Chronic Obstructive Pulmonary Disease Increases the Risk of Mortality among Patients with Colorectal Cancer: A Nationwide Population-Based Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Population
2.3. Variables Definitions and Outcome Measurement
2.4. Propensity Score Matching
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Chronic Obstructive Pulmonary Disease Causes a Higher Mortality Rate in Patients with Colorectal Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, T.H.; Wu, Y.C.; Fang, C.Y.; Wang, J.Y.; Chen, C.P.; Huang, K.W.; Jiang, J.K. Taiwan Society of Colon and Rectal Surgeons (TSCRS) Consensus for Cytoreduction Selection in Metastatic Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 1762–1776. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Huang, J.Y. Effect of nationwide screening program on colorectal cancer mortality in Taiwan: A controlled interrupted time series analysis. Int. J. Colorectal Dis. 2020, 35, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.C.; Mittal, P.K.; Sullivan, P.S.; Rutherford, R.; Staley, C.A.; Cardona, K.; Hawk, N.N.; Dixon, W.T.; Kitajima, H.D.; Kang, J.; et al. Colorectal Cancer Initial Diagnosis: Screening Colonoscopy, Diagnostic Colonoscopy, or Emergent Surgery, and Tumor Stage and Size at Initial Presentation. Clin. Colorectal Cancer 2016, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Amri, R.; Bordeianou, L.G.; Sylla, P.; Berger, D.L. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg. 2013, 148, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y. Deaths from colon cancer among farmers in Taiwan: A mortality odds ratio study. J. Toxicol. Environ. Health A 2019, 82, 1137–1142. [Google Scholar] [CrossRef]
- Riesco, J.A.; Alcázar, B.; Trigueros, J.A.; Campuzano, A.; Pérez, J.; Lorenzo, J.L. Active smoking and COPD phenotype: Distribution and impact on prognostic factors. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef]
