Quality of Life of Children and Adolescents with Multiple Sclerosis—A Literature Review of the Quantitative Evidence
Abstract
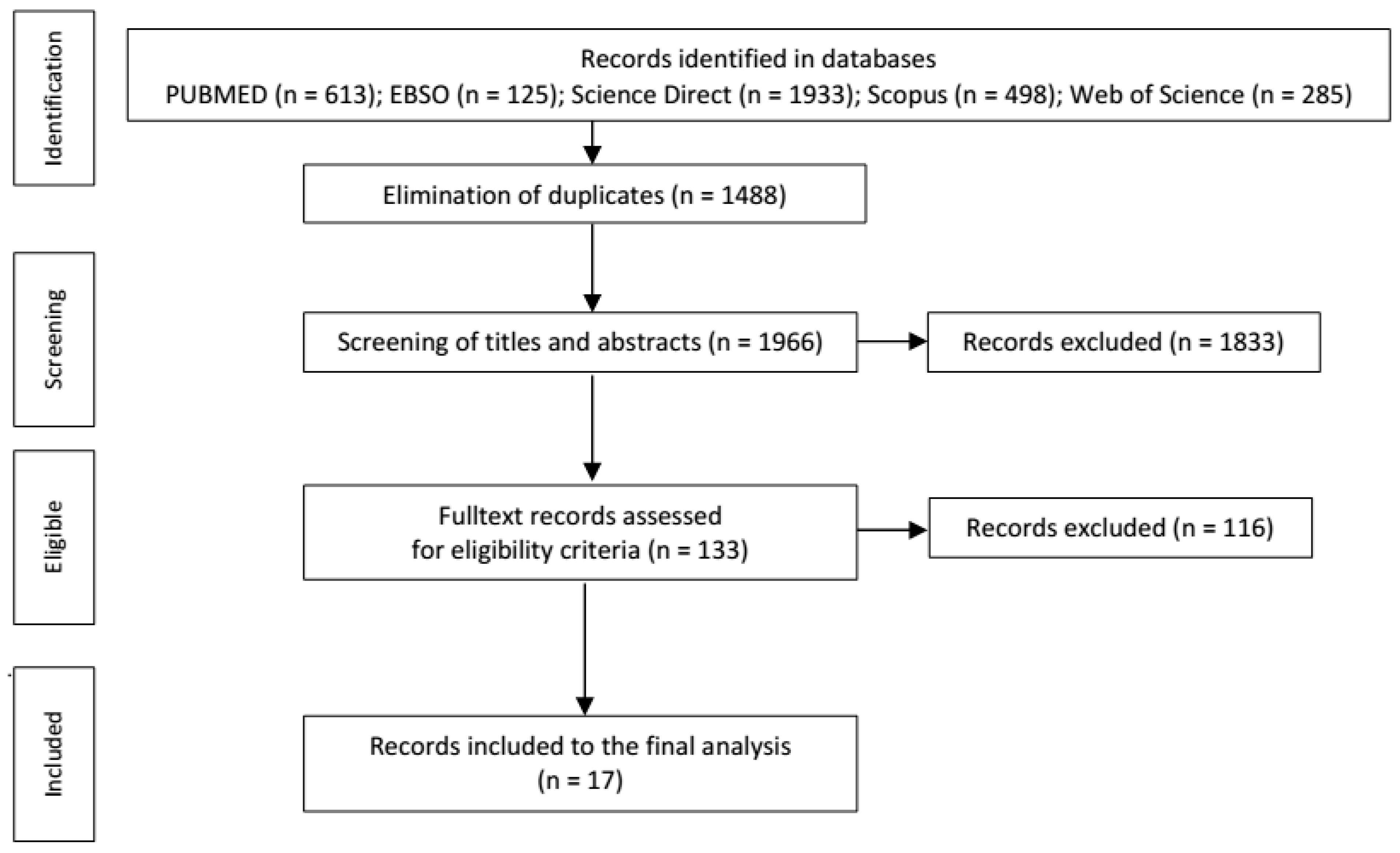
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
3.1. Tools Used for the Assessment of QoL
| Authors | Study | Country | Sample |
|---|---|---|---|
| MacAllister et al. 2009 [32] | Cross-sectional study | USA | 51 MS patients |
| Age (mean ± SD, range): 14.8 ± 2.21, 9–17 | |||
| Age at onset (mean ± SD, range): 13.1 ± 2.80, 3–16 | |||
| 64.7% female | |||
| Disease duration in months (mean ± SD, range): 20.3 ± 20.4, 2–90 | |||
| EDSS (mean ± SD, range): 1.7 ± 1.5, 0–6 | |||
| Mowry et al. 2010 [33] | Comparative study | USA | 41 MS/CIS patients |
| Age at questionnaire (mean ± SD): 14 ± 4 | |||
| 62% female | |||
| Disease duration in years (median, IQR): 2.0, 0.2–5.6 | |||
| EDSS (median, IQR): 1.5, 0–3.5 | |||
| 1. Control group (n = 12): sibling of patients | |||
| Age at questionnaire (mean ± SD): 13 ± 3 | |||
| 75% female | |||
| 2. Control group (n = 38): children with neuromuscular disorders1 | |||
| Age at questionnaire (mean ± SD): 9 ± 5 | |||
| 33% female | |||
| Lulu et al. 2014 [34] | Cross-sectional study | USA | 30 MS patients |
| Age (mean ± SD): 15.8 ± 2.5 | |||
| 53% female | |||
| EDSS (median, IQR): 1.5 (1–3) | |||
| Holland et al. 2014 [35] | Retrospective study | USA | 26 patients (MS = 23, CIS = 3) |
| Age at evaluation (mean, range): 13.96, 7–18 | |||
| 65% female | |||
| Disease duration in months (mean ± SD, range): 14.54 ± 14.38, 1–61 | |||
| Yeh et al. 2017 [38] | Randomized trial | North America | 52 MS patients |
| Age (mean ± SD): 16.03 ± 2.20 | |||
| Age at onset (mean ± SD): 13.62 ± 2.27 | |||
| 65.38% female | |||
| EDSS (mean ± SD): 1.23 ± 1.01 | |||
| Schwartzal. 2018 [39] | Randomized trial | North America | 66 MS patients |
| Age (mean ± SD): 15.74 ± 2.02 | |||
| Age at onset (mean ± SD):13.20 ± 3.91 | |||
| 67% female | |||
| Disease duration (mean ± SD): 2.27 ± 2.25 | |||
| O’Mahony et al. 2019 [41] | Comparative study | Canada | 58 MS patients |
| Age at questionnaire (median, IQR): 17.0, 14.4–19.9 | |||
| Age at onset (median, IQR): 13.9, 10.9–15.1 | |||
| 67.2% female | |||
| 178 monoADS patients | |||
| Age at questionnaire (median, IQR): 12.6, 8.5–16.3 | |||
| Age at onset (median, IQR): 9.1, 4.7–12.3 | |||
| 45.5% female | |||
| Marrie et al. 2020 [45] | Prospective, comparative study | Canada | 36 MS patients |
| Age at enrollment (mean ± SD): 13.71 ± 3.19 | |||
| Age at QoL measurement (mean ± SD): 16.61 ± 4.08 | |||
| 75.0% females | |||
| 43 monoADS patients | |||
| Age (mean ± SD): 9.67 ± 3.93 | |||
| 44.2% females | |||
| 43 healthy controls | |||
| Age (mean ± SD): 17.28 ± 4.53 | |||
| 65.1% females | |||
| Ketelslegers et al. 2010 [47] | Comparative study | Netherlands | 10 MS patients |
| Age (mean ± SD): 15.7 ± 1.47 | |||
| Boys/girls (4/6) | |||
| EDSS (mean ± SD): 2.5 ± 2.5 | |||
| 22 monophasic patients (ADEM, NO, TM) | |||
| Age (mean ± SD): 14.5 ± 1.78 | |||
| Boys/girls (14/8) | |||
| EDSS (mean ± SD): 1.1 ± 1.2 | |||
| Healthy control group | |||
| Toussaint-Duysteret al. 2018 [40] | Cross-sectional, comparative study | Netherlands | 22 MS patients |
