“I’m So Tired”: Fatigue as a Persistent Physical Symptom among Working People Experiencing Exhaustion Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Psychological Distress
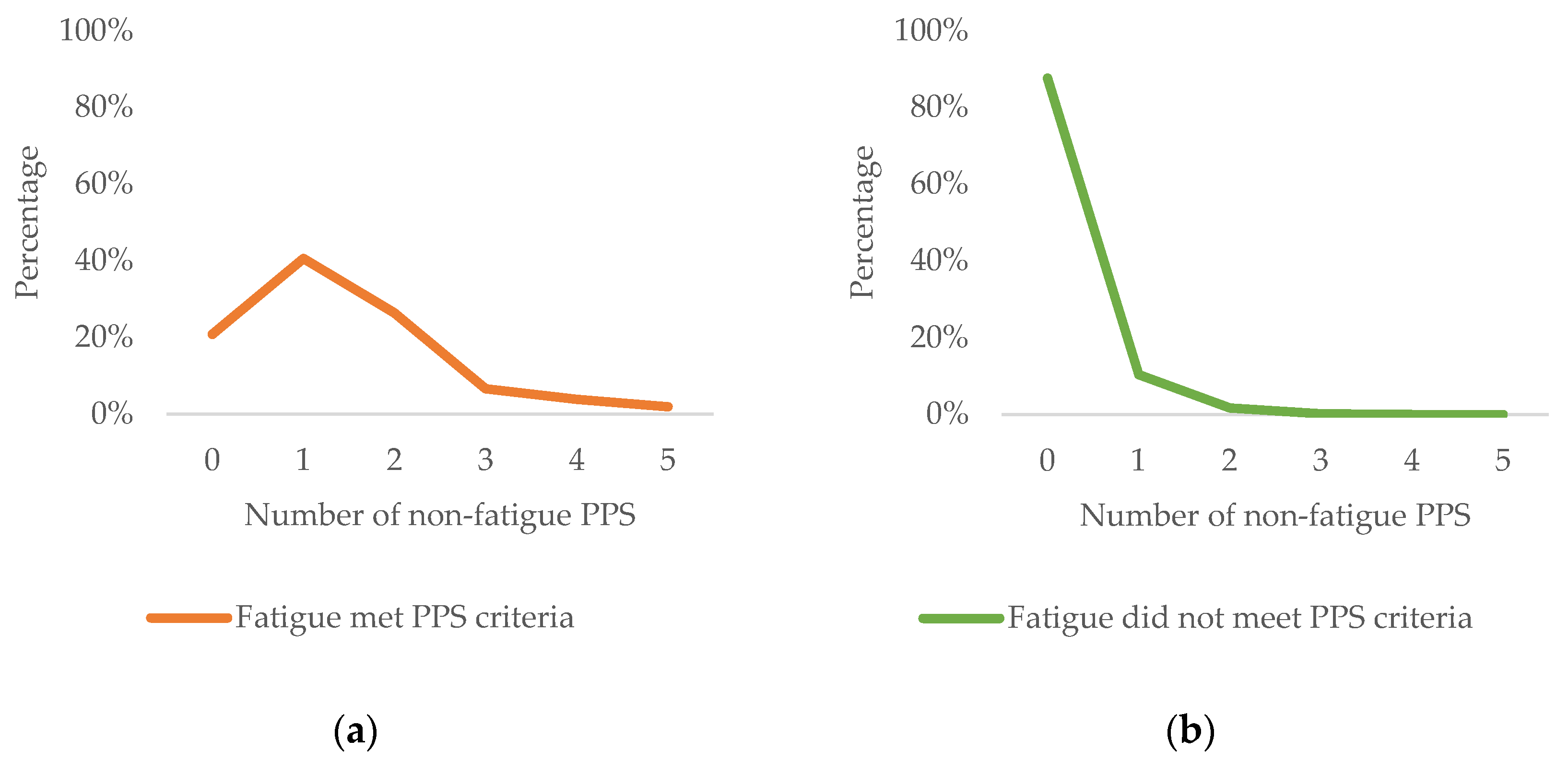
3.3. Number of Non-Fatigue PPSs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boter, H.; Mänty, M.; Hansen, A.M.; Hortobágyi, T.; Avlund, K. Self-Reported Fatigue and Physical Function in Late Mid-Life. J. Rehabil. Med. 2014, 46, 684–690. [Google Scholar] [CrossRef]
- Engberg, I.; Segerstedt, J.; Waller, G.; Wennberg, P.; Eliasson, M. Fatigue in the General Population- Associations to Age, Sex, Socioeconomic Status, Physical Activity, Sitting Time and Self-Rated Health: The Northern Sweden MONICA Study 2014. BMC Public Health 2017, 17, 654–663. [Google Scholar] [CrossRef]
- Hagelin, C.L.; Wengström, Y.; Runesdotter, S.; Fürst, C.J. The Psychometric Properties of the Swedish Multidimensional Fatigue Inventory MFI-20 in Four Different Populations. Acta Oncol. 2007, 46, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.; Kearney, Y.; Bury, G. Prevalence of Fatigue in General Practice. Ir. J. Med. Sci. 2002, 171, 10–12. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Thomas, J.M.; Hamilton, W.T.; White, P.D. Incidence of Fatigue Symptoms and Diagnoses Presenting in UK Primary Care from 1990 to 2001. J. R. Soc. Med. 2004, 97, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Flóvenz, S.Ó.; Broddadóttir, E.; Brynjólfsson, S.; Agnarsdóttir, A.S.; Salkovskis, P.M.; Sigurðsson, J.F. Prevalence of Persistent Physical Symptoms and Association with Depression, Anxiety and Health Anxiety in Iceland. Icel. Med. J. 2021, 107, 67–73. [Google Scholar] [CrossRef]
- Rose, D.M.; Seidler, A.; Nübling, M.; Latza, U.; Brähler, E.; Klein, E.M.; Wiltink, J.; Michal, M.; Nickels, S.; Wild, P.S.; et al. Associations of Fatigue to Work-Related Stress, Mental and Physical Health in an Employed Community Sample. BMC Psychiatry 2017, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Kant, I.; Bultmann, U.; Schroer, K.; Beurskens, A.; van Amelsvoort, L.G.P.M.; Swaen, G. An Epidemiological Approach to Study Fatigue in the Working Population: The Maastricht Cohort Study. Occup. Environ. Med. 2003, 60, i32–i39. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seong, S.; Park, S.; Lim, J.; Hong, S.; Cho, Y.; Kim, H. Korean Version of the Swedish Occupational Fatigue Inventory among Construction Workers: Cultural Adaptation and Psychometric Evaluation. Int. J. Environ. Res. Public Health 2021, 18, 4302. [Google Scholar] [CrossRef] [PubMed]
- Eurofound. Burnout in the Workplace: A Review of Data and Policy Responses in the EU; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Basu, N.; Yang, X.; Luben, R.N.; Whibley, D.; Macfarlane, G.J.; Wareham, N.J.; Khaw, K.-T.; Myint, P.K. Fatigue Is Associated with Excess Mortality in the General Population: Results from the EPIC-Norfolk Study. BMC Med. 2016, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- European Agency for Safety and Health at Work. Calculating the Costs of Work-Related Stress and Psychosocial Risks: Literature Review; Publications Office: Luxembourg, 2014. [Google Scholar]