- Ho, C.H.; Chen, Y.C.; Wang, J.J.; Liao, K.M. Incidence and relative risk for developing cancer among patients with COPD: A nationwide cohort study in Taiwan. BMJ Open 2017, 7, e013195. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.V.; Lee, E.; Park, B.; Jung, J.H.; Park, J.E.; Sheen, S.S.; Park, K.J.; Hwang, S.C.; Park, J.B.; Park, H.S.; et al. Cancer development in patients with COPD: A retrospective analysis of the National Health Insurance Service-National Sample Cohort in Korea. BMC Pulm. Med. 2020, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Platon, A.M.; Erichsen, R.; Christiansen, C.F.; Andersen, L.K.; Sværke, C.; Montomoli, J.; Sørensen, H.T. The impact of chronic obstructive pulmonary disease on intensive care unit admission and 30-day mortality in patients undergoing colorectal cancer surgery: A Danish population-based cohort study. BMJ Open Respir. Res. 2014, 1, e000036. [Google Scholar] [CrossRef] [Green Version]
- Flynn, D.E.; Mao, D.; Yerkovich, S.T.; Franz, R.; Iswariah, H.; Hughes, A.; Shaw, I.M.; Tam, D.P.L.; Chandrasegaram, M.D. The impact of comorbidities on post-operative complications following colorectal cancer surgery. PLoS ONE 2020, 15, e0243995. [Google Scholar] [CrossRef] [PubMed]
- Baré, M.; Montón, C.; Mora, L.; Redondo, M.; Pont, M.; Escobar, A.; Sarasqueta, C.; de Larrea, N.F.; Briones, E.; Quintana, J.M. COPD is a clear risk factor for increased use of resources and adverse outcomes in patients undergoing intervention for colorectal cancer: A nationwide study in Spain. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Han, L.; Dai, W.; Mo, S.; Xiang, W.; Li, Q.; Xu, Y.; Cai, G. Cause of death for elders with colorectal cancer: A real-world data analysis. J. Gastrointest. Oncol. 2020, 11, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Wrobel, J.P. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 871–888. [Google Scholar] [CrossRef] [Green Version]
- André, S.; Conde, B.; Fragoso, E.; Boléo-Tomé, J.P.; Areias, V.; Cardoso, J. COPD and Cardiovascular Disease. Pulmonology 2019, 25, 168–176. [Google Scholar] [CrossRef]
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.-C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2013, 22, 454–475. [Google Scholar] [CrossRef]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Senescence in COPD and Its Comorbidities. Annu. Rev. Physiol. 2017, 79, 517–539. [Google Scholar] [CrossRef]
