In Vitro and In Vivo Effects of Nobiletin on DRG Neurite Elongation and Axon Growth after Sciatic Nerve Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sciatic Nerve Injury
2.2. MTT Assay
2.3. Primary DRG Neuron and Schwann Cell Culture
2.4. Western Blot Analysis
2.5. Immunofluorescence Staining
2.6. Statistical Analysis
3. Results
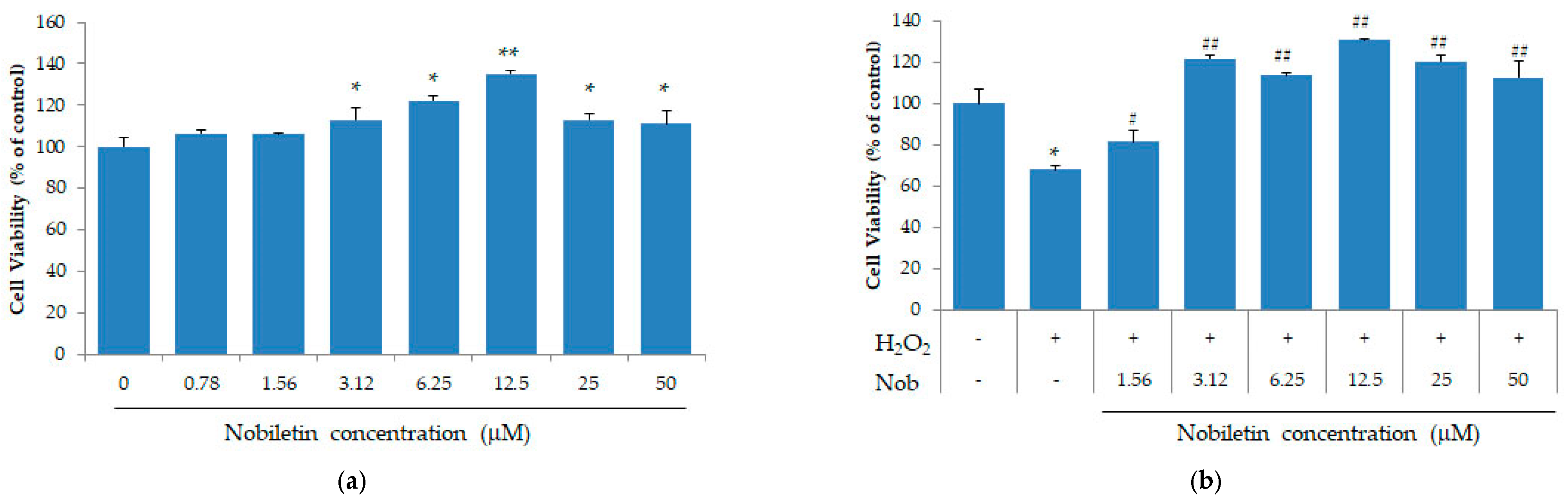
3.1. Nobiletin Suppresses the Loss of Cell Viability in SH-SY5Y Cells
3.2. Nobiletin Dose-Dependently Regulates Neurite Outgrowth of DRG Neurons
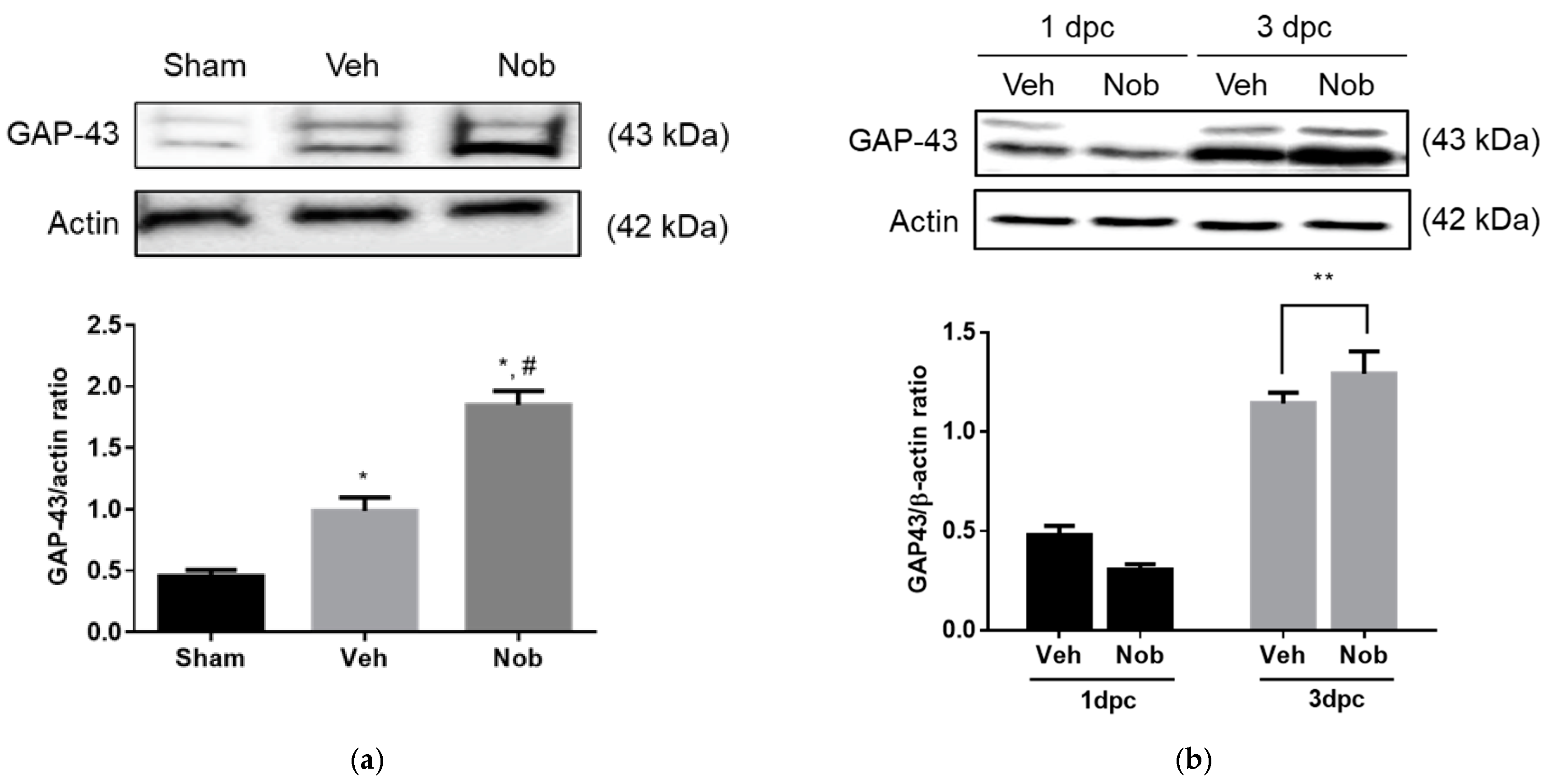
3.3. Nobiletin Increases GAP-43 Expression In Vitro and In Vivo
3.4. Nobiletin Controls Activation of BDNF, ERK1/2 and AKT Signaling Pathways at Early Stage of Nerve Regeneration
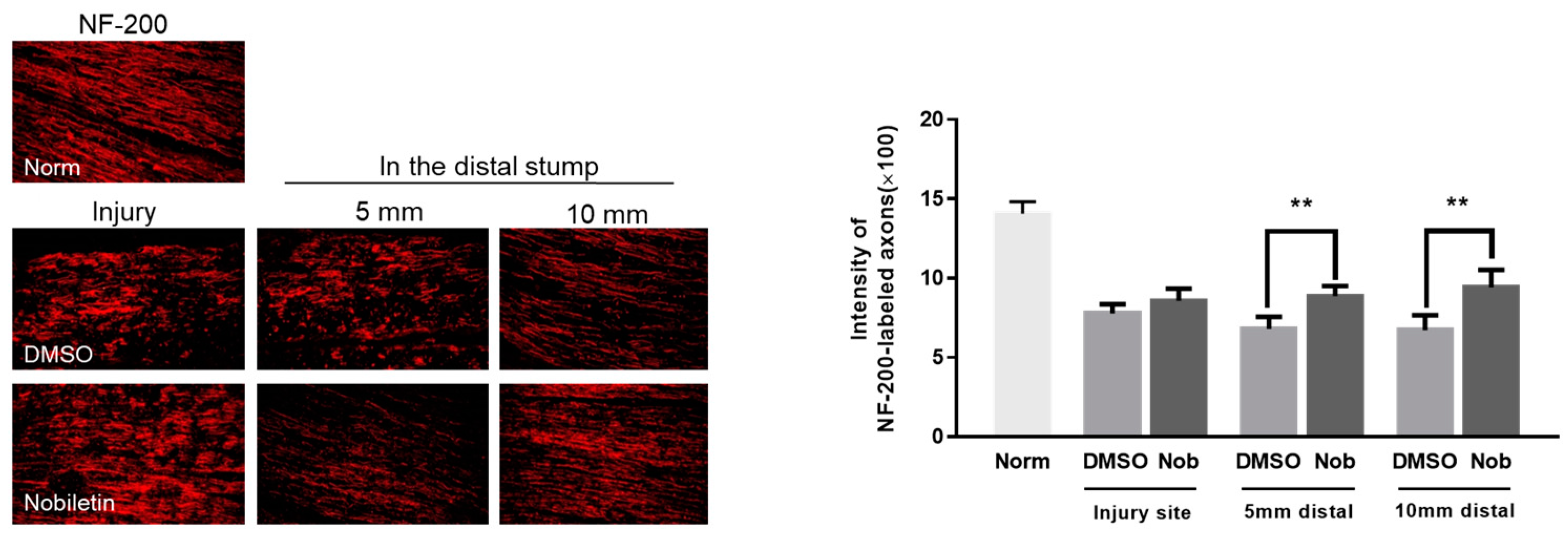
3.5. Nobiletin Facilitated Axonal Regrowth at 2 Weeks after SNI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Asmall, T.; Gunston, G.; Venter, R.; Henry, B.M.; Keet, K. Surgical anatomy of the sciatic nerve and its relationship to the piriformis muscle with a description of a rare variant. SA Orthop. J. 2020, 19, 33–39. [Google Scholar] [CrossRef]
- Pesonen, J.; Rade, M.; Könönen, M.; Marttila, J.; Shacklock, M.; Vanninen, R.; Kankaanpää, M.; Airaksinen, O. Normalization of Spinal Cord Displacement with the Straight Leg Raise and Resolution of Sciatica in Patients with Lumbar Intervertebral Disc Herniation: A 1.5-year Follow-up Study. Spine 2019, 1, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.; Heimfarth, L.; Passos, F.R.S.; Miguel-Dos-Santos, R.; Mingori, M.R.; Moreira, J.C.F.; Lauton, S.S.; Barreto, R.S.S.; Araújo, A.A.S.; Oliveira, A.P.; et al. Naringenin complexed with hydroxypropyl-β-cyclodextrin improves the sciatic nerve regeneration through inhibition of p75NTR and JNK pathway. Life Sci. 2020, 241, 117102. [Google Scholar] [CrossRef]
