Soul of the Jukskei River: The Extent of Bacterial Contamination in the Jukskei River in Gauteng Province, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Site
2.2. Sampling Collection
2.3. Microbial Analysis of Water
2.3.1. Isolation of Total Coliforms and E. coli
2.3.2. Isolation and Identification of Associated Bacteria
2.4. Molecular Detection of Identified Bacteria
2.4.1. DNA Extraction and Multiplex Polymerase Chain Reaction (m-PCR)
2.4.2. Potentially Toxic Elements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physio-Chemical Analysis
3.2. Microbiology Analysis
3.2.1. Isolation of TC (Total Coliforms) and E. coli
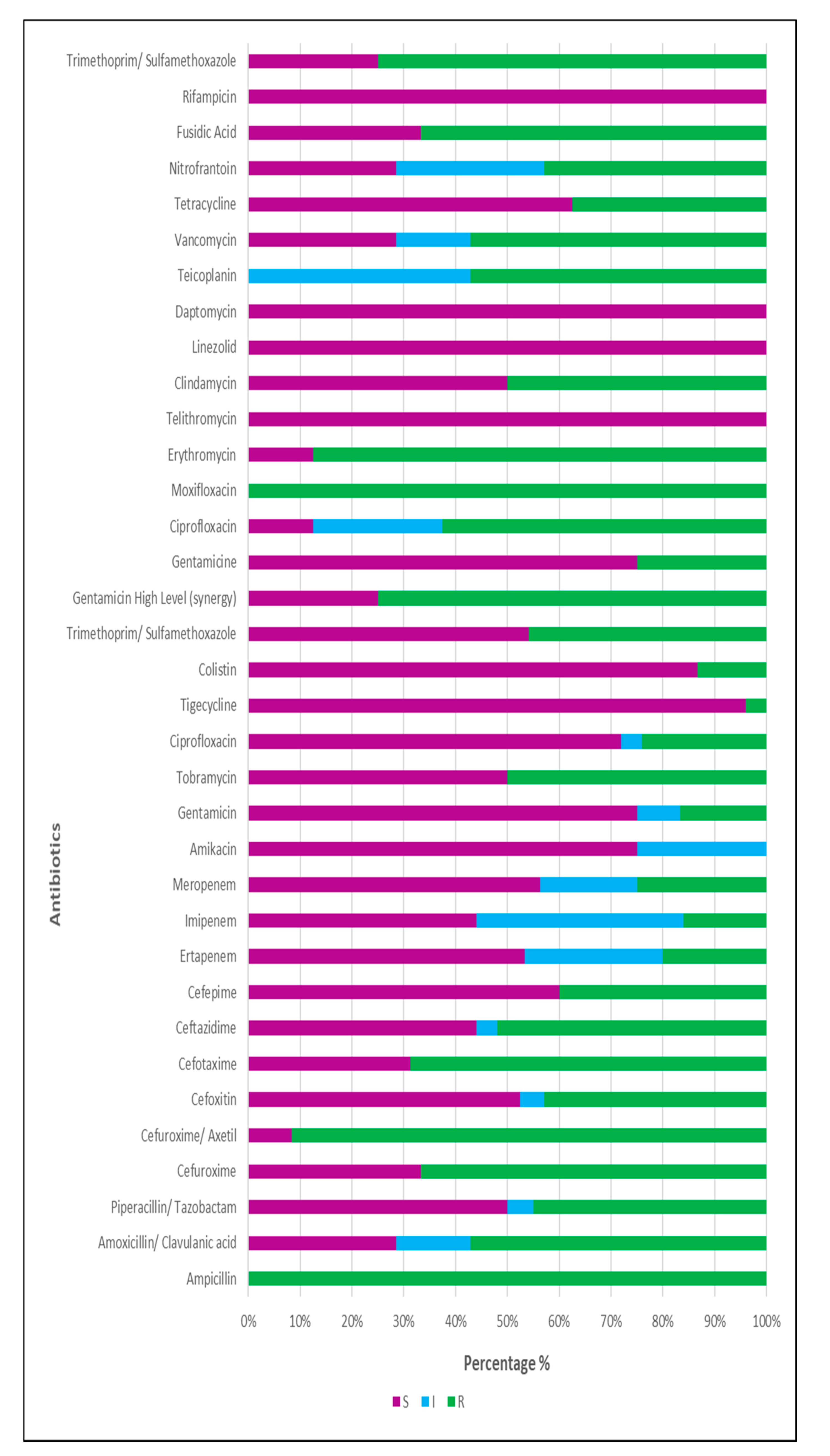
3.2.2. Isolation and Identification of Associated Bacteria
Microbiology Analysis
Molecular Biology Analysis
Potentially Toxic Elements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pindi, P.K.; Yadav, P.R.; Shanker, A.S. Identification of opportunistic Pathogenic bacteria in drinking water samples of different rural health centres and their clinical impacts on humans. Biomed. Res. Int. 2013, 2013, 348250. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Water, Sanitation, Hygiene, and Waste Management for SARS-CoV-2, the Virus That Causes COVID-19: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020; Volume 4, pp. 1–11. [Google Scholar]
- da Silva, J.M. A Case Study on the Historical Water Quality Trends Pertaining to the Jukskei River, in the Gauteng Province, South Africa; University of Johannesburg: Johannesburg, South Africa, 2012. [Google Scholar]
- Taylor, J.C.; Harding, W.R.; Archibald, C.G.M.; van Rensburg, L. Diatoms as indicators of water quality in the Jukskei—Crocodile River system in 1956 and 1957, a re-analysis of diatom count data generated by BJ Cholnoky. Water SA 2005, 31, 237–246. [Google Scholar] [CrossRef]
- Matowanyika, M. Impact of Alexandra Township on the Water Quality of the Jukskei River. Master’s Thesis, University of WITS, Johannesburg, South Africa, 2010. [Google Scholar]
- Olukenle, O.; Okonkwo, J.; Kenede, K.; Lupankwa, M. Concentration of Polybrominated Diphenyl Ethers in sediments from the Jukskei River, Gauteng South Africa. Bull. Environ. Contam. Toxicol. 2012, 88, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, J.M.; Harmse, J.T. Geological and Anthropogenic Influences on the Water Chemistry of the Jukskei River, Gauteng, South Africa. S. Afr. J. Geol. 2005, 108, 439–447. [Google Scholar] [CrossRef]
- Akanbi, O.E.; Njom, H.A.; Fri, J.; Otigbu, A.C.; Clarke, A.M. Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Recreational Waters and Beach Sand in Eastern Cape Province of South Africa. Int. J. Environ. Res. Public Health 2017, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Otigbu, A.C.; Clarke, A.M.; Fri, J.; Akanbi, E.O.; Njom, H.A. Antibiotic Sensitivity Profiling and Virulence Potential of Campylobacter jejuni Isolates from Estuarine Water in the Eastern Cape Province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 925. [Google Scholar] [CrossRef] [PubMed]
- Matlou, D.P.; Bissong, M.E.A.; Tchatchouang, C.K.; Adem, M.R.; Foka, F.E.T.; Kumar, A.; Ateba, C.N. Virulence Profiles of Vancomycin-Resistant Enterococci Isolated from Surface and Ground Water Utilized by Humans in the North-West Province, South Africa: A Public Health Perspective. Environ. Sci. Pollut. Res. Int. 2019, 26, 15105–15114. [Google Scholar] [CrossRef]
- Department of Water Affairs and Forestry (DWAF). Water Conservation and Water Demand Management Strategy for Industry, Mining and Commercial Water Use. 2003. Available online: http://www.dwaf.gov.za/WaterConservation/Programs_IMP.htm (accessed on 28 May 2021).