- Bouter, C.; Bebington, B.; Maphosa, S.; Maher, H.; Gaylard, P.; Etheredge, H.R.; Fabian, J.; Prodehl, L.; Surridge, D.; Fourie, R.L.; et al. It’s contrary—Comorbidity does not affect survival of South Africans with colorectal cancer: An analysis from the Colorectal Cancer in South Africa cohort. S. Afr. Med. J. 2020, 110, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Su, C.-C.; Shao, S.-C.; Sung, S.-F.; Lin, S.-J.; Kao Yang, Y.-H.; Lai, E.C.-C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.-J.; Wang, Y.-W.; Lee, W.-C. Taiwan’s Nationwide Cancer Registry System of 40 years: Past, present, and future. J. Formos. Med. Assoc. 2019, 118, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.-H.; Kung, P.-T.; Kuo, W.-Y.; Ke, T.-W.; Tsai, W.-C. Recurrence, death risk, and related factors in patients with stage 0 colorectal cancer: A nationwide population-based study. Medicine 2020, 99, e21688. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [Green Version]
- van de Schans, S.A.M.; Janssen-Heijnen, M.L.G.; Biesma, B.; Smeenk, F.W.J.M.; van de Poll-Franse, L.V.; Seynaeve, C.; Coebergh, J.W.W. COPD in cancer patients: Higher prevalence in the elderly, a different treatment strategy in case of primary tumours above the diaphragm, and a worse overall survival in the elderly patient. Eur. J. Cancer 2007, 43, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-R.; Tsai, Y.-Y.; Chen, K.-J.; Yang, Y.-H.; Shih, W.-T. Statin Use Improves Overall Survival of Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers 2020, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- Castejón, M.; Plaza, A.; Martinez-Romero, J.; Fernandez-Marcos, P.J.; Cabo, R.; Diaz-Ruiz, A. Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients 2020, 12, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, Y.P.; Teng, C.J.; Liu, C.J.; Hu, Y.W.; Hung, M.H.; Tzeng, C.H.; Liu, C.Y.; Yeh, C.M.; Chen, T.J.; Chiou, T.J. Cancer risk among patients with coal workers’ pneumoconiosis in Taiwan: A nationwide population-based study. Int. J. Cancer 2014, 134, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.X.; Kao, Y.W.; Qin, L.; Chen, M.C.; Shia, B.C.; Wu, S.Y. Cancer risk in chronic rhinosinusitis: A propensity score matched case-control cohort study. Am. J. Transl. Res. 2019, 11, 7146–7156. [Google Scholar]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Haram, A.; Boland, M.R.; Kelly, M.E.; Bolger, J.C.; Waldron, R.M.; Kerin, M.J. