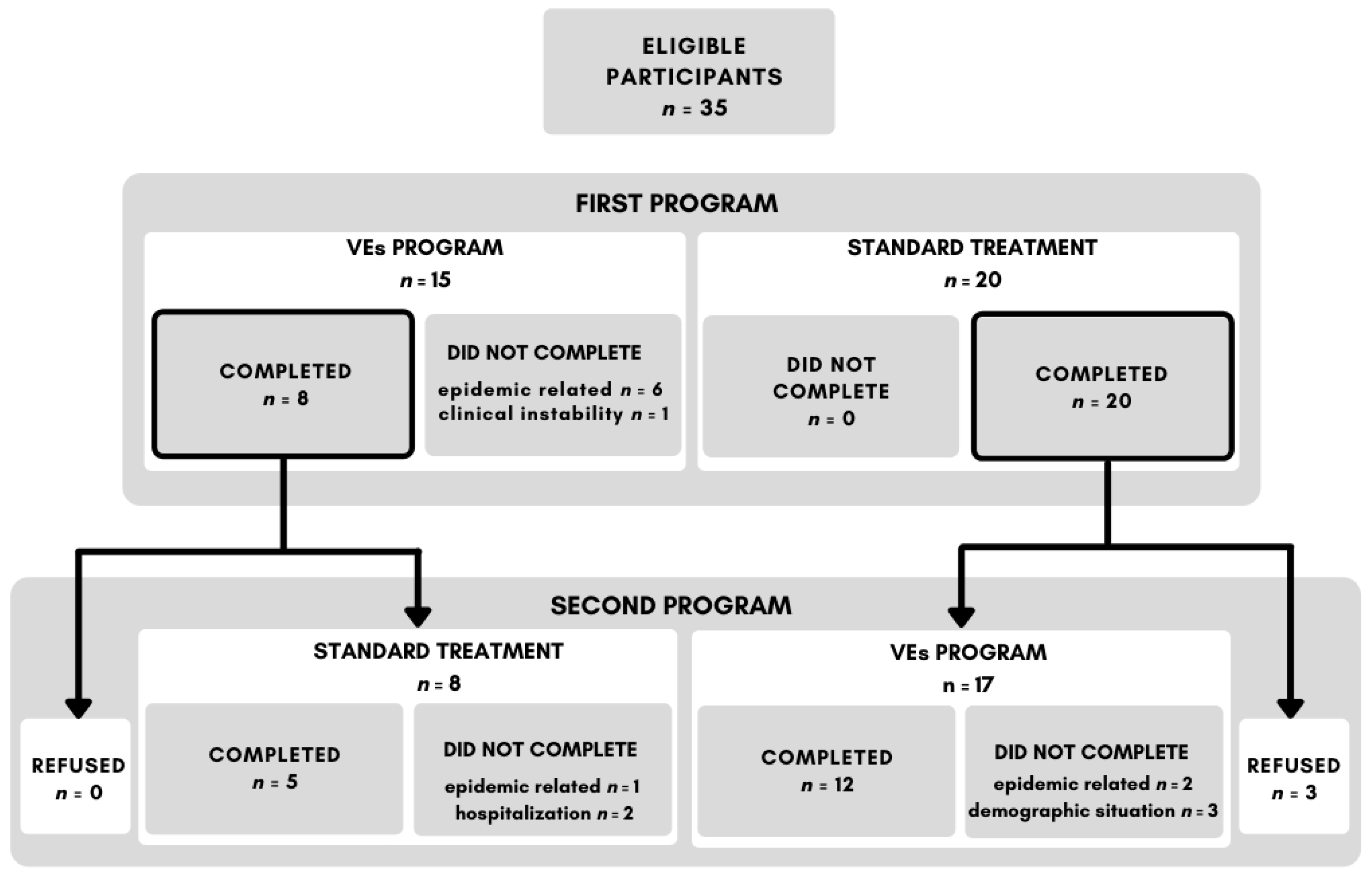
2.1. Participants
- Diagnosis of schizophrenia or other primary psychotic disorders or diagnosis of a depressive episode or recurrent depressive disorder according to ICD-11 [9].