- Sibali, L.L.; Okwonkwo, J.O.; McCrindle, R.I. Determination of selected organochlorine pesticide (OCP) compounds from the Jukskei River catchment area in Gauteng, South Africa. Water SA 2008, 34, 611–622, ISSN 0378-4738. [Google Scholar] [CrossRef]
- Fipps, G. Irrigation Water Quality Standards and Salinity Management Strategies. Tex. FARMER Collect. 2003, B1667, 1–18. [Google Scholar]
- Omar, K.B.; Potgieter, N.; Barnard, T.G. Development of a rapid screening method for the detection of pathogenic Escherichia coli using a combination of Colilert Quanti-Trays/2000 and PCR. Water Sci. Tech. Water Supply 2010, 10, 1–8. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pan Drug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.B.; Barnard, T.G. Detection of diarrhoeagenic Escherichia coli in clinical and environmental water sources in South Africa using single-step 11-gene m-PCR. World J. Microbiol. Biotechnol. 2014, 30, 2663–2671. [Google Scholar] [CrossRef]
- Ntema, V.M.; Potgieter, N.; Barnard, T.G. Detection of Vibrio cholerae and Vibrio parahaemolyticus by molecular and culture-based methods from source water to household container-stored water at the point-of-use in South African rural communities. Water Sci. Technol. 2010, 61, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Department of Water Affairs and Forestry (DWAF). South African Water Quality Guidelines, 2nd ed.; CSIR Environmental Services: Pretoria, South Africa, 1996; Volumes 1, 2 and 4.
- SABS. South African National Standard, SANS 241-2:2015, Drinking Water Part 2: Application of SANS 241-1, 2nd ed.; SABS: Pretoria, South Africa, 2015; ISBN 978-0-626-31245-9. [Google Scholar]
- Chatanga, P.; Ntuli, V.; Mugomeri, E.; Keketsi, T.; Chikowore, N.V.T. Situational analysis of physicochemical, biochemical, and microbiological quality of water along Mohokare River, Lesotho. Egypt. J. Aquat. Res. 2019, 45, 45–51. [Google Scholar] [CrossRef]
- Vadde, K.K.; Wang, J.; Cao, L.; Yuan, T.; McCarth, A.J.; Sekar, R. Assessment of Water Quality and Identification of Pollution Risk Locations in Tiaoxi River (Taihu Watershed), China. Water 2018, 10, 183. [Google Scholar] [CrossRef]
- Obasi, N.P.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Schutte, F. Handbook for the Operation of Water Treatment Works; The Water Research Commission, The Water Institute of Southern Africa: Pretoria, South Africa, 2006. [Google Scholar]
- Cloete, T.E.; Rose, J.; Nel, L.H.; Ford, T. Microbial Waterborne Pathogens; IWA Publishing: London, UK, 2004; pp. 5–10. [Google Scholar]
- Water Research Commission (WRC). The Department of Water Affairs and Forestry (DWAF) and Department of Health. In South African Water Quality Guidelines: Volume 2—Recreational Use, 2nd ed.; Water Research Commission: Pretoria, South Africa, 1996. [Google Scholar]
- Water Research Commission (WRC). The Department of Water Affairs and Forestry (DWAF), and Department of Health. In South African Water Quality Guidelines for Recreational Water Use; Holmes, S., Ed.; CSIR Environmental Services: Pretoria, South Africa, 1996; Volume 2. [Google Scholar]
- WHO. Essential Medicines and Health Products: Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria (accessed on 9 June 2021).
- Bodro, M.; Sabé, N.; Tubau, F.; Lladó, L.; Baliellas, C.; Roca, J.; Cruzado, J.M.; Carratalà, J. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation 2013, 96, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.M.; Kessel, J.; Mücke, V.T.; Schwarzkopf, K.; Hogardt, M.; Stephan, C.; Zeuzem, S.; Kempf, V.A.; Lange, C.M. The role of Enterococcus spp. and multidrug-resistant bacteria causing pyogenic liver abscesses. BMC Infect. Dis. 2017, 17, 450. [Google Scholar] [CrossRef]
- Ramsamy, Y.; Essack, S.Y.; Sartorius, B.; Patel, M.; Mlisana, K.P. Antibiotic resistance trends of ESKAPE pathogens in Kwazulu-Natal, South Africa: A five-year retrospective analysis. Afr. J. Lab. Med. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in hospitals: How understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control 2020, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi. J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef]
- Navidinia, M. The clinical importance of emerging ESKAPE pathogens in nosocomial infections. J. Paramed. Sci. 2016, 7, 43–57. [Google Scholar]
- Higgins, P.G.; Hrenovic, J.; Seifert, H.; Dekic, S. Characterization of Acinetobacter baumannii from water and sludge line of secondary wastewater treatment plant. Water Res. 2018, 140, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Regalado, N.G.; Martin, G.; Antony, S.J. Acinetobacter lwoffii: Bacteremia associated with acute gastroenteritis. Travel. Med. Infect. Dis. 2009, 7, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Senneby, E.; Göransson, L.; Weiber, S.; Rasmussen, M. A population-based study of aerococcal bacteraemia in the MALDI-TOF MS-era. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 755–762. [Google Scholar] [CrossRef]