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J. Surg. Oncol. 2017, 115, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Li, L.; Cui, N.; Hao, T.; Zou, J.; Jiao, W.; Yi, K.; Yu, W. Statins use and the prognosis of colorectal cancer: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101588. [Google Scholar] [CrossRef] [PubMed]
- Ouahoud, S.; Jacobs, R.J.; Peppelenbosch, M.P.; Fühler, G.M.; Heijmans, J.; Diks, S.; Wildenberg, M.E.; Hawinkels, L.; Kodach, L.L.; Voorneveld, P.W.; et al. Kinome-wide analysis of the effect of statins in colorectal cancer. Br. J. Cancer 2021, 124, 1978–1987. [Google Scholar] [CrossRef]

| Variables | Before Matching | After Matching a | ||||
|---|---|---|---|---|---|---|
| Non-COPD | COPD | p-Value * | Non-COPD | COPD | p-Value * | |
| (n = 26,160) | (n = 5736) | (n = 9138) | (n = 4569) | |||
| Sex | <0.001 | 0.466 | ||||
| Female | 12,506 (47.81) | 2338 (40.76) | 4102 (44.89) | 2021 (44.23) | ||
| Male | 13,654 (52.19) | 3398 (59.24) | 5036 (55.11) | 2548 (55.77) | ||
| Age, year (mean ± SD) | 63.47 ± 13.52 | 73.67 ± 11.04 | <0.001 | 70.97 ± 10.72 | 71.93 ± 11.33 | <0.001 b |
| Age-group, year | <0.001 | 0.950 | ||||
| 20–29 | 174 (0.66) | 1 (0.02) | 2 (0.02) | 1 (0.02) | ||
| 30–39 | 1021 (3.9) | 23 (0.40) | 48 (0.53) | 23 (0.51) | ||
| 40–49 | 2570 (9.82) | 120 (2.09) | 224 (2.45) | 120 (2.63) | ||
| 50–59 | 6602 (25.22) | 537 (9.36) | 1040 (11.38) | 537 (11.75) | ||
| 60–69 | 6703 (25.6) | 1147 (20) | 2299 (25.16) | 1124 (24.6) | ||
| >70 | 9090 (34.72) | 3908 (68.13) | 5525 (60.46) | 2764 (60.49) | ||
| Cancer stage (pathology) | <0.001 | 0.506 | ||||
| Stage I | 6302 (24.09) | 1330 (23.19) | 2110 (23.09) | 1093 (23.92) | ||
| Stage II | 6150 (23.51) | 1301 (22.68) | 2165 (23.69) | 1037 (22.7) | ||
| Stage III | 6963 (26.62) | 1472 (25.66) | 2402 (26.29) | 1194 (26.13) | ||
| Stage IV | 6745 (25.78) | 1633 (28.47) | 2461 (26.93) | 1245 (27.25) | ||
| Comorbidity | ||||||
| DM | 6900 (26.38) | 2268 (39.54) | <0.001 | 3344 (36.59) | 1671 (36.57) | 0.980 |
| Type I DM | 266 (1.02) | 97 (1.69) | <0.001 | 107 (1.17) | 67 (1.47) | 0.145 |