- Age 18–60 years
- Severe visual impairment
- Neurological disorder or comorbid psychiatric diagnosis
- Physical handicap preventing the participant from participating in the VE program
- Refusal to give informed consent
2.2. Procedure
2.3. Virtual Environments (VEs) Program
2.4. Standard Treatment
2.5. Cognitive Assessment
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)
2.6. Feedback Questionnaire
2.7. Adherence to the Treatment
2.8. Statistical Analysis
3. Results
3.1. Adherence to the Treatment
3.2. Feedback Questionnaire
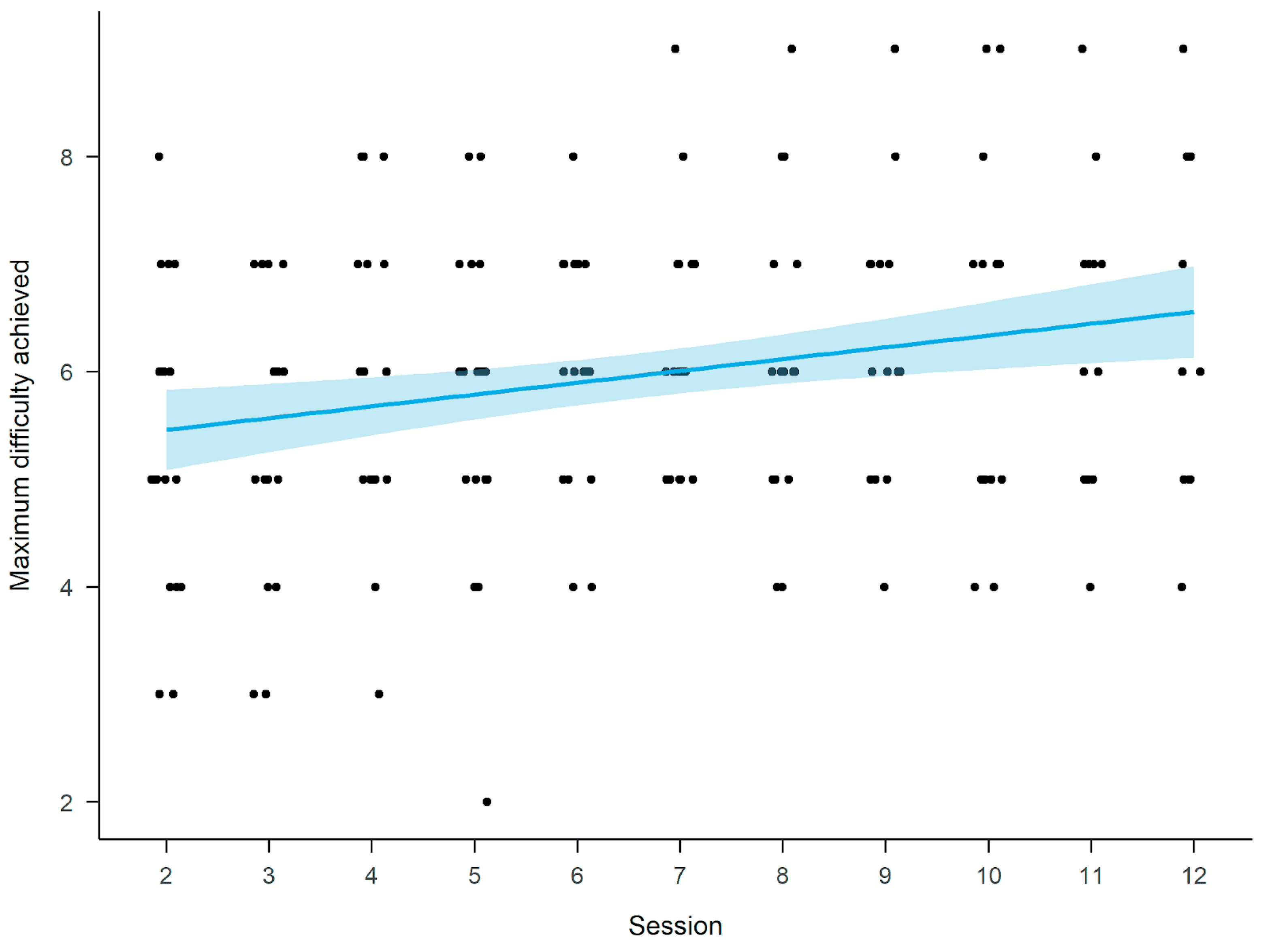
3.3. Virtual Supermarket Shopping Task
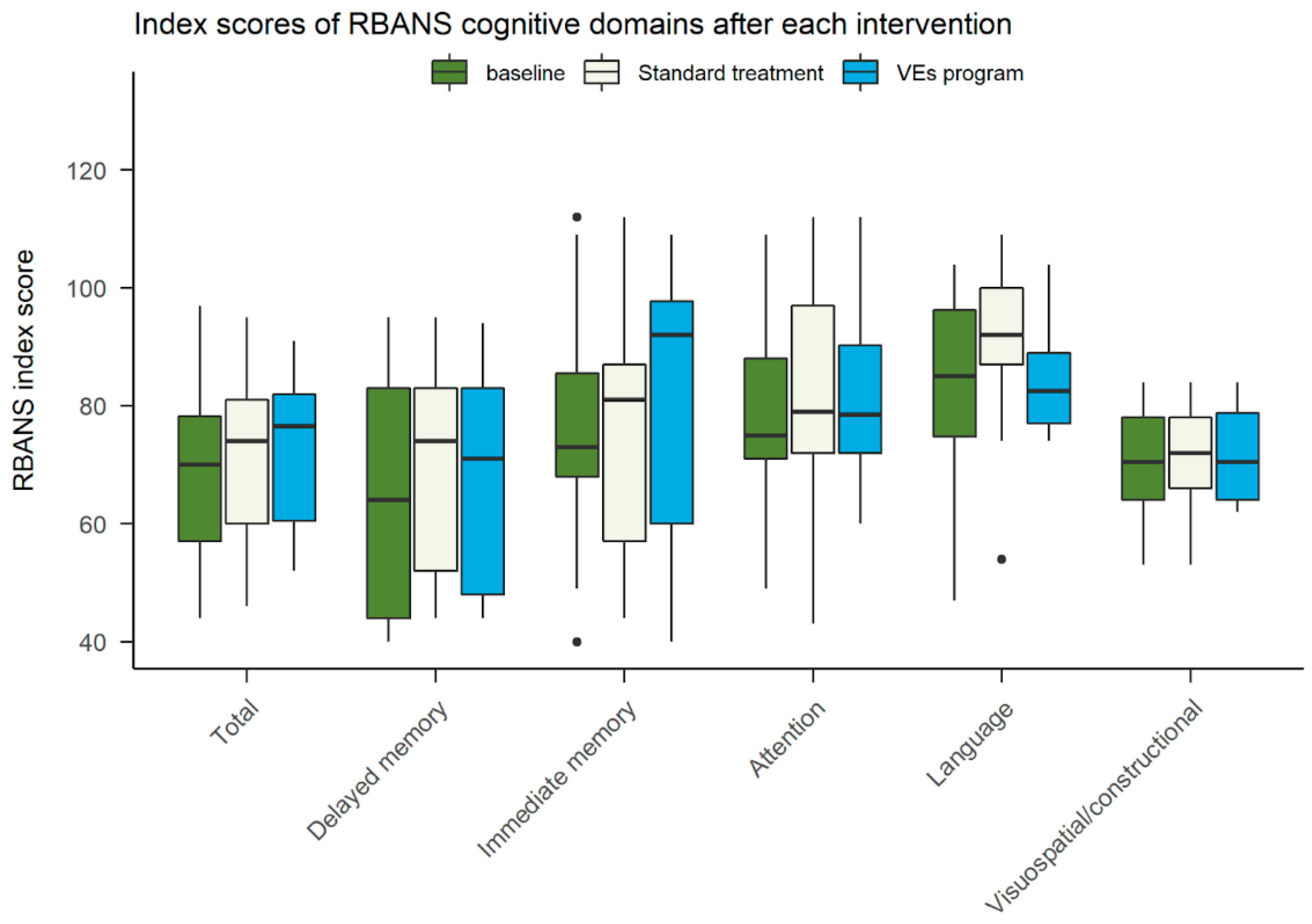
3.4. Cognitive Assessment
4. Discussion
4.1. Cognitive Assessment
4.2. VEs Program Performance
4.3. VEs Program Feasibility
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perälä, J.; Suvisaari, J.; Saarni, S.I.; Kuoppasalmi, K.; Isometsä, E.; Pirkola, S.; Partonen, T.; Tuulio-Henriksson, A.; Hintikka, J.; Kieseppä, T.; et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry 2007, 64, 19–28. [Google Scholar] [CrossRef]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Pantelis, C. Meta-analysis of Cognitive Impairment in First-Episode Bipolar Disorder: Comparison With First-Episode Schizophrenia and Healthy Controls. Schizophr. Bull. 2015, 41, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.C.; Hermens, D.F.; Scott, J.; Redoblado-Hodge, M.A.; Naismith, S.L.; Lagopoulos, J.; Griffiths, K.R.; Porter, M.A.; Hickie, I.B. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J. Psychiatr. Res. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Hammar, A.; Ardal, G. Cognitive functioning in major depression--a summary. Front. Hum. Neurosci. 2009, 3, 26. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Schatzberg, A.F.; Posener, J.A.; DeBattista, C.; Kalehzan, B.M.; Rothschild, A.J.; Shear, P.K. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am. J. Psychiatry 2000, 157, 1095–1100. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems; World Health Organization: Geneva, Switzerland, 2004; ISBN 9789241546492. [Google Scholar]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Bora, E.; Binnur Akdede, B.; Alptekin, K. Neurocognitive impairment in deficit and non-deficit schizophrenia: A meta-analysis. Psychol. Med. 2017, 47, 2401–2413. [Google Scholar] [CrossRef]
- Green, M.F.; Kern, R.S.; Heaton, R.K. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr. Res. 2004, 72, 41–51. [Google Scholar] [CrossRef]