- Hassard, J.; Teoh, K.R.H.; Visockaite, G.; Dewe, P.; Cox, T. The Cost of Work-Related Stress to Society: A Systematic Review. J. Occup. Health Psychol. 2018, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nijrolder, I.; van der Windt, D.; de Vries, H.; van der Horst, H. Diagnoses during Follow-up of Patients Presenting with Fatigue in Primary Care. Can. Med. Assoc. J. CMAJ 2009, 181, 683–687. [Google Scholar] [CrossRef] [PubMed]
- National Board of Health and Welfare. Exhaustion Disorder (Utmattningssyndrom—Stressrelaterad Psykisk Ohälsa); Socialstyrelsen: Stockholm, Sweden, 2003. [Google Scholar]
- Maslach, C.; Schaufeli, W.B.; Leiter, M.P. Job Burnout. Annu. Rev. Psychol. 2001, 52, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Österberg, K.; Viborg, N.; Jönsson, P.; Tenenbaum, A. Two Swedish Screening Instruments for Exhaustion Disorder: Cross-Sectional Associations with Burnout, Work Stress, Private Life Stress, and Personality Traits. Scand. J. Public Health 2017, 45, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, K.M.; Linton, S.J.; Fedeli, C.; Bryngelsson, I.-L. Burnout in the Working Population: Relations to Psychosocial Work Factors. Int. J. Behav. Med. 2006, 13, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lindert, J.; Müller-Nordhorn, J.; Soares, J.F. Age and Distress of Women–Results of a Representative Population-Based Study. Arch. Womens Ment. Health 2009, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Norlund, S.; Reuterwall, C.; Höög, J.; Janlert, U.; Slunga Järvholm, L. Work Situation and Self-Perceived Economic Situation as Predictors of Change in Burnout—A Prospective General Population-Based Cohort Study. BMC Public Health 2015, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lexén, A.; Kåhlin, I.; Erlandsson, L.-K.; Håkansson, C. Occupational Health among Swedish Occupational Therapists: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 3379. [Google Scholar] [CrossRef]
- Asplund, S.; Åhlin, J.; Åström, S.; Hedlund, M.; Lindgren, B.-M.; Ericson-Lidman, E. Self-Rated Exhaustion Disorder and Associated Health-Related Factors among Municipal Employees in Rural Areas of Northern Sweden. Int. Arch. Occup. Environ. Health 2021, 94, 659–668. [Google Scholar] [CrossRef]
- Persson, R.; Österberg, K. Repeated Assessment of Work-Related Exhaustion: The Temporal Stability of Ratings in the Lund University Checklist for Incipient Exhaustion. BMC Res. Notes 2020, 13, 304. [Google Scholar] [CrossRef]
- Glise, K.; Hadzibajramovic, E.; Jonsdottir, I.H.; Ahlborg, G., Jr. Self-Reported Exhaustion: A Possible Indicator of Reduced Work Ability and Increased Risk of Sickness Absence among Human Service Workers. Int. Arch. Occup. Environ. Health 2010, 83, 511–520. [Google Scholar] [CrossRef]
