Health Technology Assessment Development in Vietnam: A Qualitative Study of Current Progress, Barriers, Facilitators, and Future Strategies
Abstract
1. Introduction
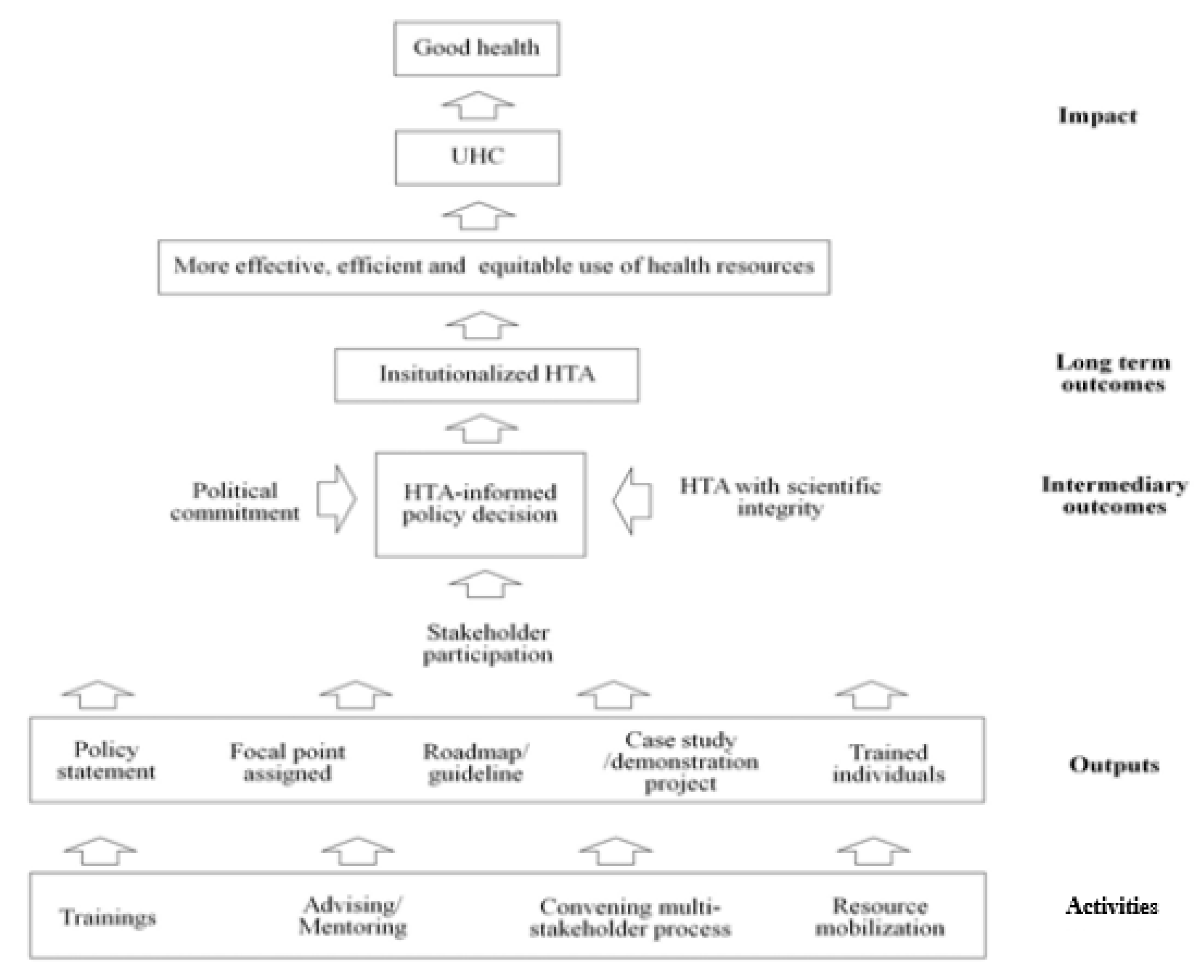
A Conceptual Framework for HTA Development in LMICs
2. Methods
2.1. Study Design
2.2. Study Participants
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Participants
3.2. Current Decision-Making Regarding the Health Insurance Benefit Package
“The osteoporosis drugs, one of the 20 highest consumed in Vietnam, had some changes, from 100 percent coverage to a co-payment of 50:50 percent. However, this was done without any information, (without) any research.”(female respondent in her 30s from academia)
“We prioritize brand-name drugs for severe or chronic diseases such as diabetes, cardiovascular disease, cancer, and for diseases (treated) in the ICU. For other mild diseases, we use generic drugs, especially Vietnamese drugs with low prices. In general, we have a recommended usage percentage between generics and brand-name drugs. The Department of Health in Ho Chi Minh City recommended that the percentage of expenditures for brand-name drugs account for 25% of the whole budget, and now we just maintain that ratio.”(female respondent in her 30s from a hospital)
3.3. Progress in HTA Development in Vietnam
3.3.1. Activities
“Now we have three researchers who are receiving training abroad with grants. One is at a university in the Netherlands for a master’s in health economics. The other two are in Thailand for master’s training in health technology assessment. Recently, we are recruiting for master’s degree students at Mahidol University for next year. Those international training programs are funded by IDSI (the International Decision Support Initiative) project and also by HITAP as well.”(female respondent in her 30s from the government)
“We had a sort of meeting to set about how to function, how often we meet. However, our job description has not been fixed yet.. it’s not that clear about the function or our value.”(male respondent in his 40s from academia)
3.3.2. Output
3.3.3. Intermediary Outcomes
3.4. Perception of HTA
“Cost-effectiveness analyses rely on models. Like Markov models. Policymakers, especially clinicians, don’t like calculation very much. They are not very clear about it. They say ‘Oh, you will do some trick, you will do some calculation, not a real one’.”(male respondent in his 50s from academia)
“There was some disagreement between pharmacoeconomic people and clinical people…. Pharmacoeconomic people, we have the same understanding of HTA and data. However, clinical people, they think this complex concept is unnecessary. They think it is not feasible.”(female respondent in her 30s from the government)
“HTA for decision-making on reimbursements should be mandatory soon because HTA is clear and transparent and everyone can follow it very easily. And (it involves) no corruption and no lobbying. Not much lobbying”(male respondent in his 50s from academia)
“I think in our condition of lacking data, we should just encourage (not mandate) HTA for the time being before making it formal.”(female respondent in her 40s from a hospital)
3.5. Barriers to the Use of HTA
“Not many researchers here in Vietnam can do health economic evaluation. I know everyone who can do… maybe fewer than ten people. Especially, modeling… very few, fewer than five people can do modeling.”(male respondent in his 40s from academia)
“Some medical universities or pharmacology universities have centers for HTA research. But, even if they are universities, their capacity is still limited.”(male respondent in his 40s from other organization)
“We have limitations in data resources… epidemiology data, disease data, prevalence, incidence…everything we don’t have, also costing data and willingness to pay…no.”(female respondent in her 30s from academia)