- Green, M.F. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J. Clin. Psychiatry 2006, 67, e12. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef]
- Uchida, H.; Suzuki, T.; Takeuchi, H.; Arenovich, T.; Mamo, D.C. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: Meta-analysis. Schizophr. Bull. 2011, 37, 788–799. [Google Scholar] [CrossRef]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Davidson, M.; Galderisi, S.; Weiser, M.; Werbeloff, N.; Fleischhacker, W.W.; Keefe, R.S.; Boter, H.; Keet, I.P.M.; Prelipceanu, D.; Rybakowski, J.K.; et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: A randomized, open-label clinical trial (EUFEST). Am. J. Psychiatry 2009, 166, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.E.; Levander, S.; Kjaersdam Telléus, G.; Jensen, S.O.W.; Østergaard Christensen, T.; Leucht, S. Second-generation antipsychotic effect on cognition in patients with schizophrenia--a meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015, 131, 185–196. [Google Scholar] [CrossRef]
- Prado, C.E.; Watt, S.; Crowe, S.F. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol. Rev. 2018, 28, 32–72. [Google Scholar] [CrossRef]
- Shilyansky, C.; Williams, L.M.; Gyurak, A.; Harris, A.; Usherwood, T.; Etkin, A. Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. Lancet Psychiatry 2016, 3, 425–435. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Kakar, R.; McIntyre, R.S. The Cognitive Effects of Antidepressants in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Neuropsychopharmacol. 2015, 19. [Google Scholar] [CrossRef]
- Benedict, R.H.; Harris, A.E.; Markow, T.; McCormick, J.A.; Nuechterlein, K.H.; Asarnow, R.F. Effects of attention training on information processing in schizophrenia. Schizophr. Bull. 1994, 20, 537–546. [Google Scholar] [CrossRef][Green Version]
- Prikken, M.; Konings, M.J.; Lei, W.U.; Begemann, M.J.H.; Sommer, I.E.C. The efficacy of computerized cognitive drill and practice training for patients with a schizophrenia-spectrum disorder: A meta-analysis. Schizophr. Res. 2019, 204, 368–374. [Google Scholar] [CrossRef]