| Age (median, IQR): 14.0, 13.0–15.0 | |||
| Boys: 18% | |||
| EDSS (range): 0–2, 46% at level 0, 9% at level 2 | |||
| 16 ADEM patients | |||
| Age (median, IQR): 4.5, 2.3–5.9 | |||
| Boys: 56% | |||
| EDSS (range): 0–3, 56% at level 0, 6% at level 3 | |||
| Lanzillo et al. 2016 [36] | Cross-sectional study | Italy | 54 MS/CIS patients |
| Age (mean ± SD): 20 ± 3.6 | |||
| 52.5% female | |||
| Disease duration in years (median): 2 | |||
| EDSS (median, range): 2.5, 0–4.5 | |||
| MSSS (mean ± SD): 5.88 ± 1.44 | |||
| Pediatric group (n = 34): disease onset ≤18 years | |||
| Juvenile group (n = 20): disease onset 18–25 years | |||
| Ghezzi etal. 2017 [37] | Observational, prospective study | Italy | 50 MS patients |
| Age (mean ± SD, range): 15 ± 2.1, 12–16 | |||
| 30% male sex | |||
| EDSS (mean): 1 | |||
| Ostojic et al. 2016 [48] | Retrospective, comparative study | Serbia | 21 MS patients |
| Age (mean ± SD, range): 16.95 ± 1.01, 14–18 | |||
| Age at onset (mean ± SD, range): 13.98 ± 2.29, 8–17.50 | |||
| 71.40% female | |||
| Disease duration in years (mean ± SD): 3.08 ± 2.50 | |||
| EDSS (mean ± SD, range): 1.71 ± 0.83, 0–3.5 | |||
| 110 healthy adolescents | |||
| Johnen et al. 2019 [42] | Prospective study | Germany | 19 MS patients |
| Age (mean ± SD, range): 15.05 ± 2.01, 10–17 | |||
| 14 females | |||
| Disease duration in months (mean ± SD): 12.95 ± 23.52 | |||
| EDSS (mean ± SD): 0.50 ± 0.61 | |||
| Non-escalated MS group (treatment by interferon, dimethyl fumarate, glatiramer acetate) (n = 13) | |||
| Escalated MS group (treatment by DMT) (n = 6) | |||
| Storm van’s Gravesande et al. 2019 [43] | Multicenter, cross-sectional, comparative study | Germany, Austria | 106 MS patients |
| Age (mean ± SD, range): 15,71 ± 1.63, 12–18 | |||
| 76 females (71.7%) | |||
| Disease duration in months (mean ± SD, range): 18.6 ± 23.7, 0–152 | |||
| EDSS (mean ± SD, range): 0.65 ± 1.09, 0–7.5 | |||
| 210 healthy subjects | |||
| Age (mean ± SD, range): 15.00 ± 2.0, 12–18 | |||
| 95 females (45.2%) | |||
| Florea et al. 2020 [44] | Cross-sectional study | France | 26 MS patients |
| Age at onset (mean ± SD): 12.4 ± 3.1 | |||
| Age at test (mean ± SD): 15.2 ± 1.11 | |||
| Boys/girls (17/9) | |||
| EDSS (mean ± SD): 0.1 ± 0.5 | |||
| Smith et al. 2020 [46] | Retrospective study | UK | 51 MS patients (QoL evaluation only in 15 patients) |
| Age at onset (median): 13.7 | |||
| EDSS (median): 1 |
3.2. Quality of Life in MS Patients
3.3. Comparison of the Quality of Life in MS Patients with a Healthy Control
3.4. Comparison of the Quality of Life in MS Patients with Other Neurological Disease Groups
3.5. Factors Determining Quality of Life
4. Discussion
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alroughani, R.; Boyko, A. Pediatric multiple sclerosis: A review. BMC Neurol. 2018, 18, 27. [Google Scholar] [CrossRef]
- Ochi, H. Clinical features, diagnosis and therapeutic strategies in pediatric multiple sclerosis. Clin. Exp. Neuroimmunol. 2017, 8, 33–39. [Google Scholar] [CrossRef]
- Multiple Sclerosis International Federation. The Atlas of MS Is Live! The 3rd Edition. 2020. Available online: https://www.atlasofms.org/map/global/epidemiology/number-of-children-with-ms (accessed on 2 October 2020).