- Soares Dos Santos Cardoso, F.; Cardoso, R.; Dos Santos Ramalho, B.; Bastos Taboada, T.; Dos Santos Nogueira, A.C.; Blanco Martinez, A.M.; Martins de Almeida, F. Inosine Accelerates the Regeneration and Anticipates the Functional Recovery after Sciatic Nerve Crush Injury in Mice. Neuroscience 2019, 15, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Okada, M.; Tamada, A.; Okuda, S.; Nozumi, M.; Ito, Y.; Kobayashi, D.; Yamasaki, T.; Yokoyama, R.; Shibata, T.; et al. Growth Cone Phosphoproteomics Reveals that GAP-43 Phosphorylated by JNK Is a Marker of Axon Growth and Regeneration. iScience 2018, 29, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Zhang, H.; Sun, F.; Lu, Z.; Reed-Maldonado, A.; Lee, Y.C.; Wang, G.; Banie, L.; Lue, T.F. Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Transl. Androl. Urol. 2016, 5, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Seo, T.B.; Han, I.S.; Yoon, J.H.; Hong, K.E.; Yoon, S.J.; Namgung, U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med. Sci. Sports Exerc. 2006, 38, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.B.; Oh, M.J.; You, B.G.; Kwon, K.B.; Chang, I.A.; Yoon, J.H.; Lee, C.Y.; Namgung, U. ERK1/2-mediated Schwann cell proliferation in the regenerating sciatic nerve by treadmill training. J. Neurotrauma 2009, 26, 1733–1744. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Yamakawa, H.; Choi, S.S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 1, 1674–1683. [Google Scholar] [CrossRef]
- Li, S.; Sang, S.; Pan, M.H.; Lai, C.S.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg. Med. Chem. Lett. 2007, 15, 5177–5181. [Google Scholar] [CrossRef]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 18, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 2, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.A. Selectivity in neurotrophin signaling: Theme and variations. Annu. Rev. Neurosci. 