- McGurk, S.R.; Twamley, E.W.; Sitzer, D.I.; McHugo, G.J.; Mueser, K.T. A Meta-Analysis of Cognitive Remediation in Schizophrenia. Am. J. Psychiatry 2007, 164, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Wykes, T.; Huddy, V.; Cellard, C.; McGurk, S.R.; Czobor, P. A Meta-Analysis of Cognitive Remediation for Schizophrenia: Methodology and Effect Sizes. Am. J. Psychiatry 2011, 168, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Grynszpan, O.; Perbal, S.; Pelissolo, A.; Fossati, P.; Jouvent, R.; Dubal, S.; Perez-Diaz, F. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: A meta-analytical study. Psychol. Med. 2011, 41, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Price, T.; Corboy, H.; Onwumere, J.; Shergill, S.; Preti, A. Cognitive remediation for inpatients with psychosis: A systematic review and meta-analysis. Psychol. Med. 2020, 50, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Hajri, M.; Abbes, Z.; Ben Yahia, H.; Boudali, M.; Halayem, S.; Othman, S.; Bouden, A.; Ouanes, S. Effects of Cognitive Remediation in Patients with Mood Disorder. Eur. Psychiatry 2015, 30, 1659. [Google Scholar] [CrossRef]
- Paquin, K.; Wilson, A.L.; Cellard, C.; Lecomte, T.; Potvin, S. A systematic review on improving cognition in schizophrenia: Which is the more commonly used type of training, practice or strategy learning? BMC Psychiatry 2014, 14, 139. [Google Scholar] [CrossRef]
- Neisser, U. Memory: What are the important questions. In Practical Aspects of Memory; Gruneberg, M.M., Morris, P.E., Sykes, R.N., Eds.; Academic Press: Cambridge, MA, USA; London, UK, 1978; pp. 3–24. [Google Scholar]
- Moreau, D.; Conway, A.R.A. The case for an ecological approach to cognitive training. Trends Cogn. Sci. 2014, 18, 334–336. [Google Scholar] [CrossRef]
- Rose, F.D.; Attree, E.A.; Brooks, B.M.; Parslow, D.M.; Penn, P.R.; Ambihaipahan, N. Training in virtual environments: Transfer to real world tasks and equivalence to real task training. Ergonomics 2000, 43, 494–511. [Google Scholar] [CrossRef]
- Parsons, T.D. Virtual Reality for Enhanced Ecological Validity and Experimental Control in the Clinical, Affective and Social Neurosciences. Front. Hum. Neurosci. 2015, 9, 660. [Google Scholar] [CrossRef]
- Samuel, R.; Thomas, E.; Jacob, K.S. Instrumental Activities of Daily Living Dysfunction among People with Schizophrenia. Indian J. Psychol. Med. 2018, 40, 134–138. [Google Scholar] [CrossRef]
- Rizzo, A.A.; Schultheis, M.; Kerns, K.A.; Mateer, C. Analysis of assets for virtual reality applications in neuropsychology. Neuropsychol. Rehabil. 2004, 14, 207–239. [Google Scholar] [CrossRef]