- Jeong, A.; Oleske, D.M.; Holman, J. Epidemiology of pediatric-onset multiple sclerosis: A systematic review of the literature. J. Child. Neurol. 2019, 34, 705–712. [Google Scholar] [CrossRef]
- Chmelařová, D. Rehabilitace kognitivních funkcií. Neurol. Praxi 2016, 17, 62–69. [Google Scholar]
- Ruet, A. Update on pediatric-onset multiple sclerosis. Rev. Neurol. 2018, 174, 398–407. [Google Scholar] [CrossRef]
- Waldman, A.; Ness, J.; Pohl, D.; Simone, I.L.; Anlar, B.; Amato, M.P.; Ghezzi, A. Pediatric multiple sclerosis. Clinical features and outcome. Neurology 2016, 87, S74–S80. [Google Scholar] [CrossRef]
- Yeh, E.A.; Parrish, J.B.; Weinstock-Guttman, B. Disease progression in pediatric multiple sclerosis: Disparities between physical and neurocognitive outcomes. Expert Rev. Neurother. 2011, 11, 433–440. [Google Scholar] [CrossRef]
- Spiro, D.B. Early onset multiple sclerosis. A review for nurse practitioners. J. Pediatr. Health Care 2012, 26, 399–408. [Google Scholar] [CrossRef]
- Carroll, S.; Chalder, T.; Hemingway, C.; Heyman, I.; Moss-Morris, R. Understanding fatigue in paediatric multiple sclerosis: A systematic review of clinical and psychosocial factors. Dev. Med. Child Neurol. 2016, 58, 229–239. [Google Scholar] [CrossRef]
- Post, M.W. Definitions of quality of life: What has happened and how to move on. Top. Spinal Cord Inj. Rehabil. 2014, 20, 167–180. [Google Scholar] [CrossRef]
- Cella, D.; Nowinski, C.J. Measuring quality of life in chronic illness: The functional assessment of chronic illness therapy measurement system. Arch. Phys. Med. Rehabil. 2002, 83, S10–S17. [Google Scholar] [CrossRef]
- Revicki, D.A.; Osoba, D.; Fairclough, D.; Barofsky, I.; Berzon, R.; Leidy, N.K.; Rothman, M. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res. 2000, 9, 887–900. [Google Scholar] [CrossRef]
- Strober, L.B. Quality of life and psychological well-being in the early stages of multiple sclerosis (MS): Importance of adopting a biopsychosocial model. Disabil. Health J. 2018, 11, 555–561. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Reflection Paper on the Regulatory Guidance for the Use of Health Related Quality of Life (HRQOL) Measures in the Evaluation of Medicinal Products. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf (accessed on 11 July 2021).
- Varni, J.W.; Burwinkle, T.M.; Katz, E.R.; Meeske, K.; Dickinson, P. The PedsQL™ in pediatric cancer reliability and validity of the pediatric quality of life Inventory™ generic core scales, multidimensional fatigue scale, and cancer module. Cancer 2002, 94, 2090–2106. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Smith Knight, T.; Burwinkle, T.; Brown, J.; Szer, I.S. The PedsQL™ in pediatric rheumatology reliability, validity, and responsiveness of the pediatric quality of life Inventory™ generic core scales and rheumatology module. Arthritis Rheum. 2002, 46, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Chromá, J.; Slaný, J. Kvalita života dětí s astmatem. Časopis Lékařů Českých 2011, 150, 660–664. [Google Scholar] [PubMed]
- Nieuwesteeg, A.; Pouwer, F.; van der Kamp, R.; van Bakel, H.; Aaanstoot, H.-J.; Hartman, E. Quality of life of children with type 1 diabetes: A systematic review. Curr. Diabetes Rev. 2012, 8, 434–443. [Google Scholar] [CrossRef]
- Goethe, E.; LoPresti, M.A.; Zhao, M.; Brayton, A.; Gadgil, N.; Pan, I.W.; Lam, S. Quality of life in pediatric neurosurgery: Comparing parent and patient perceptions. World Neurosurg. 2020, 134, e306–e310. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Limbers, C.A.; Szer, I.S. The PedsQL™ as a patient-reported outcome in children and adolescents with fibromyalgia: An analysis of OMERACT domains. Health Qual. Life Outcomes 2007, 5, 9. [Google Scholar] [CrossRef]
- Self, M.M.; Fobian, A.; Cutitta, K.; Wallace, A.; Lotze, T.E. Health-related quality of life in pediatric patients with demyelinating diseases: Relevance of disability, relapsing presentation, and fatigue. J. Pediatr. Psychol. 2018, 43, 133–142. [Google Scholar] [CrossRef]
- Iannaccone, S.T.; Hynan, L.S.; Morton, A.; Buchanan, R.; Limbers, C.A.; Varni, J.W.; the AmSMART Group. The PedsQLTM in pediatric patients with spinal muscular atrophy: Feasibility, reliability, and validity of the pediatric quality of life InventoryTM generic core scales and neuromuscular module. Neuromuscul. Disord. 2009, 19, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-L.; Wang, H.-Y.; Tseng, M.-H.; Shieh, J.-Y.; Lu, L.; Yao, K.-P.G.; Huang, C.-Y. The cerebral palsy quality of life for children (CP QOL-Child): Evidence of construct validity. Res. Dev. Disabil. 2013, 34, 994–1000. [Google Scholar] [CrossRef]