2003, 26, 299–330. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Kim, S.J.; Lee, S.R.; Kim, S.J.; Kim, S.J.; et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef] [Green Version]
- Lyu, C.; Lyu, G.W.; Mulder, J.; Martinez, A.; Shi, T.J.S. G protein-gated inwardly rectifying potassium channel subunit 3 is upregulated in rat DRGs and spinal cord after peripheral nerve injury. J. Pain Res. 2020, 13, 419. [Google Scholar] [CrossRef] [Green Version]
- Han, I.S.; Seo, T.B.; Kim, K.H.; Yoon, J.H.; Yoon, S.J.; Namgung, U. Cdc2-mediated Schwann cell migration during peripheral nerve regeneration. J. Cell Sci. 2007, 120, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 10, 3380. [Google Scholar] [CrossRef] [Green Version]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 28, 3923. [Google Scholar] [CrossRef] [Green Version]
- Sousa, D.P.; Pojo, M.; Pinto, A.T.; Leite, V.; Serra, A.T.; Cavaco, B.M. Nobiletin Alone or in Combination with Cisplatin Decreases the Viability of Anaplastic Thyroid Cancer Cell Lines. Nutr. Cancer 2020, 72, 352–363. [Google Scholar] [CrossRef]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.H.; Su, M.Y.; Huang, H.Y.; Yuan, C.G. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci. Lett. 2010, 22, 6–11. [Google Scholar] [CrossRef]
- Cho, H.W.; Jung, S.Y.; Lee, G.H.; Cho, J.H.; Choi, I.Y. Neuroprotective effect of Citrus unshiu immature peel and nobiletin inhibiting hydrogen peroxide-induced oxidative stress in HT22 murine hippocampal neuronal cells. Pharmacogn. Mag. 2015, 11 (Suppl. 2), S284–S289. [Google Scholar] [CrossRef] [Green Version]