| Type II DM | 6702 (25.62) | 2196 (38.28) | <0.001 | 3274 (35.83) | 1619 (35.43) | 0.650 |
| Hypertension | 13,233 (50.58) | 4426 (77.16) | <0.001 | 6653 (72.81) | 3286 (71.92) | 0.273 |
| Hyperlipidemia | 8780 (33.56) | 2648 (46.16) | <0.001 | 4066 (44.5) | 2050 (44.87) | 0.680 |
| Prior myocardial infarction | 5132 (19.62) | 2559 (44.61) | <0.001 | 3026 (33.11) | 1506 (32.96) | 0.857 |
| Congestive heart failure | 1576 (6.02) | 1143 (19.93) | <0.001 | 640 (7) | 354 (7.75) | 0.113 |
| Chronic kidney disease | 1252 (4.79) | 563 (9.82) | <0.001 | 587 (6.42) | 334 (7.31) | 0.051 |
| Liver diseases | 7010 (26.8) | 2102 (36.65) | <0.001 | 3002 (32.85) | 1494 (32.7) | 0.857 |
| Variables | No. of Death | Univariate Analysis | Multivariate Analysis * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||||
| COPD | |||||||||
| No | 3534 | 1 (reference) | 1 (reference) | ||||||
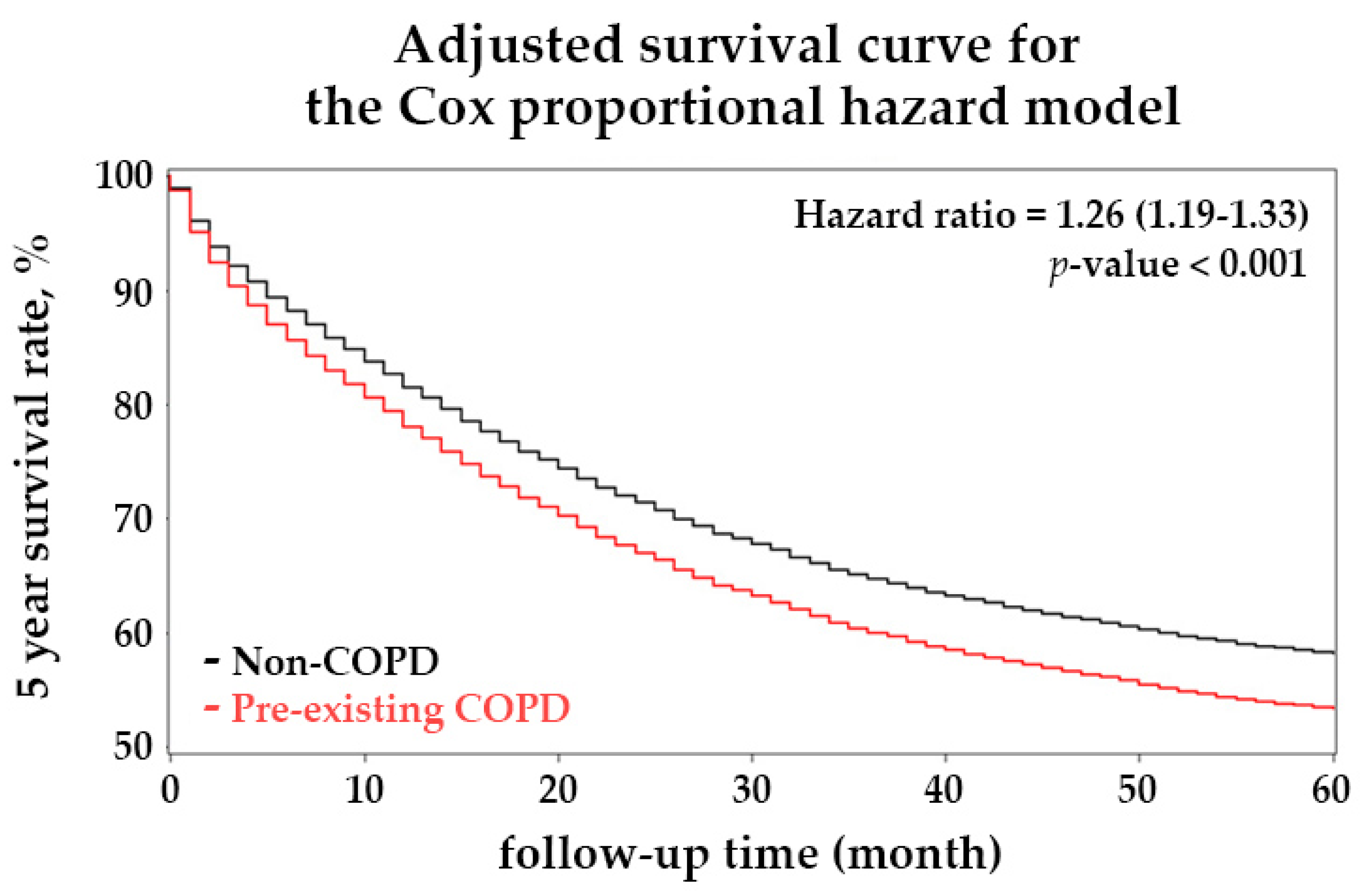
| Yes | 1943 | 1.19 | 1.13 | 1.26 | <0.001 | 1.26 | 1.19 | 1.33 | <0.001 |
| Sex | |||||||||
| Female | 2469 | 1 (reference) | 1 (reference) | ||||||
| Male | 3008 | 0.98 | 0.93 | 1.03 | 0.409 | 0.93 | 0.88 | 0.99 | 0.015 |
| Age-group, year | |||||||||
| 20–29 | 2 | 1.26 | 0.32 | 5.04 | 0.743 | 0.32 | 0.08 | 1.30 | 0.111 |
| 30–39 | 30 | 0.79 | 0.55 | 1.13 | 0.199 | 0.58 | 0.41 | 0.84 | 0.004 |
| 40–49 | 143 | 0.78 | 0.66 | 0.92 | 0.004 | 0.62 | 0.52 | 0.74 | <0.001 |
| 50–59 | 449 | 0.48 | 0.44 | 0.53 | <0.001 | 0.51 | 0.46 | 0.56 | <0.001 |
| 60–69 | 1071 | 0.55 | 0.52 | 0.59 | <0.001 | 0.60 | 0.56 | 0.64 | <0.001 |
| >70 | 3782 | 1 (reference) | 1 (reference) | ||||||
| Cancer stage (pathology) | |||||||||
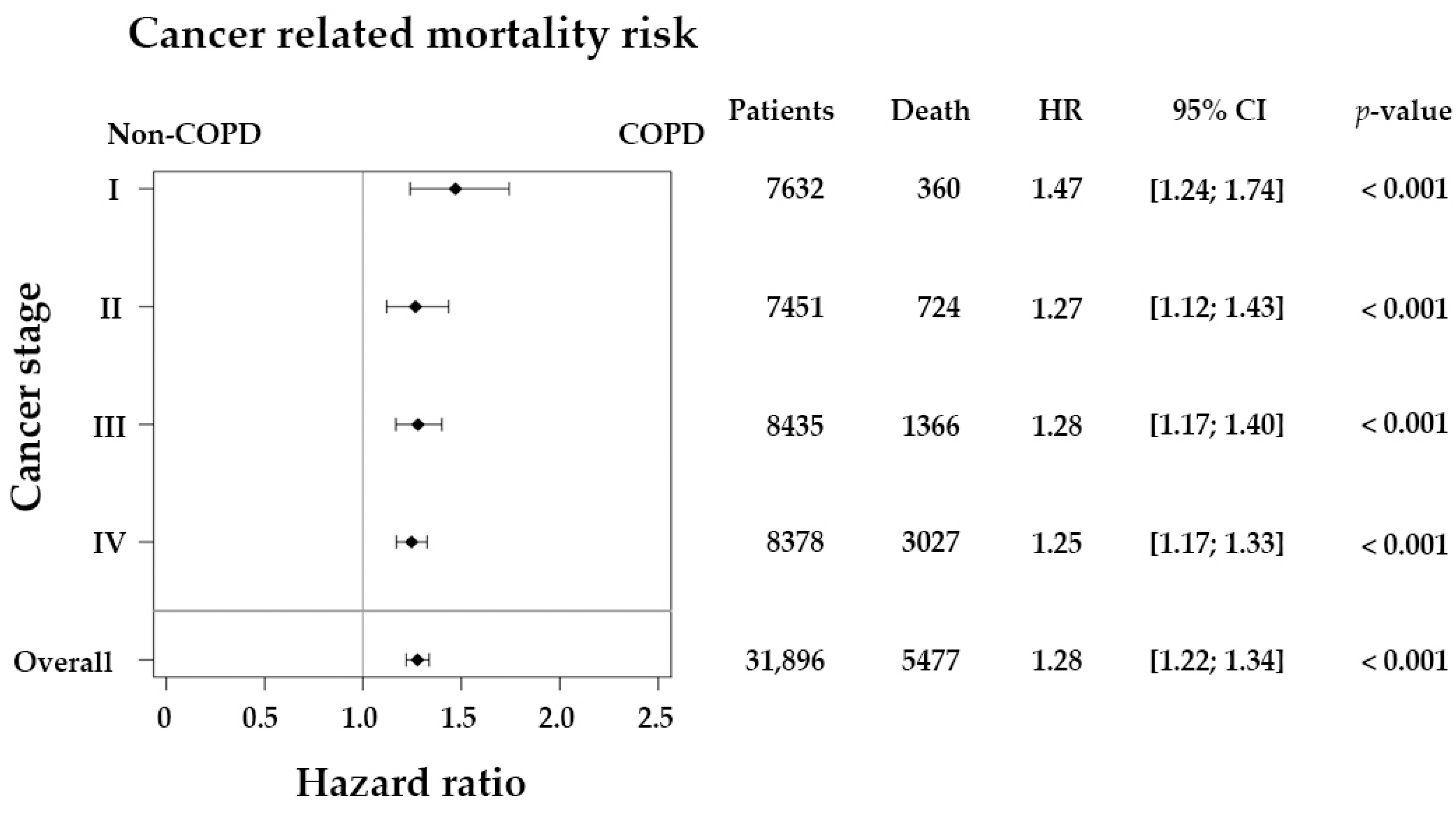
| Stage I | 360 | 1 (reference) | 1 (reference) | ||||||
| Stage II | 724 | 2.21 | 1.95 | 2.51 | <0.001 | 1.95 | 1.72 | 2.21 | <0.001 |
| Stage III | 1366 | 4.02 | 3.58 | 4.52 | <0.001 | 3.71 | 3.30 | 4.17 | <0.001 |
| Stage IV | 3027 | 18.18 | 16.27 | 20.30 | <0.001 | 17.25 | 15.43 | 19.28 | <0.001 |
| Comorbidity | |||||||||
| DM | 2026 | 1.06 | 1.00 | 1.11 | 0.054 | 0.84 | 0.63 | 1.13 | 0.246 |
| Type I DM | 67 | 0.97 | 0.76 | 1.23 | 0.782 | 0.83 | 0.65 | 1.06 | 0.140 |