- Rose, F.D.; Brooks, B.M.; Rizzo, A.A. Virtual reality in brain damage rehabilitation: Review. Cyberpsychol. Behav. 2005, 8, 241–262; discussion 263–271. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.J.; Bailenson, J.N. How Immersive Is Enough? A Meta-Analysis of the Effect of Immersive Technology on User Presence. Media Psychol. 2015, 19, 272–309. [Google Scholar] [CrossRef]
- Bisso, E.; Signorelli, M.S.; Milazzo, M.; Maglia, M.; Polosa, R.; Aguglia, E.; Caponnetto, P. Immersive Virtual Reality Applications in Schizophrenia Spectrum Therapy: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6111. [Google Scholar] [CrossRef] [PubMed]
- Donders, F.C. On the speed of mental processes. Acta Psychol. 1969, 30, 412–431. [Google Scholar] [CrossRef]
- Plechatá, A.; Hejtmánek, L.; Fajnerová, I. Virtual Supermarket Shopping Task for Cognitive Rehabilitation and Assessment of Psychiatric Patients: Validation in Chronic Schizophrenia. Ceskoslovenska Psychol. 2021, 65, 14–30. [Google Scholar] [CrossRef]
- Gold, J.M.; Queern, C.; Iannone, V.N.; Buchanan, R.W. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: Sensitivity, reliability, and validity. Am. J. Psychiatry 1999, 156, 1944–1950. [Google Scholar] [CrossRef]
- Marder, S.R.; Fenton, W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr. Res. 2004, 72, 5–9. [Google Scholar] [CrossRef]
- Krámská, L.; Dvořáková, Z.; Žalmanová, J. Adaptation and initial validation of the Czech version of Neuropsychological Assessment Battery Screening Module. In Proceedings of the International Neuropsychological Society 2020 Virtual Event “The Neuropsychology of Pleasure, Dreaming, and Memories”, Vienna, Austria, 1–2 July 2020. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. Artic. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Cnaan, A.; Laird, N.M.; Slasor, P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 1997, 16, 2349–2380. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016; ISBN 9783319242750. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.; Christensen, R. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. Artic. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- La Cascia, C.; Ferraro, L.; Seminerio, F.; Scaglione, A.; Maniaci, G.; Matranga, D.; Sideli, L.; Colli, G.; Sartorio, C.; La Paglia, F.; et al. Evaluating the feasibility of the Italian version of the computerized interactive remediation of cognition training for schizophrenia (CIRCuiTS). Minerva Psichiatr. 2020, 61. [Google Scholar] [CrossRef]