- Akao, Y.; Itoh, T.; Ohguchi, K.; Iinuma, M.; Nozawa, Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg. Med. Chem. 2008, 15, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Papakyriakou, A.; Vourloumis, D.; Tsatsakis, A.M.; Spandidos, D.A. Dietary flavonoids in cancer therapy and prevention: Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol. Ther. 2010, 126, 9–20. [Google Scholar] [CrossRef]
- Miyata, Y.; Sato, T.; Imada, K.; Dobashi, A.; Yano, M.; Ito, A. A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem. Biophys. Res. Commun. 2008, 1, 168–173. [Google Scholar] [CrossRef]
- De Siqueira-Santos, R.; Sardella-Silva, G.; Nascimento, M.A.; Teixeira de Oliveira, L.; Coelho-Sampaio, T.; Ribeiro-Resende, V.T. Biological activity of laminin/polylaminin-coated poly-ε-caprolactone filaments on the regeneration and tissue replacement of the rat sciatic nerve. Mater. Today Bio. 2019, 21, 100026. [Google Scholar] [CrossRef]
- Bucan, V.; Fliess, M.; Schnabel, R.; Peck, C.T.; Vaslaitis, D.; Fülbier, A.; Reimers, K.; Strauss, S.; Vogt, P.M.; Radtke, C. In vitro enhancement and functional characterization of neurite outgrowth by undifferentiated adipose-derived stem cells. Int. J. Mol. Med. 2019, 43, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Q.; Xie, C.; Wang, C.; Wang, Q.; Dong, C.; Fang, L.; Ding, J.; Wang, T. Blocking of BDNF-TrkB signaling inhibits the promotion effect of neurological function recovery after treadmill training in rats with spinal cord injury. Spinal Cord 2019, 57, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aigner, L.; Arber, S.; Kapfhammer, J.P.; Laux, T.; Schneider, C.; Botteri, F.; Brenner, H.R.; Caroni, P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 1995, 20, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.J.; Russo-Neustadt, A.A. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors 2007, 25, 118–131. [Google Scholar] [CrossRef]