- Ahola, K.; Toppinen-Tanner, S.; Seppänen, J. Interventions to Alleviate Burnout Symptoms and to Support Return to Work among Employees with Burnout: Systematic Review and Meta-Analysis. Burn. Res. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Wallensten, J.; Åsberg, M.; Wiklander, M.; Nager, A. Role of Rehabilitation in Chronic Stress-Induced Exhaustion Disorder: A Narrative Review. J. Rehabil. Med. 2019, 51, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.M.; Rothstein, H.R. Effects of Occupational Stress Management Intervention Programs: A Meta-Analysis. Database Abstr. Rev. Eff. DARE Qual. Assess. Rev. Internet 2008, 13, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, S.; Santoft, F.; Lindsäter, E.; Ejeby, K.; Ingvar, M.; Ljótsson, B.; Öst, L.-G.; Lekander, M.; Hedman-Lagerlöf, E. Effects of Cognitive Behavioural Therapy and Return-to-Work Intervention for Patients on Sick Leave Due to Stress-Related Disorders: Results from a Randomized Trial. Scand. J. Psychol. 2020, 61, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Lindsäter, E.; Axelsson, E.; Salomonsson, S.; Santoft, F.; Ejeby, K.; Ljótsson, B.; Åkerstedt, T.; Lekander, M.; Hedman-Lagerlöf, E. Internet-Based Cognitive Behavioral Therapy for Chronic Stress: A Randomized Controlled Trial. Psychother. Psychosom. 2018, 87, 296–305. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Boraxbekk, C.-J.; Stenlund, T.; Järvholm, L.S.; Neely, A.S. Effects of a Process-Based Cognitive Training Intervention for Patients with Stress-Related Exhaustion. Stress 2015, 18, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Glise, K.; Ahlborg, G.; Jonsdottir, I.H. Course of Mental Symptoms in Patients with Stress-Related Exhaustion: Does Sex or Age Make a Difference? BMC Psychiatry 2012, 12, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Glise, K.; Jonsdottir, I.H.; Ahlborg, G. Prevalence and Course of Somatic Symptoms in Patients with Stress-Related Exhaustion: Does Sex or Age Matter. BMC Psychiatry 2014, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Grossi, G.; Santell, B. Quasi-Experimental Evaluation of a Stress Management Programme for Female County and Municipal Employees on Long-Term Sick Leave Due to Work-Related Psychological Complaints. J. Rehabil. Med. 2009, 41, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.S.; Huibers, M.J.; Knottnerus, J.A.; Kant, Ij. A Comparison of the Course of Burnout and Prolonged Fatigue: A 4-Year Prospective Cohort Study. J. Psychosom. Res. 2008, 65, 31–38. [Google Scholar] [CrossRef]
- Salomonsson, S.; Santoft, F.; Lindsäter, E.; Ejeby, K.; Ljótsson, B.; Öst, L.-G.; Ingvar, M.; Lekander, M.; Hedman-Lagerlöf, E. Cognitive–Behavioural Therapy and Return-to-Work Intervention for Patients on Sick Leave Due to Common Mental Disorders: A Randomised Controlled Trial. Occup. Environ. Med. 2017, 74, 905–912. [Google Scholar] [CrossRef]
- Willert, M.V.; Thulstrup, A.M.; Bonde, J.P. Effects of a Stress Management Intervention on Absenteeism and Return to Work—Results from a Randomized Wait-List Controlled Trial. Scand. J. Work Environ. Health 2011, 37, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Glise, K.; Wiegner, L.; Jonsdottir, I.H. Long-Term Follow-up of Residual Symptoms in Patients Treated for Stress-Related Exhaustion. BMC Psychol. 2020, 8, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lander, F.; Friche, C.; Tornemand, H.; Andersen, J.H.; Kirkeskov, L. Can We Enhance the Ability to Return to Work among Workers with Stress-Related Disorders? BMC Public Health 2009, 9, 372–376. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics, 11th ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Marks, E.M.; Hunter, M.S. Medically Unexplained Symptoms: An Acceptable Term? Br. J. Pain 2015, 9, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Picariello, F.; Ali, S.; Moss-Morris, R.; Chalder, T. The Most Popular Terms for Medically Unexplained Symptoms: The Views of CFS Patients. J. Psychosom. Res. 2015, 78, 420–426. [Google Scholar] [CrossRef]