- Kaboré, W.A.D.; Konaté, A.; Dembélé, R.; Konaté, R.; Faye, B.; Ouédraogo, D.; Boisramé, S.; Traoré, A.S.; Barro, N.; Sangaré, L. Occurrence and Antibiotic Susceptibility of Aerococcus and Enterococcus Strains Isolated from Acute and Chronic Cellulites of Dental Origin in Ouagadougou, Burkina Faso. J. Dent. Oral Care Med. 2016, 2, 104. [Google Scholar]
- Yadav, K.; Sharma, M.; Agarwal, S.; Bhatia, N.; Yadav, N. Aortic pseudoaneurysm & endocarditis caused by Aerococcus viridans: A case report and literature review. Cardiovasc. Revasc. Med. 2018, 19, 201–203. [Google Scholar] [PubMed]
- Abboud, A.A.; Ahmad, S.O.; Habli, M.M.; Bechara, C. Aerococcus Viridians: Under-Recognized Cause of Pyomyositis. Clin. Microbiol. 2016, 5, 2. [Google Scholar]
- Mohan, B.; Zaman, K.; Anand, N.; Taneja, N. Aerococcus viridans: A rare pathogen causing urinary tract infection. J. Clin. Diagn. Res. 2017, 11, DR01–DR03. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Vila, J.; Ruiz, J.; Gallardo, F.; Vargas, M.; Soler, L.; Figueras, M.J.; Gascon, J. Aeromonas spp. and traveler’s diarrhea: Clinical features and antimicrobial resistance. Emerg. Infect. Dis. 2003, 9, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Bierbaum, G.; Molitor, E.; Götte, N.; Antwerpen, M. Genome Sequence of Bacillus pumilus Strain Bonn, Isolated from an Anthrax-Like Necrotic Skin Infection Site of a Child. Genome Announc. 2016, 4, e01741-15. [Google Scholar] [CrossRef] [PubMed]
- Tena, D.; Martínez-Torres, J.Á.; Pérez-Pomata, M.T.; Sáez-Nieto, J.A.; Rubio, V.; Bisquert, J. Cutaneous infection due to Bacillus pumilus: Report of 3 cases. Clin. Infect. Dis. 2007, 44, e40–e42. [Google Scholar] [CrossRef]
- Kimouli, M.; Vrioni, G.; Papadopoulou, M.; Koumaki, V.; Petropoulou, D.; Gounaris, A.; Friedrich, A.W.; Tsakris, A. Two cases of severe sepsis caused by Bacillus pumilus in neonatal infants. J. Med. Microbiol. 2012, 61, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Shivamurthy, V.M.; Gantt, S.; Reilly, C.; Tilley, P.; Guzman, J.; Tucker, L. Bacillus pumilus septic arthritis in a healthy child. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 3265037. [Google Scholar] [CrossRef][Green Version]
- Mishra, R.; Kelly, P.; Toolsie, O.; Ayyadurai, P.; Adrish, M. Uncommon cause of fungemia in a patient with renal cell cancer: A case report of Candida lusitaniae Fungemia. Medicine 2017, 96, e8510. [Google Scholar] [CrossRef] [PubMed]
- Bini Viotti, J.; Corzo-Pedroza, M.; Gonzales Zamora, J.A. Prosthetic joint infection caused by Candida lusitaniae: Report of a unique case. Acta Clin. Belg. 2018, 74, 286–291. [Google Scholar] [CrossRef]
- Basra, P.; Koziol, A.; Wong, A.; Carrillo, C.D. Complete genome sequences of Citrobacter braakii strains GTA-CB01 and GTA-CB04, isolated from ground beef. Genome Announc. 2015, 3, e01307–e01314. [Google Scholar] [CrossRef] [PubMed]
- Hirai, J.; Uechi, K.; Hagihara, M.; Sakanashi, D.; Kinjo, T.; Haranaga, S.; Fujita, J. Bacteremia due to Citrobacter braakii: A case report and literature review. J. Infect. Chemother. 2016, 22, 819–821. [Google Scholar] [CrossRef]
- Liu, L.-H.; Wang, N.-Y.; Wu, A.Y.-J.; Lin, C.-C.; Lee, C.-M.; Liu, C.-P. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J. Microbiol. Immunol. Infect. 2018, 51, 565–572. [Google Scholar] [CrossRef]
- Guermazi-Toumi, S.; Boujlel, S.; Assoudi, M.; Issaoui, R.; Tlili, S.; Hlaiem, M.E. Susceptibility profiles of bacteria causing urinary tract infections in Southern Tunisia. J. Glob. Antimicrob. Resist. 2018, 12, 48–52. [Google Scholar] [CrossRef]
- Salih, M.K.; Alrabadi, N.I.; Thalij, K.M.; Hussien, A.S. Isolation of pathogenic gram-negative bacteria from urinary tract infected patients. Open J. Med. Microbiol. 2016, 6, 59–65. [Google Scholar] [CrossRef][Green Version]
- Duran, A.; Abacilar, A.F.; Uyar, I.S.; Akpinar, M.B.; Sahin, V.; Okur, F.F.; Ates, M.; Alayunt, E.A. Comamonas testosteroni endocarditis in Turkey: A case report and review of the literature. Sifa. Med. J. 2015, 2, 44–47. [Google Scholar] [CrossRef]
- Farshad, S.; Norouzi, F.; Aminshahidi, M.; Heidari, B.; Alborzi, A. Two cases of bacteremia due to an unusual pathogen, Comamonas testosteroni in Iran and a review literature. J. Infect. Dev. Ctries. 2012, 6, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Orsini, J.; Tam, E.; Hauser, M.; Rajayer, S. Polymicrobial Bacteremia Involving Comamonas testosteroni. Case Rep. Med. 2014, 2014, 578127. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Farooq, R.; Nahvi, N. Comamonas testosteroni: Is It Still a Rare Human Pathogen? Case Rep. Gastroenterol. 2017, 11, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Raphael, R.; Riley, L.W. Infections Caused by Antimicrobial Drug-Resistant Saprophytic Gram-Negative Bacteria in the Environment. Front. Med. 2017, 4, 1–17. [Google Scholar] [CrossRef]