- Okada, K.; Tanaka, H.; Temporin, K.; Okamoto, M.; Kuroda, Y.; Moritomo, H.; Murase, T.; Yoshikawa, H. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp. Neurol. 2010, 222, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bhave, S.V.; Ghoda, L.; Hoffman, P.L. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: Signal transduction cascades and site of ethanol action. J. Neurosci. 1999, 1, 3277–3286. [Google Scholar] [CrossRef]
- Bonni, A.; Brunet, A.; West, A.E.; Datta, S.R.; Takasu, M.A.; Greenberg, M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 1999, 12, 1358–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, T.-B.; Jeon, Y.-A.; Kim, S.S.; Lee, Y.J. In Vitro and In Vivo Effects of Nobiletin on DRG Neurite Elongation and Axon Growth after Sciatic Nerve Injury. Int. J. Environ. Res. Public Health 2021, 18, 8988. https://doi.org/10.3390/ijerph18178988
Seo T-B, Jeon Y-A, Kim SS, Lee YJ. In Vitro and In Vivo Effects of Nobiletin on DRG Neurite Elongation and Axon Growth after Sciatic Nerve Injury. International Journal of Environmental Research and Public Health. 2021; 18(17):8988. https://doi.org/10.3390/ijerph18178988
Chicago/Turabian StyleSeo, Tae-Beom, Yoon-A Jeon, Sang Suk Kim, and Young Jae Lee. 2021. "In Vitro and In Vivo Effects of Nobiletin on DRG Neurite Elongation and Axon Growth after Sciatic Nerve Injury" International Journal of Environmental Research and Public Health 18, no. 17: 8988. https://doi.org/10.3390/ijerph18178988
APA StyleSeo, T.-B., Jeon, Y.-A., Kim, S. S., & Lee, Y. J. (2021). In Vitro and In Vivo Effects of Nobiletin on DRG Neurite Elongation and Axon Growth after Sciatic Nerve Injury. International Journal of Environmental Research and Public Health, 18(17), 8988. https://doi.org/10.3390/ijerph18178988