- Chalder, T.; Willis, C. “Lumping” and “Splitting” Medically Unexplained Symptoms: Is There a Role for a Transdiagnostic Approach? J. Ment. Health 2017, 26, 187–191. [Google Scholar] [CrossRef]
- Dimsdale, J.; Sharma, N.; Sharpe, M. What Do Physicians Think of Somatoform Disorders? Psychosomatics 2011, 52, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Wessely, S.; Nimnuan, C.; Sharpe, M. Functional Somatic Syndromes: One or Many? Lancet 1999, 354, 936–939. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schröder, A.; Jørgensen, T.; Ørnbøl, E.; Dantoft, T.M.; Eliasen, M.; Thuesen, B.H.; Fink, P. The Unifying Diagnostic Construct of Bodily Distress Syndrome (BDS) Was Confirmed in the General Population Elsevier Enhanced Reader. J. Psychosom. Res. 2020, 128, 109868. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schröder, A.; Jørgensen, T.; Ørnbøl, E.; Meinertz Dantoft, T.; Eliasen, M.; Benros, M.E.; Fink, P. Irritable Bowel, Chronic Widespread Pain, Chronic Fatigue and Related Syndromes Are Prevalent and Highly Overlapping in the General Population: DanFunD. Sci. Rep. 2020, 10, 3273. [Google Scholar] [CrossRef] [PubMed]
- Nimnuan, C.; Hotopf, M.; Wessely, S. Medically Unexplained Symptoms: An Epidemiological Study in Seven Specialities. J. Psychosom. Res. 2001, 51, 361–367. [Google Scholar] [CrossRef]
- Reid, S.; Wessely, S.; Crayford, T.; Hotopf, M. Medically Unexplained Symptoms in Frequent Attenders of Secondary Health Care: Retrospective Cohort Study. Br. Med. J. 2001, 322, 767. [Google Scholar] [CrossRef]
- Roca, M.; Gili, M.; Garcia-Garcia, M.; Salva, J.; Vives, M.; Garcia Campayo, J.; Comas, A. Prevalence and Comorbidity of Common Mental Disorders in Primary Care. J. Affect. Disord. 2009, 119, 52–58. [Google Scholar] [CrossRef]
- De Waal, M.W.M.; Arnold, I.A.; Eekhof, J.A.H.; van Hemert, A.M. Somatoform Disorders in General Practice—Prevalence, Functional Impairment and Comorbidity with Anxiety and Depressive Disorders. Br. J. Psychiatry 2004, 184, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Orav, E.; Bates, D.; Barsky, A. Somatization Increases Disability Independent of Comorbidity. J. Gen. Intern. Med. 2009, 24, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, N.; Koerber, S.; Frieser, D.; Hiller, W. The Prevalence of Medically Unexplained Symptoms in Primary Care. Psychosomatics 2011, 52, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bekhuis, E.; Boschloo, L.; Rosmalen, J.G.M.; Schoevers, R.A. Differential Associations of Specific Depressive and Anxiety Disorders with Somatic Symptoms. J. Psychosom. Res. 2015, 78, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Den Boeft, M.; Twisk, J.W.R.; Hoekstra, T.; Terluin, B.; Penninx, B.W.J.H.; van der Wouden, J.C.; Numans, M.E.; van der Horst, H.E. Medically Unexplained Physical Symptoms and Work Functioning over 2 Years: Their Association and the Influence of Depressive and Anxiety Disorders and Job Characteristics.