- Tymensen, L.; Booker, C.W.; Hannon, S.J.; Cook, S.R.; Zaheer, R.; Read, R.; McAllister, T.A. Environmental growth of enterococci and Escherichia coli in feedlot catch basins and a constructed wetland in the absence of fecal input. Environ. Sci. Technol. 2017, 51, 5386–5395. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V.; De Aguirre, M.; Divito, J. Enterococcus hirae bacteremia associated with acute pancreatitis and septic shock. Case Rep. Infect. Dis. 2015, 2015, 123852. [Google Scholar]
- Lee, G.H.; Lee, H.W.; Lee, Y.J.; Park, B.S.; Kim, Y.W.; Park, S. Acute Pyelonephritis with Enterococcus hirae and Literature Review. Urogenit. Tract. Infect. 2017, 12, 49–53. [Google Scholar] [CrossRef]
- Pãosinho, A.; Azevedo, T.; Alves, J.V.; Costa, I.A.; Carvalho, G.; Peres, S.R.; Baptista, T.; Borges, F.; Mansinho, K. Acute pyelonephritis with bacteremia caused by Enterococcus hirae: A rare infection in humans. Case Rep. Infect. Dis. 2016, 2016, 4698462. [Google Scholar]
- Bourafa, N.; Loucif, L.; Boutefnouchet, N.; Rolain, J.M. Enterococcus hirae, an unusual pathogen in humans causing urinary tract infection in a patient with benign prostatic hyperplasia: First case report in Algeria. New Microbes New Infect. 2015, 8, 7–9. [Google Scholar] [CrossRef]
- Edgeworth, J.D. Bacterial gastroenteritis. Medicine 2005, 33, 73–77. [Google Scholar] [CrossRef]
- Dib, R.W.; Matar, M.; Hallak, R.; Farra, A.; Mokhbat, J. Gemella hepatic abscesses: A case report and review of the literature. J. Infect. Dev. Ctries. 2018, 12, 146–149. [Google Scholar]
- Mneimneh, S.; Awada, H.; Shatila, A. A Child with Brain Abscess Due to Gemella haemolysans. ECPE 2016, 2, 139–142. [Google Scholar]
- Ando, A.; Kagihara, J.; Chung, H.; Bolger, D.T., Jr. Case Report: Native bivalvular endocarditis by Gemella haemolysans requiring venovenous extracorporeal membrane oxygenation. BMJ Case Rep. 2016, bcr2016216029. [Google Scholar] [CrossRef]
- Abu-Heija, A.A.; Ajam, M.; Veltman, J. Gemella morbillorum Cryptogenic Brain Abscess: A Case Report and Literature. Cureus 2018, 10, e3612. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Zheng, X.; Wang, W.; Xu, R.; Liu, K. Gemella morbillorum endocarditis of pulmonary valve: A case report. J. Cardiothorac. Surg. 2017, 12, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rial, L.; Schargel, K.; Rial, A.; Ortega, L.; Belda, J.I. Late-Onset Gemella morbillorum Flap-Margin-Related Keratitis After Laser In Situ Keratomileusis. J. Clin. Case Rep. 2016, 6, 885. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.S.; Price, L.B. Recent research examining links among Klebsiella pneumoniae from food, food animals, and human extraintestinal infections. Curr. Environ. Health Rep. 2016, 3, 128–135. [Google Scholar] [CrossRef]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Jung, Y.H.; Lee, S.; Yun, M.R.; Kim, W.; Kim, D.W. Comparative genomic analysis of Klebsiella pneumoniae subsp. pneumoniae KP617 and PittNDM01, NUHL24835, and ATCC BAA-2146 reveals unique evolutionary history of this strain. Gut Pathog. 2016, 8, 34. [Google Scholar] [PubMed]
- Sarria, J.C.; Vidal, A.M.; Kimbrough, R.C. Infections Caused by Kluyvera Species in Humans. Clin. Infect. Dis. 2001, 33, e69–e74. [Google Scholar] [CrossRef]
- Alfreijat, M. A Case of Urinary Tract Infection and Severe Sepsis Caused by Kluyvera ascorbata in a 73-Year-Old Female with a Brief Literature Review. Case Rep. Infect. Dis. 2017, 2017, 3848963. [Google Scholar]
- Yoshino, Y.; Nakazawa, S.; Otani, S.; Sekizuka, E.; Ota, Y. Nosocomial bacteremia due to Kluyvera cryocrescens: Case report and literature review. IDCases 2016, 4, 24–26. [Google Scholar] [CrossRef]
- Erbasan, F. Brain abscess caused by Micrococcus luteus in a patient with systemic lupus erythematosus: Case-based review. Rheumatol. Int. 2018, 38, 2323–2328. [Google Scholar] [CrossRef]
- Yan, S.F.; Liu, X.Y.; Cheng, Y.F.; Li, Z.Y.; Ou, J.; Wang, W.; Li, F.Q. Relationship between intrauterine bacterial infection and early embryonic developmental arrest. Chin. Med. J. 2016, 129, 1455–1458. [Google Scholar] [CrossRef]
- Baumbach, S.F.; Prall, W.C.; Scharpf, A.M.; Hererich, V.; Schmidt, M.; Suedkamp, N.P.; Stoehr, A.; Mayr, H.O. Significant increase of pathogen detection rate by dry arthroscopic biopsies at suspected low-grade infection following total knee arthroplasty: A prospective observational study. Arch. Orthop. Trauma. Surg. 2018, 138, 1583–1590. [Google Scholar] [CrossRef]
- Shima, A.; Hinenoya, A.; Samosornsuk, W.; Samosornsuk, S.; Mungkornkaew, N.; Yamasaki, S. Prevalence of Providencia strains among patients with diarrhea and in retail meats in Thailand. Jpn. J. Infect. Dis. 2016, 69, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Yoh, M.; Matsuyama, J.; Ohnishi, M.; Takagi, K.; Miyagi, H.; Mori, K.; Park, K.S.; Ono, T.; Honda, T. Importance of Providencia species as a major cause of travellers’ diarrhoea. J. Med. Microbiol. 2005, 54, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Odoyo, E.; Larson, P.S.; Apondi, E.; Kathiiko, C.; Miringu, G.; Nakashima, M.; Ichinose, Y. First report of a foodborne Providencia alcalifaciens outbreak in Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Ji, Y.S.; Yoon, K.C. A case of bilateral keratitis caused by Providencia alcalifaciens: A rarely encountered ocular pathogen. Int. Ophthalmol. 2018, 38, 1325–1328. [Google Scholar] [CrossRef]
