Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
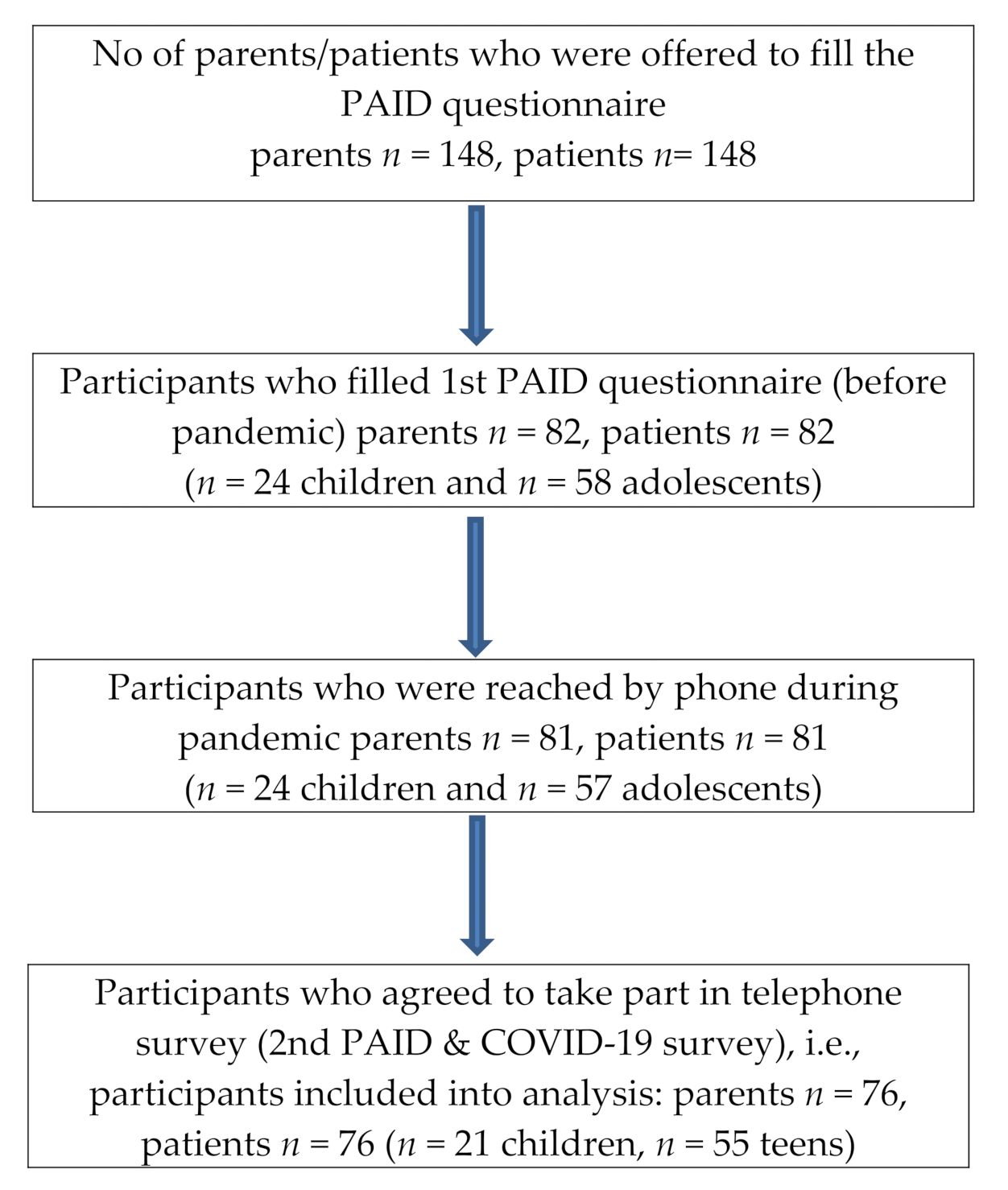
3. Results
3.1. Study Group Characteristics
3.2. Diabetes Distress before the Pandemic
3.3. Diabetes Distress during Pandemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | Children (N = 21) | Teens (N = 55) | ||
|---|---|---|---|---|
| PAID | P-PAID | PAID | P-PAID | |
| Age | R = -0.24. p = 0.2883 | R = 0.25. p = 0.2674 | R = 0.14. p = 0.3024 | R = 0.17. p = 0.2276 |
| T1DM duration | R = 0.06. p = 0.7838 | R = 0.19. p = 0.4065 | R = 0.09. p = 0.4958 | R = 0.26. p = 0.0601 |
| BMI z-score | R = 0.38. p = 0.0909 | R = 0.15. p = 0.5066 | R = 0.31. p = 0.0206 | R = 0.31. p = 0.0232 |
| HbA1c | R = 0.10. p = 0.7046 | R = 0.20. p = 0.4311 | R = 0.18. p = 0.2026 | R = 0.22. p = 0.1145 |
| PAID | N/A | R = 0.35. p = 0.1204 | N/A | R = 0.21. p = 0.1210 |
| P-PAID | R = 0.35. p = 0.1204 | N/A | R = 0.21. p = 0.1210 | N/A |
| Characteristics | Children (N = 21) | Teens (N = 55) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PAID | P-PAID | PAID | P-PAID | ||||||
| Median (Q1–Q3) | p-Value | Median (Q1–Q3) | p-Value | Median (Q1-Q3) | p-Value | Median (Q1–Q3) | p-Value | ||
| Sex | Male | 31.0 (26.0 to 42.0) | 0.6983 | 53.0 (48.0 to 70.0) | 0.8051 | 34.0 (24.0 to 42.0) | 0.0030 | 57.0 (46.0 to 64.0) | 0.8817 |
| Female | 35.5 (19.0 to 47.0) | 51.0 (46.0 to 72.0) | 44.5 (40.0 to 51.5) | 56.0 (43.0 to 67.5) | |||||
| T1DM duration | ≤6 months | 22.5 (15.0 to 27.0) | 0.0223 | 50.5 (43.5 to 61.5 | 0.5012 | 27.0 (20.0 to 30.5) | 0.0195 | 39.0 (33.5 to 54.0) | 0.0828 |
| >6 months | 36.0 (30.0 to 45.0) | 52.0 (49.0 to 72.0) | 40.0 (31.0 to 48.0) | 58.0 (47.0 to 65.0) | |||||
| BMI z-score | Normal | 31.0 (26.0 to 45.0) | 0.3684 | 52.0 (48.0 to 72.0) | 0.4355 | 36.0 (30.0 to 43.0) | 0.1367 | 55.0 (45.0 to 63.0) | 0.0990 |
| Overweight or obese | 23.5 (11.0 to 36.0) | 47.5 (42.0 to 53.0) | 47.0 (35.0 to 50.0) | 63.0 (50.0 to 67.0) | |||||
| Type of glucose monitoring | SMBG | 36.0 (19.0 to 45.0) | 0.7245 | 50.0 (42.0 to 63.0) | 0.3066 | 37.0 (31.0 to 49.0) | 0.3911 | 60.0 (50.0 to 67.0) | 0.0642 |
| CGM | 30.5 (28.0 to 40.0) | 56.5 (49.0 to 72.0) | 39.5 (29.0 to 43.5) | 52.5 (43.5 to 62.5 | |||||
| Type of insulin therapy | MDI | 40.0 (11.0 to 48.0) | 0.9199 | 53.0 (52.0 to 63.0) | 0.6507 | 31.0 (24.0 to 48.0) | 0.4901 | 58.0 (41.0 to 67.0) | 0.6983 |
| CSII | 31.0 (26.0 to 42.0) | 50.0 (47.0 to 72.0) | 39.5 (32.5 to 46.0) | 56.0 (46.0 to 64.0) | |||||
| Characteristics | PAID | P-PAID | ||