- Goodwin, S.W.; Ferro, M.A.; Speechley, K.N. Development and assessment of the Quality of life in childhood epilepsy questionnaire (QOLCE-16). Epilepsia 2018, 59, 668–678. [Google Scholar] [CrossRef]
- Cross, T.P.; Shanks, A.K.; Duffy, L.V.; Rintell, D.J. Families’ experience of pediatric onset multiple sclerosis. J. Child Adolesc. Trauma 2019, 12, 425–435. [Google Scholar] [CrossRef]
- Uccelli, M.M. The impact of multiple sclerosis on family members: A review of the literature. Neurodegener. Dis. Manag. 2014, 4, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Krysko, K.M.; O’Connor, P. Quality of life, cognition and mood in adults with pediatric multiple sclerosis. Can. J. Neurol. Sci. 2016, 43, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.I.; Weiland, T.J.; Jelinek, G.A.; Simpson, S.; Brown, C.R.; Neate, S.L.; Taylor, K.L.; O’Kearney, E.; Milanzi, E.; de Livera, A.M. Longitudinal associations of the healthy lifestyle index score with quality of life in people with multiple sclerosis: A prospective cohort study. Front. Neurol. 2018, 9, 874. [Google Scholar] [CrossRef]
- McKay, K.A.; Ernstsson, O.; Manouchehrinia, A.; Olsson, T.; Hillert, J. Determinants of quality of life in pediatric- and adult-onset multiple sclerosis. Neurology 2020, 94, e932–e941. [Google Scholar] [CrossRef]
- Gil-González, I.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M.Á. Quality of life in adults with multiple sclerosis: A systematic review. BMJ Open 2020, 10, e041249. [Google Scholar] [CrossRef] [PubMed]
- MacAllister, W.S.; Christodoulou, C.; Troxell, R.; Milazzo, M.; Block, P.; Preston, T.E.; Bender, H.A.; Belman, A.; Krupp, L.B. Fatigue and quality of life in pediatric multiple sclerosis. Mult. Scler. 2009, 15, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Mowry, E.M.; Julian, L.J.; Im-Wang, S.; Chabas, D.; Gavin, A.J.; Strober, J.B.; Waubant, E. Health-related quality of life is reduced in pediatric multiple sclerosis. Pediatr. Neurol. 2010, 43, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lulu, S.; Julian, L.; Shapiro, E.; Hudson, K.; Waubant, E. Treatment adherence and transitioning youth in pediatric multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.A.; Graves, D.; Greenberg, B.M.; Harder, L.L. Fatigue, emotional functioning, and executive dysfunction in pediatric multiple sclerosis. Child Neuropsychol. 2014, 20, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, R.; Chiodi, A.; Carotenuto, A.; Magri, V.; Napolitano, A.; Liuzzi, R.; Costabile, T.; Rainone, N.; Freda, M.F.; Valerio, P.; et al. Quality of life and cognitive functions in early onset multiple sclerosis. Eur. J. Paediatr. Neurol. 2016, 20, 158–163. [Google Scholar] [CrossRef]
- Ghezzi, A.; Bianchi, A.; Baroncini, D.; Bertolotto, A.; Malucchi, S.; Bresciamorra, V.; Lanzillo, R.; Milani, N.; Martinelli, V.; Patti, F.; et al. A multicenter, observational, prospective study of self and parent-reported quality of life in adolescent multiple sclerosis patients self-administering interferon-β1a using RebiSmart™—The FUTURE study. Neurol. Sci. 2017, 38, 1999–2005. [Google Scholar] [CrossRef]
- Yeh, E.A.; Grover, S.A.; Powell, V.E.; Alper, G.; Banwell, B.L.; Edwards, K.; Gorman, M.; Graves, J.; Lotze, T.E.; Mah, J.K.; et al. Impact of an electronic monitoring device and behavioral feedback on adherence to multiple sclerosis therapies in youth: Results of a randomized trial. Qual. Life Res. 2017, 26, 2333–2349. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Grover, S.A.; Powell, V.E.; Noguera, A.; Mah, J.K.; Mar, S.; Mednick, L.; Banwell, B.L.; Alper, G.; Rensel, M.; et al. Risk factors for non-adherence to disease-modifying therapy in pediatric multiple sclerosis. Mult. Scler. 2018, 24, 175–185. [Google Scholar] [CrossRef]
- Toussaint-Duyster, L.C.C.; Wong, Y.Y.M.; van der Cammen-van Zijp, M.H.M.; van Pelt-Gravesteijn, D.; Catsman-Berrevoets, C.E.; Hintzen, R.Q.; Neuteboom, R.F. Fatigue and physical functioning in children with multiple sclerosis and acute disseminated encephalomyelitis. Mult. Scler. 2018, 24, 982–990. [Google Scholar] [CrossRef]
- O’Mahony, J.; Marrie, R.A.; Laporte, A.; Bar-Or, A.; Yeh, E.A.; Brown, A.; Dilenge, M.-E.; Banwell, B. Pediatric-onset multiple sclerosis is associated with reduced parental health–related quality of life and family functioning. Mult. Scler. 2019, 25, 1661–1672. [Google Scholar] [CrossRef]
- Johnen, A.; Elpers, C.; Riepl, E.; Landmeyer, N.C.; Krämer, J.; Polzer, P.; Lohmann, H.; Omran, H.; Wiendl, H.; Göbel, K.; et al. Early effective treatment may protect from cognitive decline in paediatric multiple sclerosis. Eur. J. Paediatr. Neurol. 2019, 23, 783–791. [Google Scholar] [CrossRef]