- Luczkiewicz, A.; Kotlarska, E.; Artichowicz, W.; Tarasewicz, K.; Fudala-Ksiazek, S. Antimicrobial resistance of Pseudomonas spp. isolated from wastewater and wastewater-impacted marine coastal zone. Environ. Sci. Pollut. Res. 2015, 22, 19823–19834. [Google Scholar] [CrossRef] [PubMed]
- Gershman, M.D.; Kennedy, D.J.; Noble-Wang, J.; Kim, C.; Gullion, J.; Kacica, M.; Jensen, B.; Pascoe, N.; Saiman, L.; McHale, J.; et al. Multistate outbreak of Pseudomonas fluorescens bloodstream infection after exposure to contaminated heparinized saline flush prepared by a compounding pharmacy. Clin. Infect. Dis. 2008, 47, 1372–1379. [Google Scholar] [CrossRef]
- Nishimura, T.; Hattori, K.; Inoue, A.; Ishii, T.; Yumoto, T.; Tsukahara, K.; Nakao, A.; Ishihara, S.; Nakayama, S. Bacteremia or pseudobacteremia? Review of Pseudomonas fluorescens infections. World J. Emerg. Med. 2017, 8, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.; Lien, C.Y.; Tsai, W.C.; Lai, W.A.; Hsu, C.W.; Tsai, N.W.; Chang, C.C.; Lu, C.H.; Chien, C.C.; Chang, W.N. The clinical characteristics of adult bacterial meningitis caused by non-Pseudomonas (Ps.) aeruginosa Pseudomonas species: A clinical comparison with Ps. aeruginosa meningitis. Kaohsiung. J. Med. Sci. 2018, 34, 49–55. [Google Scholar] [CrossRef]
- Kalra, D.; Sati, A.; Shankar, S.; Jha, A. Corneal infection by Pseudomonas stutzeri following excision of trigeminal nerve schwannoma. BMJ Case Rep. 2015, 2015, bcr2014207496. [Google Scholar] [CrossRef]
- Halabi, Z.; Mocadie, M.; El Zein, S.; Kanj, S.S. Pseudomonas stutzeri prosthetic valve endocarditis: A case report and review of the literature. J. Infect. Public Health 2019, 12, 434–437. [Google Scholar] [CrossRef]
- Shalabi, A.; Ehrlich, T.; Schäfers, H.J.; Becker, S.L. Infective endocarditis caused by Pseudomonas stutzeri in a patient with Marfan syndrome: Case report and brief literature review. IDCases 2017, 10, 22–25. [Google Scholar] [CrossRef]
- Bonares, M.J.; Vaisman, A.; Sharkawy, A. Prosthetic vascular graft infection and prosthetic joint infection caused by Pseudomonas stutzeri. IDCases 2016, 6, 106–108. [Google Scholar] [CrossRef][Green Version]
- Kumar, M.S.; Das, A.P. Molecular identification of multi drug resistant bacteria from urinary tract infected urine samples. Microb. Pathog. 2016, 98, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Cardoso, C.; Cruz, C.S.; Machado, C.; Vesza, Z.; Tosatto, V.; Maia, D.; Cardoso, S.; Pinto, M.; et al. Infections on the rise: Raoultella spp., clinical and microbiological findings from a retrospective study, 2010–2014. Infect. Dis. 2016, 48, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, T.; Naito, H.; Ihoriya, H.; Tsukahara, K.; Ota, T.; Watanabe, T.; Nakao, A. Raoultella planticola bacteremia-induced fatal septic shock following burn injury. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Demiray, T.; Koroglu, M.; Ozbek, A.; Altindis, M. A rare cause of infection, Raoultella planticola: Emerging threat and new reservoir for carbapenem resistance. Infection 2016, 44, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Jung, E.J.; Seong, J.S.; Woo, Y.M.; Jeong, B.J.; Kang, Y.M.; Lee, E. A case of pneumonia caused by Raoultella planticola. Tuberc. Respir. Dis. 2016, 79, 42–45. [Google Scholar] [CrossRef]
- Skelton, W.P., IV; Taylor, Z.; Hsu, J. A rare case of Raoultella planticola urinary tract infection in an immunocompromised patient with multiple myeloma. IDCases 2017, 8, 9–11. [Google Scholar] [CrossRef]
- Carrero, P.; Garrote, J.A.; Pacheco, S.; García, A.I.; Gil, R.; Carbajosa, S.G. Report of six cases of human infection by Serratia plymuthica. J. Clin. Microbiol. 1995, 33, 275–276. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Jain, R.; Kaur, I.R. Serratia plymuthica: A community-acquired uropathogen. Indian J. Med. Sci. 2017, 69, 31–32. [Google Scholar] [CrossRef]
- Vignier, N.; Barreau, M.; Olive, C.; Baubion, E.; Théodose, R.; Hochedez, P.; Cabié, A. Human infection with Shewanella putrefaciens and S. algae: Report of 16 cases in Martinique and review of the literature. Am. J. Trop. Med. Hyg. 2013, 89, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- De Vecchi, E.; George, D.A.; Romanò, C.L.; Pregliasco, F.E.; Mattina, R.; Drago, L. Antibiotic sensitivities of coagulase-negative staphylococci and Staphylococcus aureus in hip and knee periprosthetic joint infections: Does this differ if patients meet the International Consensus Meeting Criteria? Infect. Drug. Resist. 2018, 11, 539–546. [Google Scholar] [CrossRef]
- Czekaj, T.; Ciszewski, M.; Szewczyk, E.M. Staphylococcus haemolyticus—An emerging threat in the twilight of the antibiotics age. Microbiology 2015, 161, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Williford, S.; Heavner, M.; Lambing, T.; Wian, B.; Ma, S.; Gonzales, J. 704: When “contaminants” become pathogens Staphylococcus auricularis bacteremia in the critically ill. Crit. Care Med. 2018, 46, 338. [Google Scholar] [CrossRef]