“There is no standard procedure pharmaceutical companies can follow. So they submit different documents, different levels of information, and different levels of evidence.”(male respondent in his 40s from academia)
“They (government staff) say ‘I am the director now and I care about the budget right now and I do not care about the budget 15 years later.”(male respondent in his 50s from academia)
“For example, for the treatment of hepatitis C, we reviewed if the health insurance (was willing to) spend more money for patient treatment to save costs for the treatment of liver cancer and cirrhosis occurring in ten years later (from preventing hepatitis C). They know (the benefit) but they have budget constraints right now so they can’t (allow) more expenditures today to save the money for a future treatment.”(male respondent in his 50s from academia)
“For example, uh.. the health insurance… they would like to cut the budget for the biologic treatments because they are not very cost-effective. I think that’s reasonable, but they cannot. The reason they explain to us is because, in the newspaper, some interest group write a lot of letters and they say “I’m now on that treatment and if you cut the treatment, it means you will kill me” or something like that… You can imagine.”(male respondent in his 50s from academia)
3.6. Facilitators of the Use of HTA
“If government had enough money to do all the HTA on its own, it would be the most transparent option. When I look at other countries’ models, very few countries can pay 100% of HTA studies. So the important thing is whether we (government) have personnel with good qualifications to assess the results of HTA (submitted from manufacturers of health technologies).”(female respondent in her 30s from academia)
“We should make connections between researchers, policymakers and even suppliers. These connection should be more regular and larger-scale in the future.”(male respondent in his 50s from the government)
“Currently, costs are sent daily to Vietnamese Social Security from about 97–98% of nationwide facilities, approximately 12,000 clinics throughout the country. This is a good data resource for future HTA in Vietnam. At least it is better than before, when HTA researchers only had small and empirical data sets collected directly in hospitals.”(female respondent in her 20s from academia)
“Vietnamese Social Security only has medial expenditure data covered by healthcare funds. We don’t have data on expenditures paid by patients and other parties. We are requesting to add non-insurance expenditures to the central database, but most hospitals still do not agree.”(male respondent in his 40s from the government)
“We should set a standard for quality of research, issued by the MoH or relevant organizations so that we can check the quality of research to be more confident in applications.”(female respondent in her 30s from hospital)
“It should be a fixed willingness to pay, a very clear and simple threshold to prevent controversy..… I know, not many people have that kind of thinking. I think that among the health economics (people), about only 10–15% like this idea.”(male respondent in his 50s from academia)
4. Discussion
Policy Suggestions for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekman, B.; Liem, N.T.; Duc, H.A.; Axelson, H. Health insurance reform in Vietnam: A review of recent developments and future challenges. Health Policy Plan. 2008, 23, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Long, G.T. Social health insurance in Vietnam: Current issues and policy recommendations. Munich MPRA Pap. 2008, 9926. [Google Scholar]
- Ministry of Health. Annual Report on Healthcare Activities 2016; Ministry of Health: Hanoi, Vietnam, 2017.