- Storm Van’s Gravesande, K.; Blaschek, A.; Calabrese, P.; Rostásy, K.; Huppke, P.; Kessler, J.; Kalbe, E.; Mall, V.; MUSICADO Study group. Fatigue and depression predict health-related quality of life in patients with pediatric-onset multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 39, 101368. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Maurey, H.; le Sauter, M.; Bellesme, C.; Sevin, C.; Deiva, K. Fatigue, depression, and quality of life in children with multiple sclerosis: A comparative study with other demyelinating diseases. Dev. Med. Child Neurol. 2020, 62, 241–244. [Google Scholar] [CrossRef]
- Marrie, R.A.; O’Mahony, J.; Maxwell, C.; Ling, V.; Till, C.; Barlow-Krelina, E.; Yeh, E.A.; Arnold, D.L.; Bar-Or, A.; Banwell, B. Canadian pediatric demyelinating disease network. Factors associated with health care utilization in pediatric multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 38, 101511. [Google Scholar] [CrossRef]
- Smith, A.L.; Benetou, C.; Bullock, H.; Kuczynski, A.; Rudebeck, S.; Hanson, K.; Crichton, S.; Mankad, K.; Siddiqui, A.; Byrne, S.; et al. Progress in the management of paediatric-onset multiple sclerosis. Children 2020, 7, 222. [Google Scholar] [CrossRef]
- Ketelslegers, I.A.; Catsman-Berrevoets, C.E.; Boon, M.; Eikelenboom, M.J.; Stroink, H.; Neuteboom, R.F.; Aarsen, F.K.; van de Putte, E.M.; Hintzen, R.Q. Fatigue and depression in children with multiple sclerosis and monophasic variants. Eur. J. Paediatr. Neurol. 2010, 14, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.; Stevanovic, D.; Jancic, J. Quality of life and its correlates in adolescent multiple sclerosis patients. Mult. Scler. Relat. Disord. 2016, 10, 57–62. [Google Scholar] [CrossRef]
- Baumstarck, K.; Boyer, L.; Boucekine, M.; Michel, P.; Pelletier, J.; Auquier, P. Measuring the quality of life in patients with multiple sclerosis in clinical practice: A necessary challenge. Mult. Scler. Int. 2013, 524894. [Google Scholar] [CrossRef]
- Health Measures. Neuro QoL. List of Pediatric Measures. 2021. Available online: https://www.healthmeasures.net/explore-measurement-systems/neuro-qol/intro-to-neuro-qol/list-of-pediatric-measures (accessed on 11 July 2021).
- Hyarat, S.Y.; Subih, M.; Rayan, A.; Salami, I.; Harb, A. Health related quality of life among patients with multiple sclerosis: The role of psychosocial adjustment to illness. Arch. Psychiatr. Nurs. 2019, 33, 11–16. [Google Scholar] [CrossRef]
- Cioncoloni, D.; Innocenti, I.; Bartalini, S.; Santarnecchi, E.; Rossi, S.; Rossi, A.; Ulivelli, M. Individual factors enhance poor health-related quality of life outcome in multiple sclerosis patients. Significance of predictive determinants. J. Neurol. Sci. 2014, 345, 213–219. [Google Scholar] [CrossRef]
- Garg, H.; Bush, S.; Gappmaier, E. Associations between fatigue and disability, functional mobility, depression, and quality of life in people with multiple sclerosis. Int. J. MS Care 2016, 18, 71–77. [Google Scholar] [CrossRef]
- Ochoa-Morales, A.; Hernández-Mojica, T.; Paz-Rodríguez, F.; Jara-Prado, A.; de los Santos, Z.T.; Sánchez-Guzmán, M.A.; Guerrero-Camacho, J.L.; Corona-Vázquez, T.; Flores, J.; Camacho-Molina, A.; et al. Quality of life in patients with multiple sclerosis and its association with depressive symptoms and physical disability. Mult. Scler. Relat. Disord. 2019, 36, 101386. [Google Scholar] [CrossRef]
- Amtmann, D.; Bamer, A.M.; Kim, J.; Chung, H.; Salem, R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil. Health, J. 2018, 11, 99–107. [Google Scholar] [CrossRef]
- Rezapour, A.; Kia, A.A.; Goodarzi, S.; Hasoumi, M.; Motlagh, S.N.; Vahedi, S. The impact of disease characteristics on multiple sclerosis patients’ quality of life. Epidemiol. Health. 2017, 39, e2017008. [Google Scholar] [CrossRef]
- Schmidt, S.; Jöstingmeyer, P. Depression, fatigue and disability are independently associated with quality of life in patients with multiple Sclerosis: Results of a cross-sectional study. Mult. Scler. Relat. Disord. 2019, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Yalachkov, Y.; Soydas, D.; Bergmann, J.; Frisch, S.; Behrens, M.; Foerch, C.; Gehrig, J. Determinants of quality of life in relapsing-remitting and progressive multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 33–37. [Google Scholar] [CrossRef]
- Belman, A.L.; Krupp, L.B.; Olsen, C.S.; Rose, J.W.; Aaen, G.; Benson, L.; Chitnis, T.; Gorman, M.; Graves, J.; Harris, Y.; et al. Characteristics of children and adolescents with multiple sclerosis. Pediatrics 2016, 138, e20160120. [Google Scholar] [CrossRef] [PubMed]