|---|---|---|---|---|
| Children (N = 21) | Teens (N = 55) | Children (N = 21) | Teens (N = 55) | |
| 1st PAID—at baseline | 31 (26 to 42) | 39 (30 to 47) | 52 (48 to 70) | 57 (46 to 65) |
| 2nd PAID—during pandemic | 28 (21 to 37) | 33 (27 to 43) | 53 (42 to 66) | 58 (48 to 69) |
| PAID change | −3 (−14 to 7) | −3 (−11 to 3) | −5 (−9 to 1) | 3 (−9 to 10) |
| p for within-group change | 0.1305 | 0.0183 | 0.2273 | 0.3767 |
References
- Hannonen, R.; Eklund, K.; Tolvanen, A.; Komulainen, J.; Riikonen, R.; Delamater, A.M.; Ahonen, T. Psychological distress of children with early-onset type 1 diabetes and their mothers’ well-being. Acta Paediatr. 2015, 104, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Butwicka, A.; Fendler, W.; Zalepa, A.; Szadkowska, A.; Zawodniak-Szalapska, M.; Gmitrowicz, A.; Mlynarski, W. Psychiatric Disorders and Health-Related Quality of Life in Children with Type 1 Diabetes Mellitus. J. Psychosom. Res. 2016, 57, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Arabiat, D.; Al Jabery, M.; Whitehead, L. A concept analysis of psychological distress in parents related to diabetes management in children and adolescents. J. Spéc. Pediatr. Nurs. 2020, 25, e12287. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Gonzalez, J.S.; Polonsky, W.H. The confusing tale of depression and distress in patients with diabetes: A call for greater clarity and precision. Diabet. Med. 2014, 31, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hagger, V.; Hendrieckx, C.; Sturt, J.; Skinner, T.C.; Speight, J. Diabetes Distress Among Adolescents with Type 1 Diabetes: A Systematic Review. Curr. Diabetes Rep. 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hessler, D.; Fisher, L.; Polonsky, W.; Johnson, N. Understanding the Areas and Correlates of Diabetes-Related Distress in Parents of Teens With Type 1 Diabetes. J. Pediatr. Psychol. 2016, 41, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef] [PubMed]
- Joensen, L.E.; Madsen, K.P.; Holm, L.; Nielsen, K.A.; Rod, M.H.; Petersen, A.A.; Rod, N.H.; Willaing, I. Diabetes and COVID-19: Psychosocial consequences of the COVID-19 pandemic in people with diabetes in Denmark—What characterizes people with high levels of COVID-19-related worries? Diabet. Med. 2020, 37, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.J.B.; Vesco, A.; Weil, M.L.E.G.; Evans, M.; Hood, K.K.; Weissberg-Benchell, J. Psychometric Properties of the Problem Areas in Diabetes: Teen and Parent of Teen Versions. J. Pediatr. Psychol. 