- Geller, J.A.; MacCallum, K.P.; Murtaugh, T.S.; Patrick, D.A., Jr.; Liabaud, B.; Jonna, V.K. Prospective comparison of blood culture bottles and conventional swabs for microbial identification of suspected periprosthetic joint infection. J. Arthroplast. 2016, 31, 1779–1783. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ali, F.A. Microbiological Study of Pathogens Isolated From Women with Genital Tract Infection. Int. J. Med. Res. 2015, 1, 95–105. [Google Scholar]
- Bhattacharya, G.; Dey, D.; Bhattacharya, A.; Das, S.; Banerjee, A. Bacteraemia by Staphylococcus cohnii subsp. urealyticus in a diabetic patient: A case report. World J. Pharm. Pharm. Sci. 2016, 5, 1864–1869. [Google Scholar]
- Garg, S. Staphylococcus cohnii: Not so innocuous. J. Acute Dis. 2017, 6, 239–240. [Google Scholar] [CrossRef]
- Shahandeh, Z.; Shafi, H.; Sadighian, F. Association of staphylococcus cohnii subspecies urealyticum infection with recurrence of renal staghorn stone. Caspian J. Intern. Med. 2015, 6, 40–42. [Google Scholar]
- Hetsa, B.A.; Kumar, A.; Ateba, C.N. Characterization of multiple antibiotic resistant clinical strains of Staphylococcus isolated from pregnant women vagina. Folia Microbiol. 2018, 63, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Visai, L.; Corazzari, T.; Poggio, C.; Pegreffi, F.; Maso, A.; Pirini, V.; Ravaioli, S.; Cangini, I.; et al. Characterization of 26 Staphylococcus warneri isolates from orthopedic infections. Int. J. Artif. Organs 2010, 33, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Dakić, I.; Martel, A.; Vaneechoutte, M.; Morrison, D.; Shittu, A.; Ježek, P.; Decostere, A.; Devriese, L.A.; Haesebrouck, F. A comparative evaluation of phenotypic and molecular methods in the identification of members of the Staphylococcus sciuri group. Syst. Appl. Microbiol. 2005, 28, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Krzymińska, S.; Kaznowski, A. Clonality, virulence and the occurrence of genes encoding antibiotic resistance among Staphylococcus warneri isolates from bloodstream infections. J. Med. Microbiol. 2016, 65, 828–836. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Bhatnagar, U.B.; Conaway, D.G. An Unusual Presentation of Native Valve Endocarditis caused by Staphylococcus warneri. Rev. Cardiovasc. Med. 2016, 17, 140–143. [Google Scholar]
- Kuvhenguhwa, M.S.; Belgrave, K.O.; Shah, S.U.; Bayer, A.S.; Miller, L.G. A Case of Early Prosthetic Valve Endocarditis Caused by Staphylococcus warneri in a Patient Presenting with Congestive Heart Failure. Cardiol. Res. 2017, 8, 236–240. [Google Scholar] [CrossRef]
- Ivić, I.; Karanović, J.; Pavičić-Ivelja, M. Sepsis with multiple abscesses caused by staphylococcus warneri: A case report. Cent. Eur. J. Med. 2013, 8, 45–47. [Google Scholar] [CrossRef]
- Dimitriadi, D.; Charitidou, C.; Charvalos, E. Urinary tract infection due to beta-lactams-resistant Staphylococcus warneri: A case report. Int. J. Antimicrob. Agents 2015, 4, 3. [Google Scholar] [CrossRef]
- Zhi, A.; Ma, B.; Wu, Y.; Fang, J.; Yu, X.; Zhang, M. Detection of Viable Vibrio cholerae Cells in Seafood Using a Real-Time Visual Loop-Mediated Isothermal Amplification Combined with Propidium Monoazide. Food Anal. Methods 2018, 11, 99–110. [Google Scholar] [CrossRef]
- Karaiskos, I.; Giamarellou, H. Multidrug-Resistant and Extensively Drug-Resistant Gram-Negative Pathogens: Current and Emerging Therapeutic Approaches. Exp. Opin. Pharmacother. 2014, 15, 1351–1370. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Pandya, H. Comparison of Colistin Susceptibility Testing by VITEK 2 Compact and Broth Microdilution Method for Carbapenem Resistant Isolates in a Tertiary Diagnostic Centre. J. Clin. Nephrol. Kidney Dis. 2019, 4, 1024. [Google Scholar]
- Müller, H.; Sib, E.; Gajdiss, M.; Klanke, U.; Lenz-Plet, F.; Barabasch, V.; Albert, C.; Schallenberg, A.; Timm, C.; Zacharias, N.; et al. Dissemination of multi-resistant gram-negative bacteria into Germanwastewater and surfacewaters. FEMS Microbiol. Ecol. 2018, 94, fiy057. [Google Scholar] [CrossRef]
- Roberts, R.R.; Hota, B.; Ahmad, I.; Scott, R.D.; Foster, S.D.; Abbasi, F.; Schabowski, S.; Kampe, L.M.; Ciavarella, G.G.; Supino, M.; et al. Hospital and Societal Costs of Antimicrobial-Resistant Infections in a Chicago Teaching Hospital: Implications for Antibiotic Stewardship. Clin. Infect. Dis. 2009, 49, 1175–1184. [Google Scholar] [CrossRef]
- Schreiber, C.; Zacharias, N.; Essert, S.M.; Wasser, F.; Müller, H.; Siba, E.; Precht, T.; Parcina, M.; Bierbaumb, G.; Schmithausen, R.M.; et al. Clinically relevant antibiotic-resistant bacteria in aquatic environments—An optimized culture-based approach. Sci. Total Environ. 2021, 750, 142265. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef]
- Jafari, A.; Aslani, M.M.; Bouzari, S. Escherichia coli: A brief review of diarrhoeagenic patho-types and their role in diarrheal diseases in Iran. Iran. J. Microbiol. 2012, 4, 102–117. [Google Scholar]