(Report). BMC Fam. Pract. 2016, 17, 46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Löwe, B.; Spitzer, R.L.; Williams, J.B.W.; Mussell, M.; Schellberg, D.; Kroenke, K. Depression, Anxiety and Somatization in Primary Care: Syndrome Overlap and Functional Impairment. Gen. Hosp. Psychiatry 2008, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Lilly, A.; Vestergaard, M.; Fink, P.; Carlsen, A.H.; Rosendal, M. Patient Characteristics and Frequency of Bodily Distress Syndrome in Primary Care: A Cross-Sectional Study. Br. J. Gen. Pract. 2015, 65, e617–e623. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gilmour, H. Medically Unexplained Physical Symptoms (MUPS) among Adults in Canada: Comorbidity, Health Care Use and Employment. Health Rep. 2017, 28, 3. [Google Scholar] [PubMed]
- Aamland, A.; Malterud, K.; Werner, E.L. Patients with Persistent Medically Unexplained Physical Symptoms: A Descriptive Study from Norwegian General Practice. BMC Fam. Pract. 2014, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Momsen, A.H.; Nielsen, C.V.; Nielsen, M.B.D.; Rugulies, R.; Jensen, C. Work Participation and Health-Related Characteristics of Sickness Absence Beneficiaries with Multiple Somatic Symptoms. Public Health 2016, 133, 75–82. [Google Scholar] [CrossRef]
- Loengaard, K.; Bjorner, J.B.; Fink, P.K.; Burr, H.; Rugulies, R. Medically Unexplained Symptoms and the Risk of Loss of Labor Market Participation--a Prospective Study in the Danish Population. BMC Public Health 2015, 15, 844. [Google Scholar] [CrossRef] [PubMed]
- Rask, M.T.; Ørnbøl, E.; Rosendal, M.; Fink, P. Long-Term Outcome of Bodily Distress Syndrome in Primary Care: A Follow-up Study on Health Care Costs, Work Disability, and Self-Rated Health. Psychosom. Med. 2017, 79, 345–357. [Google Scholar] [CrossRef]
- Barsky, A.J.; Orav, E.J.; Bates, D.W. Somatization Increases Medical Utilization and Cost Independent of Psychiatric and Medical Comorbidity. Arch. Gen. Psychiatry 2005, 62, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gill, N.S.; Teodorczuk, A.; Li, Z.; Sun, J. The Efficacy of Cognitive Behavioural Therapy in Somatoform Disorders and Medically Unexplained Physical Symptoms: A Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2019, 245, 98–112. [Google Scholar] [CrossRef]
- Sanders, S.; Coppin, S.; Moulson, H.; Meola, J.; Meyrick, J. What adaptions are effective to cognitive behavioural interventions for adults with long-term conditions and medically unexplained symptoms? A systematic review. Ansiedad Estrés 2020, 26, 188–201. [Google Scholar] [CrossRef]
- Kleinstäuber, M.; Witthöft, M.; Hiller, W. Efficacy of Short-Term Psychotherapy for Multiple Medically Unexplained Physical Symptoms: A Meta-Analysis. Clin. Psychol. Rev. 2011, 31, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Kleinstäuber, M.; Allwang, C.; Bailer, J.; Berking, M.; Brünahl, C.; Erkic, M.; Gitzen, H.; Gollwitzer, M.; Gottschalk, J.-M.; Heider, J.; et al. Cognitive Behaviour Therapy Complemented with Emotion Regulation Training for Patients with Persistent Physical Symptoms: A Randomised Clinical Trial. Psychother. Psychosom. 