- WHO. Health technology assessment and incorporation into health systems. In Proceedings of the 28th Pan American Sanitary Conference, 64th Session of the Regional Committee, Washington, DC, USA, 17–21 September 2012. [Google Scholar]
- WHO. Health Technology Assessment of Medical Devices; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Tantivess, S.; Chalkidou, K.; Tritasavit, N.; Teerawattananon, Y. Health Technology Assessment capacity development in low-and middle-income countries: Experiences from the international units of HITAP and NICE. F1000Research 2017, 6, 2119. [Google Scholar] [CrossRef] [PubMed]
- Eddama, O.; Coast, J. A systematic review of the use of economic evaluation in local decision-making. Health Policy 2008, 86, 129–141. [Google Scholar] [CrossRef]
- Hoffmann, C.; Stoykova, B.A.; Nixon, J.; Glanville, J.M.; Misso, K.; Drummond, M.F. Do health-care decision makers find economic evaluations useful? The findings of focus group research in UK health authorities. Value Health 2002, 5, 71–78. [Google Scholar] [CrossRef]
- Abelson, J.; Giacomini, M.; Lehoux, P.; Gauvin, F.-P. Bringing ‘the public’ into health technology assessment and coverage policy decisions: From principles to practice. Health Policy 2007, 82, 37–50. [Google Scholar] [CrossRef]
- Fischer, K.E. A systematic review of coverage decision-making on health technologies—Evidence from the real world. Health Policy 2012, 107, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Bombard, Y.; Abelson, J.; Simeonov, D.; Gauvin, F.-P. Eliciting ethical and social values in health technology assessment: A participatory approach. Soc. Sci. Med. 2011, 73, 135–144. [Google Scholar] [CrossRef]
- Erntoft, S. Pharmaceutical priority setting and the use of health economic evaluations: A systematic literature review. Value Health 2011, 14, 587–599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vo, T.Q.; Van Hoang, M.; Riewpaiboon, A. Quality of Health Economic Evaluation in Developing Countries: A Systematic Review in Vietnam. Syst. Rev. Pharm. 2017, 8, 97. [Google Scholar] [CrossRef]
- Tran, B.X.; Nong, V.M.; Maher, R.M.; Nguyen, P.K.; Luu, H.N. A systematic review of scope and quality of health economic evaluation studies in Vietnam. PLoS ONE 2014, 9, e103825. [Google Scholar] [CrossRef] [PubMed]
- My, N.T.T.; Thanh, N.X. Current state of health-related cost-effectiveness analyses in Vietnam: A literature review. Vietnam J. Public Health 2014, 2, 51–58. [Google Scholar]
- Ritchie, J.; Lewis, J.; Nicholls, C.M.; Ormston, R. Qualitative Research Practice: A Guide for Social Science Students and Researchers; Sage Publications: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Corbin, J.; Strauss, A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory; Sage Publications: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Glaser, B.G. The constant comparative method of qualitative analysis. Soc. Probl. 1965, 12, 436–445. [Google Scholar] [CrossRef]
- Due, O.T.; Thakkinstian, A.; Thavorncharoensap, M.; Sobhonslidsuk, A.; Wu, O.; Phuong, N.K.; Chaikledkaew, U. Cost-utility analysis of direct-acting antivirals for treatment of chronic hepatitis C genotype 1 and 6 in Vietnam. Value Health 2020, 23, 1180–1190. [Google Scholar] [CrossRef]
- Marseille, E.; Larson, B.; Kazi, D.S.; Kahn, J.G.; Rosen, S. Thresholds for the cost–effectiveness of interventions: Alternative approaches. Bull. World Health Org. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Duthie, T.; Trueman, P.; Chancellor, J.; Diez, L. Research into the use of health economics in decision making in the United Kingdom—Phase II: Is health economics ‘for good or evil’? Health Policy 1999, 46, 143–157. [Google Scholar] [CrossRef]
- Hoffmann, C.; von der Schulenburg, J.-M.G. The influence of economic evaluation studies on decision making: A European survey. Health Policy 2000, 52, 179–192. [Google Scholar] [CrossRef]
- Drummond, M.; Cooke, J.; Walley, T. Economic evaluation under managed competition: Evidence from the UK. Soc. Sci. Med. 1997, 45, 583–595. [Google Scholar] [CrossRef]
- Jönsson, B. Economic evaluation of medical technologies in Sweden. Soc. Sci. Med. 1997, 45, 597–604. [Google Scholar] [CrossRef]