- Gomi, R.; Matsuda, T.; Fujimori, Y.; Harada, H.; Matsui, Y.; Yoneda, M. Characterization of pathogenic Escherichia coli in river water by simultaneous detection and sequencing of 14 virulence genes. Environ. Sci. Technol. 2015, 49, 6800–6807. [Google Scholar]
- Morabito, S. Pathogenic Escherichia coli, Molecular and Cellular Microbiology; Caister Academic Press: Norfolk, UK, 2014; pp. 1–273. [Google Scholar]
- Fratamico, P.M.; Liu, Y.; Sommers, H. Pathogenic Escherichia coli, Evolution omics, Detection and Control; Caister Academic Press: Norfolk, UK, 2018; pp. 1–249. [Google Scholar]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed]
- Marie, V.; Lin, J. Microbial Indicators and Environmental Relationships in the Umhlangane River, Durban, South Africa; De Gruyter: Berlin, Germany, 2018; Available online: https://doi.org/10.1515/biol-2018-0047 (accessed on 28 May 2021).
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Manaka, T.; Araoka, D.; Yoshimura, T.; Hossain, H.M.Z.; Nishio, Y.; Suzuki, A.; Kawahata, H. Downstream and seasonal changes of lithium isotope ratios in the Ganges-Brahmaputra river system. Adv. Earth Space Sci. 2017, 18, 3003–3015. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Effects of Heavy Metal Toxicity in Humans, Poisoning in the Modern World—New Tricks for an Old Dog? Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2018; Available online: https://doi.org/10.5772/intechopen82511 (accessed on 5 June 2021). [CrossRef]
- Campbell, L.A. The Waste Assimilation Capacity of a Reach of a Jukskei River Just Downstream of Alexandra Township Johannesburg: Research Group; University of the Witwatersrand: Johannesburg, South Africa, 1996. [Google Scholar]
- Herbig, F.J.W. Talking dirty-effluent and sewage irreverence in South Africa: A conservation crime perspective. Cogent Soc. Sci. 2019, 5, 1701359. [Google Scholar] [CrossRef]
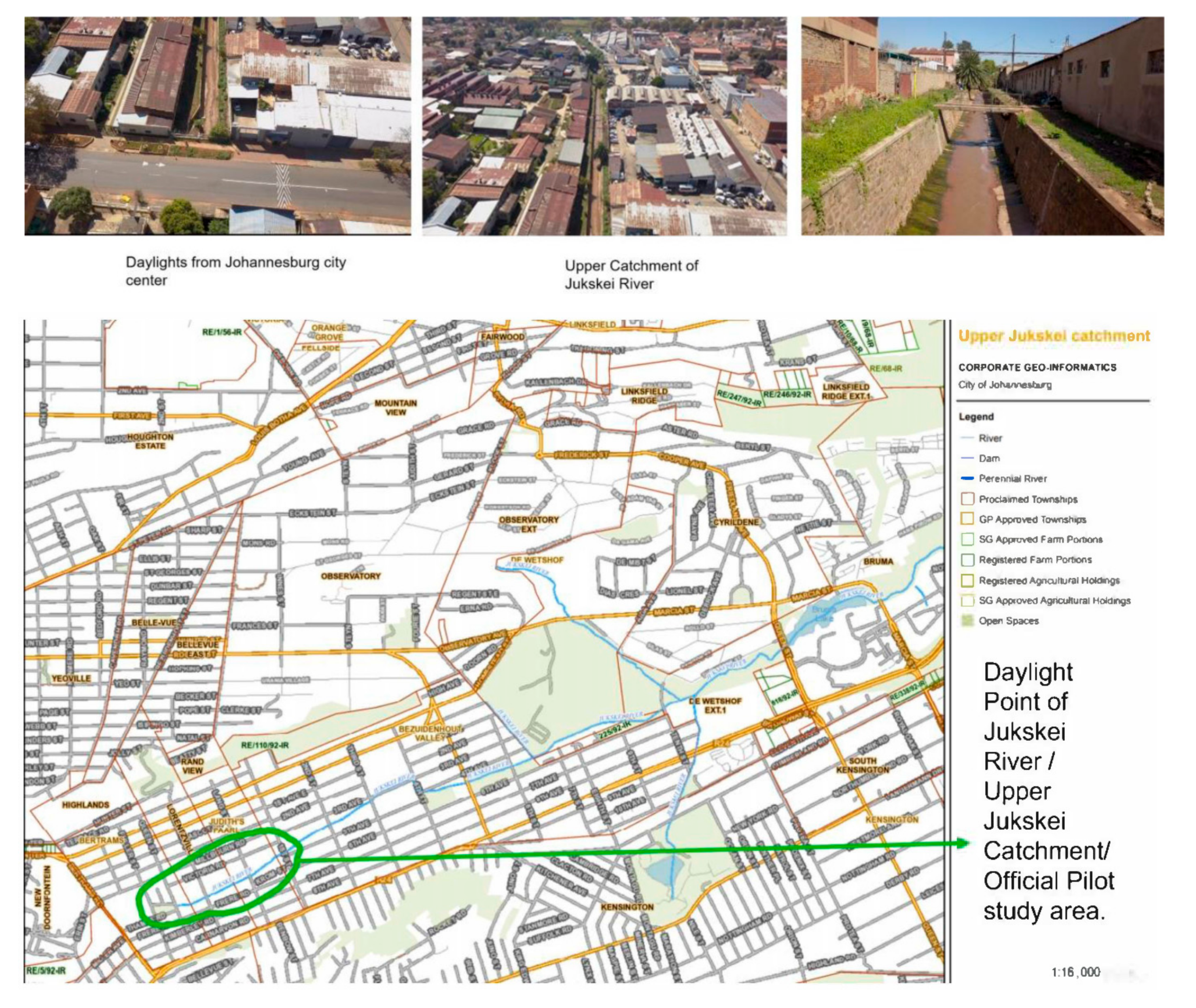
| Test | Unit | Min | Max | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Physio-Chemical Analysis | |||||
| pH | 7.11 | 7.38 | 7.31 | 0.09 | |
| Turbidity | NTU | 35.9 | 85.7 | 52.4 | 5.5 |
| Electrical conductivity | µS/m | 420 | 472 | 441 | 15.8 |
| Temperature | °C | 13.8 | 19 | 15.9 | 0.64 |
| Total dissolved solids | mg/L | 269 | 302.1 | 282 | 9.91 |
| Microbiology Analysis | |||||
| Total coliforms | MPN/100 mL | 4.1 × 106 | 3.9 × 107 | 1.8 × 107 | 3.5 × 106 |
| Escherichia coli | MPN/100 mL | 8.9 × 105 | 4.0 × 106 | 2.1 × 106 | 1.3 × 105 |
| Organism | Nr. of Isolate/s per Specie | Associated Human Disease | Reference |
|---|---|---|---|
| Acinetobacter baumannii | 2 | Bloodstream infection, Endocarditis, Meningitis, Ophthalmitis/keratitis, Peritonitis, neumonia, Soft tissue/skin infections, Urinary tract infections, Wound infections | [33,34,35] |
| Acinetobacter lwoffii | 1 | Gastroenteritis, Meningitis, Pneumonia, Septicaemia, Skin infections, Urinary tract infections | [36] |
| Aerococcus viridans | 2 | Bacteraemia, Cellulitis, Endocarditis, Soft tissue infection, Urinary tract infections | [37,38,39,40,41] |
| Aeromonas sobria | 10 | Bacteraemia, Sepsis, Traveller’s diarrhoea, Urinary tract infections | [42,43] |
| Aeromonas hydrophila | 22 | Gastroenteritis, Peritonitis, Sepsis, Septicaemia, Traveller’s diarrhoea | [42,43] |
| Aeromonas caviae | 22 | Keratitis, Traveller’s diarrhoea, Urinary tract infections | [42,43] |
| Bacillus pumilus | 1 | Catheter infection, Necrotic skin infection, Sepsis, Septic arthritis | [43,44,45,46,47] |
| Candida lusitaniae | 1 | Fungemia and Prosthetic joint infection | [48,49] |
| Citrobacter braakii | 4 | Bacteraemia and Urinary tract infections | [50,51] |
| Citrobacter freundii | 4 | Bacteraemia, Diarrheal, Urinary tract infections | [52,53,54] |
| Comamonas testosterone | 1 | Appendicitis, Bacteraemia, Catheter-related infection, Cholesteatoma, Endocarditis, Gastroenteritis | [55,56,57,58] |
| Enterobacter asburiae | 1 | Bloodstream infections, Osteomyelitis, Pneumonia, Soft-tissue and skin infections, Urinary tract infections | [59] |
| Enterococcus faecalis | 8 | Sepsis and Urinary tract infection | [60] |
| Enterococcus hirae | 1 | Bacteraemia, Pyelonephritis, Urinary tract infection | [61,62,63,64] |
| Escherichia coli | 14 | Diarrheal, Gastroenteritis, Urinary tract infections | [53,54,60,65] |
| Gemella haemolysans | 1 | Bacteraemia, Brain abscess, Endocarditis | [66,67,68] |
| Gemella morbillorum | 1 | Brain abscess, Endocarditis, Keratitis | [69,70,71] |
| Klebsiella pneumoniae | 1 | Bacteraemia, Cystitis, Meningitis, Pneumonia, Pyelonephritis, Pyogenic liver abscess, Sepsis, Septicaemia, Soft-tissue infections, Urinary tract infections, Wound infections | [72,73,74,75] |
| Kluyvera ascorbate | 2 | Diarrheal, Sepsis, Urinary tract infection | [76,77] |
| Kluyvera cryocrescens | 1 | Bacteraemia, Cholecystitis, Sepsis, Soft-tissue infections | [76,77,78] |
| Micrococcus luteus | 4 | Brain abscess, Meningitis, Myocarditis, Periprosthetic joint infection, Pyogenic liver abscess | [79,80,81] |