2019, 88, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Lee, J.-S.; Park, S.-Y.; Kim, S.-J.; Son, C.-G. Systematic Review of Randomized Controlled Trials for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Malouff, J.M.; Thorsteinsson, E.B.; Rooke, S.E.; Bhullar, N.; Schutte, N.S. Efficacy of Cognitive Behavioral Therapy for Chronic Fatigue Syndrome: A Meta-Analysis. Clin. Psychol. Rev. 2008, 28, 736–745. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Goldsmith, K.; Johnson, A.; Potts, L.; Walwyn, R.; DeCesare, J.; Baber, H.; Burgess, M.; Clark, L.; Cox, D.; et al. Comparison of Adaptive Pacing Therapy, Cognitive Behaviour Therapy, Graded Exercise Therapy, and Specialist Medical Care for Chronic Fatigue Syndrome (PACE): A Randomised Trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef]
- Huibers, M.J.H.; Beurskens, A.; Prins, J.B.; Kant, I.J.; Bazelmans, E.; Van Schayck, C.P.; Knottnerus, J.A.; Bleijenberg, G. Fatigue, Burnout, and Chronic Fatigue Syndrome among Employees on Sick Leave: Do Attributions Make the Difference? Occup. Environ. Med. 2003, 60, i26–i31. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.S.; Huibers, M.J.H.; Knottnerus, J.A.; Kant, I.J. Similarities, Overlap and Differences between Burnout and Prolonged Fatigue in the Working Population. QJM 2007, 100, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Maroti, D.; Bileviciute-Ljungar, I. Similarities and Differences between Health-Related Quality of Life in Patients with Exhaustion Syndrome and Chronic Fatigue Syndrome. Fatigue Biomed. Health Behav. 2018, 6, 208–219. [Google Scholar] [CrossRef]
- Maroti, D.; Molander, P.; Bileviciute-Ljungar, I. Differences in Alexithymia and Emotional Awareness in Exhaustion Syndrome and Chronic Fatigue Syndrome. Scand. J. Psychol. 2017, 58, 52–61. [Google Scholar] [CrossRef]
- Wiegner, L.; Hange, D.; Björkelund, C.; Ahlborg, G. Prevalence of Perceived Stress and Associations to Symptoms of Exhaustion, Depression and Anxiety in a Working Age Population Seeking Primary Care—An Observational Study. BMC Fam. Pract. 2015, 16, 38–46. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.M.D. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Mundt, J.; Marks, I.; Shear, M.; Greist, J. The Work and Social Adjustment Scale: A Simple Measure of Impairment in Functioning. Br. J. Psychiatry 2002, 180, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.S.; Wessely, S.; Huibers, M.J.; Knottnerus, J.A.; Kant, Ij. Two Sides of the Same Coin? On the History and Phenomenology of Chronic Fatigue and Burnout. Psychol. Health 2011, 26, 449–464. [Google Scholar] [CrossRef]
- Toft, T.; Fink, P.E.R.; Oernboel, E.V.A.; Christensen, K.A.J.; Frostholm, L.; Olesen, F. Mental Disorders in Primary Care: Prevalence and Co-Morbidity among Disorders. Results from the Functional Illness in Primary Care (FIP) Study. Psychol. Med. 2005, 35, 1175–1184. [Google Scholar] [CrossRef]
- Flóvenz, S.Ó.; Salkovskis, P.M.; Svansdóttir, E.; Andersen, K. Non-Cardiac Chest Pain as a Persistent Physical Symptom and Its Relationship with Other Persistent Physical Symptoms, Psychological Distress and Workability. (submitted).