- La Paglia, F.; La Cascia, C.; Rizzo, R.; Sanna, M.; Cangialosi, F.; Sideli, L.; Francomano, A.; Riva, G.; La Barbera, D. Virtual reality environments to rehabilitation attention deficits in schizophrenic patients. Annu. Rev. Cybertherapy Telemed. 2016, 14, 143–148. [Google Scholar]
- La Paglia, F.; La Cascia, C.; Rizzo, R.; Sideli, L.; Francomano, A.; La Barbera, D. Cognitive rehabilitation of schizophrenia through NeuroVr training. Stud. Health Technol. Inform. 2013, 191, 158–162. [Google Scholar] [PubMed]
- Revell, E.R.; Neill, J.C.; Harte, M.; Khan, Z.; Drake, R.J. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr. Res. 2015, 168, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.A.; Herman, Y.; Virdee, G.; Bowie, C.R.; Velligan, D.; Plagiannakos, C.; Voineskos, A. A comparison of compensatory and restorative cognitive interventions in early psychosis. Schizophr Res Cogn 2020, 19, 100157. [Google Scholar] [CrossRef] [PubMed]
- Mahncke, H.W.; Kim, S.-J.; Rose, A.; Stasio, C.; Buckley, P.; Caroff, S.; Duncan, E.; Yasmin, S.; Jarskog, L.F.; Lamberti, J.S.; et al. Evaluation of a plasticity-based cognitive training program in schizophrenia: Results from the eCaesar trial. Schizophr. Res. 2019, 208, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Rass, O.; Forsyth, J.K.; Bolbecker, A.R.; Hetrick, W.P.; Breier, A.; Lysaker, P.H.; O’Donnell, B.F. Computer-assisted cognitive remediation for schizophrenia: A randomized single-blind pilot study. Schizophr. Res. 2012, 139, 92–98. [Google Scholar] [CrossRef]
- Lejeune, J.A.; Northrop, A.; Kurtz, M.M. A Meta-analysis of Cognitive Remediation for Schizophrenia: Efficacy and the Role of Participant and Treatment Factors. Schizophr. Bull. 2021. [Google Scholar] [CrossRef]
- Gsottschneider, A.; Keller, Z.; Pitschel-Walz, G.; Froböse, T.; Bäuml, J.; Jahn, T. The role of encoding strategies in the verbal memory performance in patients with schizophrenia. J. Neuropsychol. 2011, 5, 56–72. [Google Scholar] [CrossRef]
- Bonner-Jackson, A.; Barch, D.M. Strategic Manipulations for Associative Memory and the Role of Verbal Processing Abilities in Schizophrenia. J. Int. Neuropsychol. Soc. 2011, 17, 796–806. [Google Scholar] [CrossRef]