- Lavery, A.M.; Banwell, B.L.; Liu, G.; Waldman, A.T. Hospital admission rates for pediatric multiple sclerosis in the United States using the Pediatric Health Information System (PHIS). Mult. Scler. Relat. Disord. 2016, 9, 5–10. [Google Scholar] [CrossRef][Green Version]
- Carroll, S.; Chalder, T.; Hemingway, C.H.; Heyman, I.; Bear, H.; Sweeney, L.; Moss-Morris, R. Adolescent and parent factors related to fatigue in paediatric multiple sclerosis and chronic fatigue syndrome: A comparative study. Eur. J. Paediatr. Neurol. 2019, 23, 70–80. [Google Scholar] [CrossRef]
- Parrish, J.B.; Fields, E. Cognitive functioning in patients with pediatric-onset multiple sclerosis, an updated review and future focus. Children 2019, 6, 21. [Google Scholar] [CrossRef]
- Nunan-Saah, J.; Paulraj, S.R.; Waubant, E.; Krupp, L.B.; Gomez, R.G. Neuropsychological correlates of multiple sclerosis across the lifespan. Mult. Scler. 2015, 21, 1355–1364. [Google Scholar] [CrossRef]
- Carroll, S.; Chalder, T.; Hemingway, C.; Heyman, I.; Moss-Morris, R. It feels like wearing a giant sandbag. Adolescent and parent perceptions of fatigue in paediatric multiple sclerosis. Eur. J. Paediatr. Neurol. 2016, 20, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Rintell, D.; Charvet, L.E.; Milazzo, M.; Wassmer, E. Pediatric multiple sclerosis. Perspectives from adolescents and their families. Neurology 2016, 87, S4–S7. [Google Scholar] [CrossRef]
- Morsa, M.; Lombrail, P.; Boudailliez, B.; Godot, C.; Jeantils, V.; Gagnayre, R. A qualitative study on the educational needs of young people with chronic conditions transitioning from pediatric to adult care. Patient Prefer. Adherence 2018, 12, 2649–2660. [Google Scholar] [CrossRef]
- Karatepe, A.G.; Kaya, T.; Günaydn, R.; Demirhan, A.; Ce, P.; Gedizlioglu, M. Quality of life in patients with multiple sclerosis: The impact of depression, fatigue, and disability. Int. J. Rehabil. Res. 2011, 34, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Salehpoor, G.; Rezaei, S.; Hosseininezhad, M. Quality of life in multiple sclerosis (MS) and role of fatigue, depression, anxiety, and stress: A bicenter study from north of Iran. Iran. J. Nurs. Midwifery Res. 2014, 19, 593–599. [Google Scholar]
- Hayter, A.L.; Salkovskis, P.M.; Silber, E.; Morris, R.G. The impact of health anxiety in patients with relapsing remitting multiple sclerosis: Misperception, misattribution and quality of life. Br. J. Clin. Psychol. 2016, 55, 371–386. [Google Scholar] [CrossRef]
- Rainone, N.; Chiodi, A.; Lanzillo, R.; Magri, V.; Napolitano, A.; Morra, V.B.; Valerio, P.; Freda, M.F. Affective disorders and Health-Related Quality of Life (HRQoL) in adolescents and young adults with Multiple Sclerosis (MS): The moderating role of resilience. Qual. Life Res. 2017, 26, 727–736. [Google Scholar] [CrossRef]
- Geng, Y.; Gu, J.; Zhu, X.; Yang, M.; Shi, D.; Shang, J.; Zhao, F. Negative emotions and quality of life among adolescents: A moderated mediation model. Int. J. Clin. Health Psychol. 2020, 20, 118–125. [Google Scholar] [CrossRef]
- Grossman, P.; Kappos, L.; Gensicke, H.; D’Souza, M.; Mohr, D.C.; Penner, I.K.; Steiner, C. MS quality of life, depression, and fatigue improve after mindfulness training. A randomized trial. Neurology 2010, 75, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, C.; Schweikert, B.; Ecari, U.; Oentrich, W.; Bugge, J.-P. Quality of life and depression in multiple sclerosis patients: Longitudinal results of the BetaPlus study. J. Neurol. 2012, 259, 2319–2328. [Google Scholar] [CrossRef]
- Lysandropoulos, A.P.; Havrdova, E.; ParadigMS Group. ‘Hidden’ factors influencing quality of life in patients with multiple sclerosis. Eur. J. Neurol. 2015, 22, 28–33. [Google Scholar] [CrossRef]
- Grover, S.A.; Sawicki, C.P.; Kinnett-Hopkins, D.; Finlayson, M.; Schneiderman, J.E.; Banwell, B.; Till, C.; Motl, R.W.; Yeh, E.A. Physical activity and its correlates in youth with multiple sclerosis. J. Pediatr. 2016, 179, 197–203. [Google Scholar] [CrossRef]
- Boesen, M.S.; Thygesen, L.C.; Uldall, P.V.; Eriksson, F.; Born, A.P.; Blinkenberg, M.; Koch-Henriksen, N.; Greisen, G.; Magyari, M. Psychiatric morbidity develops after onset of pediatric multiple sclerosis: A Danish nationwide population-based study. Mult. Scler. Relat. Disord. 2018, 19, 30–34. [Google Scholar] [CrossRef]
- Stephens, S.; Shams, S.; Lee, J.; Grover, S.A.; Longoni, G.; Berenbaum, T.; Finlayson, M.; Motl, R.W.; Yeh, E.A. Benefits of physical activity for depression and fatigue in multiple sclerosis: A longitudinal analysis. J. Pediatr. 2019, 209, 226–232. [Google Scholar] [CrossRef]