- Ubel, P.A.; Jepson, C.; Baron, J.; Hershey, J.C.; Asch, D.A. The influence of cost-effectiveness information on physicians’ cancer screening recommendations. Soc. Sci. Med. 2003, 56, 1727–1736. [Google Scholar] [CrossRef]
- Iglesias, C.P.; Drummond, M.F.; Rovira, J. Health-care decision-making processes in Latin America: Problems and prospects for the use of economic evaluation. Int. J. Technol. Assess. Health Care 2005, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.; Belizán, M.; Discacciati, V. Are economic evaluations and health technology assessments increasingly demanded in times of rationing health services? The case of the Argentine financial crisis. Int. J. Technol. Assess. Health Care 2007, 23, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Hiligsmann, M.; Mathew, J.L.; Evers, S.M. Analysis of a small group of stakeholders regarding advancing health technology assessment in India. Value Health Reg. Issues 2014, 3, 167–171. [Google Scholar] [CrossRef][Green Version]
- Addo, R.; Hall, J.; Haas, M.; Goodall, S. The knowledge and attitude of Ghanaian decision-makers and researchers towards health technology assessment. Soc. Sci. Med. 2020, 250, 112889. [Google Scholar] [CrossRef]
- Nixon, J.; Stoykova, B.; Glanville, J.; Drummond, M.; Christie, J.; Kleijnen, J. The UK NHS economic evaluation database: Economic issues in evaluations of health technology. Int. J. Health Technol. Assess. 2000, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F. Economic evaluation and the rational diffusion and use of health technology. Health Policy 1987, 7, 309–324. [Google Scholar] [CrossRef]
- van Dongen, J.M.; Tompa, E.; Clune, L.; Sarnocinska-Hart, A.; Bongers, P.M.; van Tulder, M.W.; van der Beek, A.J.; van Wier, M.F. Bridging the gap between the economic evaluation literature and daily practice in occupational health: A qualitative study among decision-makers in the healthcare sector. Implement. Sci. 2013, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.F.; Jones, C. Patient-based health technology assessment: A vision of the future. Int. J. Technol. Assess. Health Care 2007, 23, 30–35. [Google Scholar] [CrossRef] [PubMed]
| Categories | N/% |
|---|---|
| Male/female | 11/13 (45.8%/54.2%) |
| Age (years) | |
| 20–29 | 2 (8.3%) |
| 30–39 | 9 (37.5%) |
| 40–49 | 5 (20.8%) |
| 50–59 | 7 (29.2%) |
| 60+ | 1 (4.2%) |
| Occupational setting | |
| Academia | 9 (37.5%) |
| Government | 8 (33.3%) |
| Hospital | 6 (25.0%) |
| Other organization | 1 (4.2%) |
| ABC | VEN |
|---|---|
| A: Drugs that account for 70–80% of the budget with 20% of Qty * | V: Vital |
| B: Drugs that account for 10–20% of the budget with 30–40% of Qty | E: Essential |
| C: Drugs that account for 5–10% of the budget with 50–60% of Qty | N: Non-essential |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Nguyen, T.T.-T.; Park, S.; Hoang, V.M.; Kim, W.-H. Health Technology Assessment Development in Vietnam: A Qualitative Study of Current Progress, Barriers, Facilitators, and Future Strategies. Int. J. Environ. Res. Public Health 2021, 18, 8846. https://doi.org/10.3390/ijerph18168846
Lee H-Y, Nguyen TT-T, Park S, Hoang VM, Kim W-H. Health Technology Assessment Development in Vietnam: A Qualitative Study of Current Progress, Barriers, Facilitators, and Future Strategies. International Journal of Environmental Research and Public Health. 2021; 18(16):8846. https://doi.org/10.3390/ijerph18168846
Chicago/Turabian StyleLee, Hwa-Young, Thuy Thi-Thu Nguyen, Saeun Park, Van Minh Hoang, and Woong-Han Kim. 2021. "Health Technology Assessment Development in Vietnam: A Qualitative Study of Current Progress, Barriers, Facilitators, and Future Strategies" International Journal of Environmental Research and Public Health 18, no. 16: 8846. https://doi.org/10.3390/ijerph18168846
APA StyleLee, H.-Y., Nguyen, T. T.-T., Park, S., Hoang, V. M., & Kim, W.-H. (2021). Health Technology Assessment Development in Vietnam: A Qualitative Study of Current Progress, Barriers, Facilitators, and Future Strategies. International Journal of Environmental Research and Public Health, 18(16), 8846. https://doi.org/10.3390/ijerph18168846