- Rask, M.T.; Rosendal, M.; Fenger-Grøn, M.; Bro, F.; Ørnbøl, E.; Fink, P. Sick Leave and Work Disability in Primary Care Patients with Recent-Onset Multiple Medically Unexplained Symptoms and Persistent Somatoform Disorders: A 10-Year Follow-up of the FIP Study. Gen. Hosp. Psychiatry 2015, 37, 53–59. [Google Scholar] [CrossRef]
- Mellner, C.; Krantz, G.; Lundberg, U. Medically Unexplained Symptoms in Women as Related to Physiological Stress Responses. Stress Health 2005, 21, 45–52. [Google Scholar] [CrossRef]
- Conversano, C.; Carmassi, C.; Bertelloni, C.A.; Marchi, L.; Micheloni, T.; Carbone, M.G.; Pagni, G.; Tagliarini, C.; Massimetti, G.; Bazzichi, L. Potentially Traumatic Events, Post-Traumatic Stress Disorder and Post-Traumatic Stress Spectrum in Patients with Fibromyalgia. Clin. Exp. Rheumatol. 2019, 37, 39–43. [Google Scholar] [PubMed]
- Fischer, S.; Doerr, J.M.; Strahler, J.; Mewes, R.; Thieme, K.; Nater, U.M. Stress Exacerbates Pain in the Everyday Lives of Women with Fibromyalgia Syndrome—The Role of Cortisol and Alpha-Amylase. Psychoneuroendocrinology 2016, 63, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Van Houdenhove, B.; Luyten, P. Stress, Depression and Fibromyalgia. Acta Neurol. Belg. 2006, 106, 149. [Google Scholar] [PubMed]
- Grossi, G.; Perski, A.; Osika, W.; Savic, I. Stress-Related Exhaustion Disorder–Clinical Manifestation of Burnout? A Review of Assessment Methods, Sleep Impairments, Cognitive Disturbances, and Neuro-Biological and Physiological Changes in Clinical Burnout. Scand. J. Psychol. 2015, 56, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Ridsdale, L.; Evans, A.; Jerrett, W.; Mandalia, S.; Osler, K.; Vora, H. Patients with Fatigue in General Practice: A Prospective Study. Br. Med. J. 1993, 307, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.; Chalder, T. Overcoming Chronic Fatigue. A Self-Help Guide Using Cognitive Behavioural Techniques; Robinson: London, UK, 2009. [Google Scholar]
| Demographic Variables | All Participants | Fatigue Met PPS Criteria | Fatigue Did Not Meet PPS Criteria | χ2 |
|---|---|---|---|---|
| Gender | ||||
| Female | 892 (81.8%) | 100 (94.3%) | 792 (80.5%) | 12.351 *** |
| Male | 198 (18.2%) | 6 (5.7%) | 192 (19.5%) | |
| Age | ||||
| 30 years or younger | 60 (5.5%) | 6 (5.7%) | 54 (5.5%) | 0.006 |
| 31–50 years | 699 (64.1%) | 68 (64.2%) | 631 (64.1%) | |
| 51–70 years | 331 (30.4%) | 32 (30.2%) | 299 (30.4%) | |
| Education completed | ||||
| Undergraduate degree | 465 (42.7%) | 52 (49.1%) | 413 (42.0%) | 1.964 |
| Graduate degree | 625 (57.3%) | 54 (50.9%) | 571 (58.0%) | |
| Marital status | ||||
| Single | 128 (11.8%) | 17 (16.2%) | 111 (11.4%) | 2.971 |