- Thorová, K. Vývojová Psychologie. Proměny Lidské Psychiky od Početí po Smrt; Portál: Prague, Czech Republic, 2015; pp. 414–432. [Google Scholar]
- Pendley, J.S.; Kasmen, L.J.; Miller, D.L.; Donze, J.; Swenson, C.; Reeves, G. Peer and family support in children and adolescents with type 1 diabetes. J. Pediatr. Psychol. 2002, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Kakleas, K.; Kandyla, B.; Karayianni, C.; Karavanaki, K. Psychosocial problems in adolescents with type 1 diabetes mellitus. Diabetes Metab. 2009, 35, 339–350. [Google Scholar] [CrossRef]
- Jamieson, N.; Fitzgerald, D.; Singh-Grewal, D.; Hanson, C.S.; Craig, J.C.; Tong, A. Children’s experiences of cystic fibrosis: A systematic review of qualitative studies. Pediatrics 2014, 133, e1683–e1697. [Google Scholar] [CrossRef]
- Kirk, S.; Hinton, D. “I’m not what I used to be”: A qualitative study exploring how young people experience being diagnosed with a chronic illness. Child Care Health Dev. 2019, 45, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Magrin, M.E.; D’Addario, M.; Greco, A.; Miglioretti, M.; Sarini, M.; Scrignaro, M.; Steca, P.; Vecchio, L.; Crocetti, E. Social support and adherence to treatment in hypertensive patients: A meta-analysis. Ann. Behav. Med. 2015, 49, 307–318. [Google Scholar] [CrossRef]
- Wallander, J.L.; Varni, J.W. Social support and adjustment in chronically ill and handicapped children. Am. J. Community Psychol. 1989, 17, 185–201. [Google Scholar] [CrossRef]
- Von Weiss, R.T.; Rapoff, M.A.; Varni, J.W.; Lindsley, C.B.; Olson, N.Y.; Madson, K.L.; Bernstein, B.H. Daily hassles and social support as predictors of adjustment in children with pediatric rheumatic disease. J. Pediatr. Psychol. 2002, 27, 155–165. [Google Scholar] [CrossRef]
- Uccelli, M.M.; Traversa, S.; Ponzio, M. A survey study comparing young adults with MS and healthy controls on self-esteem, self-efficacy, mood and quality of life. J. Neurol. Sci. 2016, 368, 369–373. [Google Scholar] [CrossRef]
- McCabe, M.P.; McKern, S. Quality of life and multiple sclerosis: Comparison between people with multiple sclerosis and people from the general population. J. Clin. Psychol. Med. Setting 2002, 9, 287–295. [Google Scholar] [CrossRef]
- Neuteboom, R.; Wilbur, C.; van Pelt, D.; Rodriguez, M.; Yeh, A. The spectrum of inflammatory acquired demyelinating syndromes in children. Semin. Pediatr. Neurol. 2017, 24, 189–200. [Google Scholar] [CrossRef]
- Brola, W.; Sobolewski, P.; Fudala, M.; Flaga, S.; Jantarski, K. Multiple sclerosis: Patient-reported quality of life in the Świętokrzyskie Region. Med Stud. Studia Med. 2017, 33, 191–198. [Google Scholar] [CrossRef]
- Wilski, M.; Gabryelsku, J.; Brola, W.; Tomasz, T. Health-related quality of life in multiple sclerosis: Links to acceptance, coping strategies and disease severity. Disabil. Health J. 2019, 12, 608–614. [Google Scholar] [CrossRef]
| Authors | Assessment of QoL | QoL in MS Group |
|---|---|---|
| MacAllister et al. 2009 | PedsQL | Severe difficulties (score ≥ 2 SD): |
| Physical scale: 20% | ||
| Emotional scale: 10% | ||
| Social scale: 4% | ||
| School scale: 28% | ||
| Mowry et al. 2010 | PedsQL | Mean score (SD): |
| Physical scale: 73 (23) | ||
| Psychosocial scale: 70 (17) | ||
| Emotional scale: 65 (24) | ||
| Social scale: 85 (15) | ||
| School scale: 60 (23) | ||
| Global score: 71 (17) | ||
| Lulu et al. 2014 | PedsQL | Median score (IQR): |
| Physical scale: 53.1 (36.3–68.8) | ||
| Psychosocial scale: 51 (40–61.7) | ||
| Global score: 53.8 (36.1–64.1) | ||
| Holland et al. 2014 | PedsQL (used only emotional scale) | Means score (SD): 56.28 (18.79) |
| Severe difficulties (score ≥2 SD): 26.9% | ||
| Yeh et al. 2017 | PedsQL | Baseline mean score (SD): |
| Physical scale: 82.23 (17.85) | ||
| Emotional scale: 71.15 (20.52) | ||
| Social scale: 83.56 (15.85) | ||
| School scale: 66.44 (16.87) | ||
| Schwartz al. 2018 | PedsQL | Mean score (SD): |
| Physical scale: 80.17 (18.50) | ||
| Emotional scale: 68.03 (23.05) | ||
| Social scale: 83.18 (17.22) | ||
| School scale: 63.56 (18.50) | ||
| O’Mahony et al. 2019 | PedsQL | Median score (IQR): |
| Physical scale: 87.5 (75.0–93.8) | ||
| Psychosocial scale: 80.0 (65.0–90.0) | ||
| Emotional scale: 75.0 (55.0–90.0) | ||
| Social scale: 90.0 (80.0–100.0) | ||
| School scale: 75.0 (56.7–90.0) | ||
| Global score: 81.5 (73.9–91.3) | ||
| Marrie et al. 2020 | PedsQL | Mean score (SD): |
| Psychosocial scale: 76.13 (15.50) | ||
| Physical scale: 81.14 (19.49) | ||
| Ketelslegers et al. 2010 | TACQOL CF 12–15 | - |