| Type II DM | 1979 | 1.06 | 1.00 | 1.12 | 0.039 | 1.33 | 1.00 | 1.78 | 0.053 |
| Hypertension | 3983 | 1.06 | 1.00 | 1.12 | 0.060 | 1.03 | 0.96 | 1.09 | 0.459 |
| Hyperlipidemia | 2214 | 0.78 | 0.74 | 0.82 | <0.001 | 0.82 | 0.77 | 0.87 | <0.001 |
| Prior myocardial infarction | 1743 | 0.95 | 0.89 | 1.00 | 0.057 | 0.97 | 0.91 | 1.03 | 0.283 |
| Congestive heart failure | 428 | 1.25 | 1.13 | 1.38 | <0.001 | 1.44 | 1.30 | 1.59 | <0.001 |
| Chronic kidney disease | 364 | 1.16 | 1.04 | 1.29 | 0.006 | 1.28 | 1.15 | 1.42 | <0.001 |
| Liver diseases | 1712 | 0.90 | 0.85 | 0.95 | <0.001 | 1.08 | 1.02 | 1.15 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-J.; Chiang, C.-C.; Peng, M.-T.; Huang, Y.-T.; Huang, J.-L.; Chang, S.-H.; Yang, H.-T.; Chen, W.-C.; Kuo, J.-J.; Hwang, T.-L. Chronic Obstructive Pulmonary Disease Increases the Risk of Mortality among Patients with Colorectal Cancer: A Nationwide Population-Based Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 8742. https://doi.org/10.3390/ijerph18168742
Cheng W-J, Chiang C-C, Peng M-T, Huang Y-T, Huang J-L, Chang S-H, Yang H-T, Chen W-C, Kuo J-J, Hwang T-L. Chronic Obstructive Pulmonary Disease Increases the Risk of Mortality among Patients with Colorectal Cancer: A Nationwide Population-Based Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(16):8742. https://doi.org/10.3390/ijerph18168742
Chicago/Turabian StyleCheng, Wei-Jen, Chih-Chao Chiang, Meng-Ting Peng, Yu-Tung Huang, Jhen-Ling Huang, Shang-Hung Chang, Hsuan-Tzu Yang, Wei-Chun Chen, Jong-Jen Kuo, and Tsong-Long Hwang. 2021. "Chronic Obstructive Pulmonary Disease Increases the Risk of Mortality among Patients with Colorectal Cancer: A Nationwide Population-Based Retrospective Cohort Study" International Journal of Environmental Research and Public Health 18, no. 16: 8742. https://doi.org/10.3390/ijerph18168742
APA StyleCheng, W.-J., Chiang, C.-C., Peng, M.-T., Huang, Y.-T., Huang, J.-L., Chang, S.-H., Yang, H.-T., Chen, W.-C., Kuo, J.-J., & Hwang, T.-L. (2021). Chronic Obstructive Pulmonary Disease Increases the Risk of Mortality among Patients with Colorectal Cancer: A Nationwide Population-Based Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 18(16), 8742. https://doi.org/10.3390/ijerph18168742