2017, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.A.; Weil, L.E.G.; Shapiro, J.B.; Anderson, L.M.; Vesco, A.; Rychlik, K.; Hilliard, M.; Antisdel, J.; Weissberg-Benchell, J. Psychometric Properties of the Parent and Child Problem Areas in Diabetes Measures. J. Pediatr. Psychol. 2019, 44, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Passanisi, S.; Pecoraro, M.; Pira, F.; Alibrandi, A.; Donia, V.; Lonia, P.; Pajno, G.B.; Salzano, G.; Lombardo, F. Quarantine Due to the COVID-19 Pandemic From the Perspective of Pediatric Patients With Type 1 Diabetes: A Web-Based Survey. Front. Pediatr. 2020, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Charleer, S.; Gillard, P.; Vandoorne, E.; Cammaerts, K.; Mathieu, C.; Casteels, K. Intermittently scanned continuous glucose monitoring is associated with high satisfaction but increased HbA1c and weight in well-controlled youth with type 1 diabetes. Pediatr. Diabetes 2020, 21, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, H.; Wilmot, E.G.; Gregory, R.; Barnes, D.; Narendran, P.; Saunders, S.; Furlong, N.; Kamaruddin, S.; Banatwalla, R.; Herring, R.; et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care 2020, 43, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Albanese-O’Neill, A.; Muñoz, C.E.; Maahs, D.M. COVID-19 and Children With Diabetes—Updates, Unknowns, and Next Steps: First, Do No Extrapolation. Diabetes Care 2020, 43, 2631–2634. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, E.; Coraggio, L.; Pieralice, S.; Carlone, A.; Pozzilli, P.; Buzzetti, R. Effects of COVID-19 Lockdown on Glucose Control: Continuous Glucose Monitoring Data From People With Diabetes on Intensive Insulin Therapy. Diabetes Care 2020, 43, e86–e87. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Cortazar, A.; Bellido, V. Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res. Clin. Pr. 2020, 166, 108348. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, B.; Annuzzi, G.; Creanza, A.; Giglio, C.; De Angelis, R.; Lupoli, R.; Masulli, M.; Riccardi, G.; Rivellese, A.A.; Bozzetto, L. Blood Glucose Control During Lockdown for COVID-19: CGM Metrics in Italian Adults with Type 1 Diabetes. Diabetes Care 2020, 43, e88–e89. [Google Scholar] [CrossRef] [PubMed]