- Barlati, S.; Deste, G.; Galluzzo, A.; Perin, A.P.; Valsecchi, P.; Turrina, C.; Vita, A. Factors Associated With Response and Resistance to Cognitive Remediation in Schizophrenia: A Critical Review. Front. Pharmacol. 2018, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Bezdicek, O.; Michalec, J.; Kališová, L.; Kufa, T.; Děchtěrenko, F.; Chlebovcová, M.; Havlík, F.; Green, M.F.; Nuechterlein, K.H. Profile of cognitive deficits in schizophrenia and factor structure of the Czech MATRICS Consensus Cognitive Battery. Schizophr. Res. 2020, 218, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bryce, S.; Sloan, E.; Lee, S.; Ponsford, J.; Rossell, S. Cognitive remediation in schizophrenia: A methodological appraisal of systematic reviews and meta-analyses. J. Psychiatr. Res. 2016, 75, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.W. Persistent negative symptoms in schizophrenia: An overview. Schizophr. Bull. 2007, 33, 1013–1022. [Google Scholar] [CrossRef]
- Blanchard, J.J.; Cohen, A.S. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull. 2006, 32, 238–245. [Google Scholar] [CrossRef]
- Wilk, C.M.; Gold, J.M.; Bartko, J.J.; Dickerson, F.; Fenton, W.S.; Knable, M.; Randolph, C.; Buchanan, R.W. Test-retest stability of the Repeatable Battery for the Assessment of Neuropsychological Status in schizophrenia. Am. J. Psychiatry 2002, 159, 838–844. [Google Scholar] [CrossRef]
- Brodská, V. Evaluation of Psychometric Characteristics of Czech Version RBANS. Ph.D. Thesis, Charles University, Prague, Czechia, 2017. [Google Scholar]

| Question Area | Standard Treatment (n =27) | VEs Program (n = 24) | t-Test |
|---|---|---|---|
| perceived benefit | 4.259(0.656) | 4.083(0.654) | t(48.34) = 0.96, p = 0.343 |
| perceived difficulty | 2.889(0.934) | 2.917(0.929) | t(48.36) = −0.11, p = 0.916 |
| Enjoyment | 4.259(0.712) | 4.042(1.042) | t(39.98) = 0.86, p = 0.395 |
| subjective improvement | 3.556(0.934) | 3.542(0.884) | t(48.79) = 0.05, p = 0.957 |
| willingness to repeat | 3.889(1.121) | 3.231(1.366) | t(48.38) = 1.91, p = 0.062 |
| RBANS Domain | Depressive Disorder (n = 6) | Schizophrenia (n = 22) | t-Test |
|---|---|---|---|
| Mean(SD) | |||
| delayed memory | 77(19.411) | 61.909(18.296) | t(23.42) = 1.50, p = 0.147 |
| immediate memory | 85.667(18.129) | 72.5(17.88) | t(23.27) = 1.65, p = 0.112 |
| attention | 86.5(14.293) | 78.273(18.224) | t(24.43) = 1.95, p = 0.063 |
| language | 93.5(6.504) | 82.182(14.634) | t(35.27) = 2.88, p = 0.007 |
| visuospatial/constructional | 73.667(7.501) | 70.409(9.854) | t(26.42) = 1.51, p = 0.144 |
| RBANS TOTAL INDEX SCORE | 78.5(11.467) | 66(13.238) | t(24.09) = 2.51, p = 0.019 |