| Married/relationship | 880 (81.4%) | 79 (75.2%) | 801 (82.1%) | |
| Separated/widowed | 73 (6.8%) | 9 (8.6%) | 564 (6.6%) | |
| Employment | ||||
| Executives and managers | 290 (26.9%) | 32 (30.8%) | 258 (26.5%) | 3.292 |
| Specialists | 398 (36.9%) | 32 (30.8%) | 366 (37.6%) | |
| Specialists in human services | 335 (31.1%) | 36 (34.6%) | 299 (30.7%) | |
| Specialised workers | 9 (0.8%) | 0 (0.0%) | 9 (0.9%) | |
| Retail, service and other | 46 (4.3%) | 4 (3.8%) | 42 (4.3%) | |
| Total | 1090 (100%) | 106 (9.7%) | 984 (90.3%) |
| Measures | All Participants | Fatigue Met PPS Criteria | Fatigue Did Not Meet PPS Criteria | Sig. | Effect Size |
|---|---|---|---|---|---|
| Means | M (SD) | M (SD) | M (SD) | T | r |
| PHQ-9 | 9.34 (4.6) | 11.63 (5.0) | 9.08 (4.5) | −5.36 *** | 0.17 |
| GAD-7 | 7.73 (4.6) | 9.49 (5.1) | 7.52 (4.5) | −4.14 *** | 0.13 |
| WSAS | 13.04 (8.4) | 20.07 (8.6) | 12.22 (8.0) | −9.33 *** | 0.28 |
| Cut-offs | N (%) | N (%) | N (%) | χ2 | OR [95% CI] |
| PHQ-9≥10 | 389 (35.7%) | 60 (56.6%) | 392 (33.4%) | 16.44 *** | 2.43 [1.6–3.8] |
| GAD-7≥10 | 290 (26.6%) | 44 (41.5%) | 246 (25.0%) | 10.98 *** | 2.04 [1.3–3.1] |
| WSAS≥10 | 581 (53.3%) | 91 (85.8%) | 490 (49.8%) | 44.37 *** | 9.29 [4.3–20.3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broddadóttir, E.; Flóvenz, S.Ó.; Gylfason, H.F.; Þormar, Þ.; Einarsson, H.; Salkovskis, P.; Sigurðsson, J.F. “I’m So Tired”: Fatigue as a Persistent Physical Symptom among Working People Experiencing Exhaustion Disorder. Int. J. Environ. Res. Public Health 2021, 18, 8657. https://doi.org/10.3390/ijerph18168657
Broddadóttir E, Flóvenz SÓ, Gylfason HF, Þormar Þ, Einarsson H, Salkovskis P, Sigurðsson JF. “I’m So Tired”: Fatigue as a Persistent Physical Symptom among Working People Experiencing Exhaustion Disorder. International Journal of Environmental Research and Public Health. 2021; 18(16):8657. https://doi.org/10.3390/ijerph18168657
Chicago/Turabian StyleBroddadóttir, Elín, Sigrún Ólafsdóttir Flóvenz, Haukur Freyr Gylfason, Þórey Þormar, Hjalti Einarsson, Paul Salkovskis, and Jón Friðrik Sigurðsson. 2021. "“I’m So Tired”: Fatigue as a Persistent Physical Symptom among Working People Experiencing Exhaustion Disorder" International Journal of Environmental Research and Public Health 18, no. 16: 8657. https://doi.org/10.3390/ijerph18168657
APA StyleBroddadóttir, E., Flóvenz, S. Ó., Gylfason, H. F., Þormar, Þ., Einarsson, H., Salkovskis, P., & Sigurðsson, J. F. (2021). “I’m So Tired”: Fatigue as a Persistent Physical Symptom among Working People Experiencing Exhaustion Disorder. International Journal of Environmental Research and Public Health, 18(16), 8657. https://doi.org/10.3390/ijerph18168657