- Schiaffini, R.; Barbetti, F.; Rapini, N.; Inzaghi, E.; Deodati, A.; Patera, I.P.; Matteoli, M.C.; Ciampalini, P.; Carducci, C.; Lorubbio, A.; et al. School and pre-school children with type 1 diabetes during Covid-19 quarantine: The synergic effect of parental care and technology. Diabetes Res. Clin. Pract. 2020, 166, 108302. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Maltoni, G.; Bongiorno, C.; Tucci, L.; Di Natale, V.; Moscatiello, S.; Laffi, G.; Pession, A.; Zucchini, S.; Pagotto, U. Comparison of the effects of lockdown due to COVID-19 on glucose patterns among children, adolescents, and adults with type 1 diabetes: CGM study. BMJ Open Diabetes Res. Care 2020, 8, e001664. [Google Scholar] [CrossRef] [PubMed]
- Brener, A.; Mazor-Aronovitch, K.; Rachmiel, M.; Levek, N.; Barash, G.; Pinhas-Hamiel, O.; Lebenthal, Y.; Landau, Z. Lessons learned from the continuous glucose monitoring metrics in pediatric patients with type 1 diabetes under COVID-19 lockdown. Acta Diabetol. 2020, 57, 1511–1517. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients (N = 76) | Children (N = 21) | Teens (N = 55) | p-Value | |
|---|---|---|---|---|---|
| Continuous Characteristics [Median (Q1–Q3)] | |||||
| Age(years) | 13.6 (11.8–15.2) | 10.1 (9.5–11.1) | 14.4 (13.6–16.1) | N/C | |
| T1DM duration (years) | 3.7 (1.7–6.8) | 3.0 (1.1–3.9) | 4.5 (1.8–8.8) | 0.0208 | |
| Time between 1st and 2nd PAID survey (days) | 58.5 (45.5–98.5) | 64.0 (45.0–93.0) | 58.0 (46.0–99.0) | 0.9028 | |
| HBA1c (%) | 7.4 (7.2–8.1) | 7.3 (6.8–7.5) | 7.6 (7.2–8.7) | 0.0786 | |
| HbA1c (mmol/mol) | 57.4 (55.2–65) | 56 (51–58) | 60 (55–72) | 0.0786 | |
| PAID | 36.5 (29.0–46.0) | 31.0 (26.0–42.0) | 39.0 (30.0–47.0) | 0.1383 | |
| P-PAID | 56.0 (46.5–65.5) | 52.0 (48.0–70.0) | 57.0 (46.0–65.0) | 0.9167 | |
| Nominal characteristics [N (%)] | |||||
| Gender structure (N (%) of boys)) | 46 (60.5%) | 11 (52.4%) | 35 (63.6%) | 0.4356 | |
| T1DM duration ≤6 months | 8 (10.5%) | 4 (19%) | 4 (7.3%) | 0.2058 | |
| Body weight category (based on BMI z-score) | Normal | 60 (80%) | 19 (90.5%) | 41 (76.9%) | 0.3667 |
| Overweight | 8 (10.7%) | 1 (4.8%) | 7 (13%) | ||
| Obesity | 7 (9.3%) | 1 (4.8%) | 6 (11.1%) | ||
| Type of insulin therapy | MDI | 18 (23.7%) | 15 (27.3%) | 3 (14.3%) | 0.3663 |
| CSII | 58 (76.3%) | 40 (72.7%) | 18 (85.7%) | ||
| Type of glucose monitoring | SMBG | 42 (55.3%) | 11 (52.4%) | 31 (56.4%) | 0.8005 |
| CGM | 34 (44.7%) | 10 (47.6%) | 23 (43.6%) | ||
| Responding parent | Mother | 64 (84.2%) | 19 (90.5%) | 45 (81.8%) | N/C |
| Father | 5 (6.6%) | 1 (4.8%) | 4 (7.3%) | ||
| Both parents | 1 (1.3%) | 0 (0%) | 1 (1.8%) | ||
| Missing data | 6 (7.9%) | 1 (4.8%) | 5 (9.9%) | ||