| RBANS Standardized Domain | Linear Mixed-Effect Model Result of the Virtual Environments Program in Comparison to Standard Treatment | ||
|---|---|---|---|
| Beta | 95% Confidence Interval | Statistic | |
| immediate memory | 0.19 | −0.1, 0.49 | t(16.56) = 1.27, p = 0.221 |
| delayed memory | −0.14 | −0.34, 0.07 | t(15.89) = −1.3, p = 0.212 |
| attention | −0.03 | −0.4, 0.33 | t(16.16) = −0.18, p = 0.862 |
| language | 0.25 | −0.21, 0.71 | t(17.07) = 1.06, p = 0.302 |
| visuospatial/constructional | −0.37 | −0.93, 0.19 | t(20.09) = −1.29, p = 0.210 |
| RBANS TOTAL SCORE | 0.02 | −0.22, 0.26 | t(15.7) = 0.16, p = 0.875 |
| RBANS Standardized Domain | Beta | 95% Confidence Interval | Statistic |
|---|---|---|---|
| immediate memory | 0.19 | −0.16, 0.54 | t(43.78) = 1.08, p = 0.285 |
| delayed memory | 0.21 | −0.08, 0.5 | t(43.69) = 1.44, p = 0.157 |
| attention | 0.07 | −0.34, 0.49 | t(43.94) = 0.34, p = 0.732 |
| language | −0.18 | −0.72, 0.35 | t(44.39) = −0.68, p = 0.502 |
| visuospatial/constructional | −0.1 | −0.56, 0.36 | t(43.94) = −0.42, p = 0.675 |
| RBANS TOTAL SCORE | 0.13 | −0.14, 0.4 | t(43.4) = 0.95, p = 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plechatá, A.; Hejtmánek, L.; Bednářová, M.; Fajnerová, I. Cognitive Remediation in Virtual Environments for Patients with Schizophrenia and Major Depressive Disorder: A Feasibility Study. Int. J. Environ. Res. Public Health 2021, 18, 9081. https://doi.org/10.3390/ijerph18179081
Plechatá A, Hejtmánek L, Bednářová M, Fajnerová I. Cognitive Remediation in Virtual Environments for Patients with Schizophrenia and Major Depressive Disorder: A Feasibility Study. International Journal of Environmental Research and Public Health. 2021; 18(17):9081. https://doi.org/10.3390/ijerph18179081
Chicago/Turabian StylePlechatá, Adéla, Lukáš Hejtmánek, Martina Bednářová, and Iveta Fajnerová. 2021. "Cognitive Remediation in Virtual Environments for Patients with Schizophrenia and Major Depressive Disorder: A Feasibility Study" International Journal of Environmental Research and Public Health 18, no. 17: 9081. https://doi.org/10.3390/ijerph18179081
APA StylePlechatá, A., Hejtmánek, L., Bednářová, M., & Fajnerová, I. (2021). Cognitive Remediation in Virtual Environments for Patients with Schizophrenia and Major Depressive Disorder: A Feasibility Study. International Journal of Environmental Research and Public Health, 18(17), 9081. https://doi.org/10.3390/ijerph18179081