| Providencia alcalifaciens | 1 | Diarrheal, Gastroenteritis, Keratitis | [82,83,84,85] |
| Pseudomonas aeruginosa | 2 | Respiratory tract infections, Soft-tissue infections | [86] |
| Pseudomonas fluorescens | 2 | Bloodstream infection and Meningitis | [87,88,89] |
| Pseudomonas stutzeri | 2 | Corneal infection, Endocarditis, Prosthetic joint infection, Urinary tract infection | [90,91,92,93,94] |
| Raoultella planticola | 2 | Bacteraemia, Bloodstream infections, Cholecystitis, Necrotizing fasciitis, Pancreatitis, Pneumonia, Urinary tract infection | [95,96,97,98,99] |
| Serratia plymuthica | 2 | Osteomyelitis, Sepsis, Urinary tract infection | [100,101] |
| Shewanella putrefaciens | 2 | Bacteraemia, Ear infection, Skin, and soft-tissue infections | [102] |
| Staphylococcus aureus | 1 | Endocarditis, Food poisoning, Meningitis, Osteomyelitis, Pneumonia, Prosthetic joint infection, Septic arthritis, Septic shock, Septic Thrombophlebitis, Skin and soft-tissue infections, Skin disease, Staphylococcal scalded skin syndrome, Systemic infections, Toxic shock syndrome, Urinary tract infections | [47,65,103,104,105,106,107] |
| Staphylococcus auricularis | 1 | Bacteraemia, Periprosthetic joint infection, Vaginitis | [108,109,110] |
| Staphylococcus cohnii | 4 | Bacteraemia, Meningitis, Urinary tract infections | [111,112,113] |
| Staphylococcus haemolyticus | 2 | Bloodstream infections, Endocarditis, Meningitis, Peritonitis, Prosthetic joint infection, Urinary tract infections, Vaginitis | [103,106,107,110,114,115] |
| Staphylococcus vitulinus | 2 | Bloodstream infections, Endocarditis, Pelvic inflammatory disease, Peritonitis, Prosthetic joint infection, Septic shock, Urinary tract infections, Wound infections | [114,116] |
| Staphylococcus warneri | 1 | Bloodstream infections, Discitis, Endocarditis, Infection of CSF shunts, Meningitis, Osteomyelitis, Peritonitis, Prosthetic joint infection, Sepsis, Subdural empyema, Urinary tract infections | [103,106,108,114,117,118,119,120,121] |
| Vibrio cholerae | 1 | Cholera and Gastroenteritis | [65,105,122] |
| Sample Id | Li 7 µg/L | Be 9 µg/L | B 11 µg/L | Na 23 µg/L | Al 27 µg/L | V 51 µg/L | Cr 52 µg/L | Mn 55 µg/L | Fe 57 µg/L | Co 59 µg/L | Ni 60 µg/L | Cu 63 µg/L | Zn 66 µg/L | As 75 µg/L | Se 82 µg/L | Mo 98 µg/L | Cd 111 µg/L | Pb 208 µg/L | U 238 µg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upstream 1st | 4.097 | <0.1 | 35.5 | 48,282 | 68.9 | 1.3 | 0.6 | 52.4 | 211 | 0.6 | 3.403 | 15.2 | 23.8 | 0.8 | <0.1 | 0.5 | <0.1 | 1.1 | 0.5 |
| Downstream 1st | 3.254 | <0.1 | 45.0 | 49,581 | 77.1 | 1.4 | 0.6 | 50.6 | 173 | 0.6 | 3.121 | 25.0 | 37.6 | 0.6 | <0.1 | 0.5 | <0.1 | 0.9 | 0.7 |
| Upstream 2nd | 4.340 | <0.1 | 12.7 | 39,586 | 85.3 | 1.5 | 0.6 | 51.8 | 220 | 0.6 | 3.470 | 28.5 | 267 | 0.7 | <0.1 | 1.4 | 0.2 | 21.6 | 1.1 |
| Downstream 2nd | 4.507 | <0.1 | 8.7 | 44,734 | 112 | 2.3 | 0.8 | 55.6 | 257 | 0.7 | 3.665 | 24.2 | 255 | 1.0 | <0.1 | 1.4 | 0.2 | 15.2 | 1.0 |
| Upstream 3rd | 2.9 | 95.6 | <0.1 | 54,168 | 74.3 | 1.2 | 0.8 | 53.1 | 166 | 0.6 | 5.0 | 10.2 | 40.3 | 0.3 | <0.1 | 0.5 | <0.1 | 1.2 | 0.7 |
| Downstream 3rd | 3.2 | 106.6 | <0.1 | 46,958 | 64.8 | 1.1 | 0.7 | 54.3 | 221 | 0.5 | 7.8 | 9.6 | 48.7 | 0.2 | 9.4 | 0.5 | <0.1 | 1.6 | 0.6 |
| SANS 241 Drinking water Guideline (SABS, 2015) | <2400 | * | * | <200 | <300 | * | <50 | <100 | <2000 | * | <70 | <2000 | <5 | <10 | <40 | * | <3 | <10 | <30 |
| SA Irrigation water guideline (DWAF, 1996) | <2500 | 100–5000 | 500–1000 | 2000 | 5–20,000 | 100–1000 | 100 | 20–10,000 | 500–20,000 | 50–500 | 200–2000 | 200–5000 | 100–2000 | 100 | 20–50 | 10–50 | 10–50 | 200–2000 | 10–100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoorzook, K.B.; Pieterse, A.; Heine, L.; Barnard, T.G.; van Rensburg, N.J. Soul of the Jukskei River: The Extent of Bacterial Contamination in the Jukskei River in Gauteng Province, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 8537. https://doi.org/10.3390/ijerph18168537
Hoorzook KB, Pieterse A, Heine L, Barnard TG, van Rensburg NJ. Soul of the Jukskei River: The Extent of Bacterial Contamination in the Jukskei River in Gauteng Province, South Africa. International Journal of Environmental Research and Public Health. 2021; 18(16):8537. https://doi.org/10.3390/ijerph18168537
Chicago/Turabian StyleHoorzook, Kousar Banu, Anton Pieterse, Lee Heine, Tobias George Barnard, and Nickey Janse van Rensburg. 2021. "Soul of the Jukskei River: The Extent of Bacterial Contamination in the Jukskei River in Gauteng Province, South Africa" International Journal of Environmental Research and Public Health 18, no. 16: 8537. https://doi.org/10.3390/ijerph18168537
APA StyleHoorzook, K. B., Pieterse, A., Heine, L., Barnard, T. G., & van Rensburg, N. J. (2021). Soul of the Jukskei River: The Extent of Bacterial Contamination in the Jukskei River in Gauteng Province, South Africa. International Journal of Environmental Research and Public Health, 18(16), 8537. https://doi.org/10.3390/ijerph18168537