| Toussaint-Duysteret al. 2018 | PedsQL | Impaired QoL in MS group (score of 1 SD below the mean of healthy age-related reference norm): |
| Physical scale: 45% | ||
| Emotional scale: 18% | ||
| Social scale: 32% | ||
| School scale: 46% | ||
| Global score: 41% | ||
| Lanzillo et al. 2016 | PedsQoL | - |
| Ghezzi et al. 2017 | PedsQL | Baseline mean score (SD): |
| Physical scale: 81.3 (15.9) | ||
| Emotional scale: 73.1 (17.9) | ||
| Social scale: 90.3 (13.3) | ||
| School scale: 75.6 (18.5) | ||
| Psychosocial scale: 79.7 (13.8) | ||
| Global score: 80.3 (13.5) | ||
| Ostojic et al. 2016 | KIDSCREEN-52 | Mean score (SD): |
| Physical well-being: 47.00 (11.25) | ||
| Psychological well-being: 49.82 (12.76) | ||
| Moods and emotions: 51.65 (12.48) | ||
| Self-perception: 50.74 (10.19) | ||
| Autonomy: 53.40 (10.88) | ||
| Parent relation and home life: 55.12 (9.68) | ||
| Social support and peers: 52.91 (13.55) | ||
| School environment: 47.65 (10.24) | ||
| Social acceptance: 50.86 (12.67) | ||
| Financial resources: 51.96 (8.70) | ||
| Johnen et al. 2019 | PedsQL (psychosocial scale = emotional, social, school scale) | Baseline mean (SD) psychosocial scale in non-escalated: 4.50 (14.00), and in escalated group: 12.00 (13.00) |
| Storm van’s Gravesande et al. 2019 | PedsQL | Mean score (SD): |
| physical scale: 74.62 (22.1) | ||
| emotional scale: 63.35 (24.89) | ||
| social scale: 88.73 (17.01) | ||
| school scale: 58.15 (24.74) | ||
| global score: 71.81 (18.36) | ||
| Florea et al. 2020 | PedsQL | Poor QoL (below ≥75 points): |
| physical scale: 20% | ||
| emotional scale: 50% | ||
| social scale: 5% | ||
| school scale: 50% | ||
| global score: 40% | ||
| Smith et al. 2020 | PedsQL | Median global score (IQR): 81.7 (65.3–92.4) |
| Authors | Non-Significant Factors | Significant Factors |
|---|---|---|
| MacAllister et al. 2009 | Disease duration | Age |
| Total relapses. Relapse rate | Disability (EDSS) | |
| Age at onset disease | Fatigue (MFS) | |
| Mowry et al. 2010 | Age. Sex. Race. Ethnicity. | Disability (EDSS) |
| Drug therapy (DMT). Disease duration | ||
| Holland et al. 2014 | Sleep fatigue | Fatigue—general, cognitive, emotional, total (MFS) |
| Lulu et al. 2014 | Treatment adherence | Disability (EDSS) |
| Cognitive score (SDMT) | ||
| Lanzillo et al. 2016 | Disease duration | Disease severity (MSSS) |
| Age at onset disease | ||
| Ostojic et al. 2016 | Number of relapses. | Age |
| Disability (EDSS) | Age at disease onset. Disease duration | |
| Anxiety and depression (RCADS) | ||
| Fatigue (PedsFACIT-F) | ||
| Ghezzi et al. 2017 | Age. Sex | - |
| Age at onset disease. | ||
| Disease severity. | ||
| Drug treatment (interferon-β1). | ||
| Treatment naïveness | ||
| Yeh et al. 2017 | - | Non-pharmacologic interventions |
| O’Mahony et al. 2019 | Disease severity | - |
| Storm van’s Gravesande et al. 2019 | Disease course (acute relapse, remision) | Disability (EDSS) |
| Cognitive fatigue (MFS) | ||
| Depression (Depressionstest fur Kinder—DTK, German self-report questionnaire, BDI) | ||
| Johnen et al. 2019 | Drug treatment | - |
| Marrie et al. 2020 | Number of relapses. Hospitalization. | - |
| Physician visits. | ||
| Neurologic abnormality | ||
| (neurological examination). | ||
| Cognitive accuracy/cognitive response time (Penn Neurocognitive Battery) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrosková, S.; Klímová, E.; Majerníková, Ľ.; Tkáčová, Ľ. Quality of Life of Children and Adolescents with Multiple Sclerosis—A Literature Review of the Quantitative Evidence. Int. J. Environ. Res. Public Health 2021, 18, 8645. https://doi.org/10.3390/ijerph18168645
Mrosková S, Klímová E, Majerníková Ľ, Tkáčová Ľ. Quality of Life of Children and Adolescents with Multiple Sclerosis—A Literature Review of the Quantitative Evidence. International Journal of Environmental Research and Public Health. 2021; 18(16):8645. https://doi.org/10.3390/ijerph18168645
Chicago/Turabian StyleMrosková, Slávka, Eleonóra Klímová, Ľudmila Majerníková, and Ľubomíra Tkáčová. 2021. "Quality of Life of Children and Adolescents with Multiple Sclerosis—A Literature Review of the Quantitative Evidence" International Journal of Environmental Research and Public Health 18, no. 16: 8645. https://doi.org/10.3390/ijerph18168645
APA StyleMrosková, S., Klímová, E., Majerníková, Ľ., & Tkáčová, Ľ. (2021). Quality of Life of Children and Adolescents with Multiple Sclerosis—A Literature Review of the Quantitative Evidence. International Journal of Environmental Research and Public Health, 18(16), 8645. https://doi.org/10.3390/ijerph18168645