| Clinical Characteristics | PAID-Ch Change | p-Value | P-PAID-Ch Change | p-Value | PAID-T Change | p-Value | P-PAID-T Change | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Subgroup Comparisons. Median (Q1−Q3) | |||||||||
| Sex | Male | 0 (−10 to 9) | 0.121 | −7 (−14 to 6) | 0.397 | 0 (−9 to 5) | 0.028 | 3 (−6 to 10) | 0.930 |
| Female | −4 (−17 to −2) | −4.5 (−7 to 1) | −7 (−17 to −2.5) | 2.5 (−11.5 to 10.5) | |||||
| T1DM duration | >6 months | −3 (−16 to 1) | 0.394 | −4 (−7 to 6) | 0.022 | −4 (−13 to 2) | 0.277 | 2 (−9 to 10) | 0.065 |
| <6 months | 2 (−7 to 8.5) | −12.5 (−15.5 to −9) | 0.5 (−4.5 to 11) | 12 (5 to 20) | |||||
| Glucose monitoring method | SMBG | −4 (−14 to 0) | 0.217 | −7 (−11 to 6) | 0.724 | −6 (−13 to 2) | 0.592 | 5 (−6 to 10) | 0.541 |
| CGM | 2.5 (−16 to 9) | −4.5 (−7 to 0) | −2.5 (−11 to 3.5) | 1.5 (−9 to 10) | |||||
| Type of insulin therapy | MDI | −10 (−17 to 9) | 0.801 | −14 (−17 to 1) | 0.246 | −4 (−7 to 5) | 0.570 | 2 (−14 to 10) | 0.533 |
| CSII | −2.5 (−14 to 7) | −5 (−7 to 6) | −2 (−13 to 2) | 3 (−5.5 to 10.5) | |||||
| COVID-19 pandemic-related difficulties | Yes | −3 (−10 to 0) | 0.859 | −4 (−7 to 6) | 0.354 | −6.5 (−13 to 4) | 0.410 | 2 (−12 to 7.5) | 0.411 |
| No | −2.5 (−15.5 to 7.5) | −6.5 (−11.5 to 0.5) | −1 (−11 to 2) | 5 (−6 to 10) | |||||
| COVID-19 pandemic-related worries | Yes | −3 (−16 to 7) | 0.938 | 0 (−7 to 8) | 0.021 | −4.5 (−11 to 1) | 0.298 | 2.5 (−6 to 10) | 0.921 |
| No | −2.5 (−10 to 7) | −10.5 (−17 to −6) | −1 (−8 to 8) | 7 (−11 to 10) | |||||
| Association with continuous variables. Spearman R correlations | |||||||||
| Age (years) | R = −0.12 | 0.594 | R = −0.47 | 0.032 | R = −0.03 | 0.851 | R = 0.02 | 0.908 | |
| T1DM duration (years) | R = −0.08 | 0.722 | R = 0.11 | 0.645 | R = 0.19 | 0.160 | R = 0.01 | 0.942 | |
| 1st PAID | R = −0.57 | 0.007 | R = 0.41 | 0.064 | R = −0.55 | 0.0001 | R = −0.06 | 0.662 | |
| 1st P-PAID | R = −0.36 | 0.111 | R = −0.19 | 0.402 | R = −0.02 | 0.895 | R = −0.32 | 0.017 | |
| BMI z-score | R = −0.19 | 0.408 | R = −0.13 | 0.56 | R = 0.03 | 0.832 | R = −0.01 | 0.927 | |
| HbA1 (%) | R = −0.39 | 0.109 | R = −0.46 | 0.052 | R = 0.09 | 0.518 | R = 0.23 | 0.102 | |
| P-PAID change | R = −0.05 | 0.821 | NA | NA | R = 0.13 | 0.353 | NA | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mianowska, B.; Fedorczak, A.; Michalak, A.; Pokora, W.; Barańska-Nowicka, I.; Wilczyńska, M.; Szadkowska, A. Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020. Int. J. Environ. Res. Public Health 2021, 18, 8527. https://doi.org/10.3390/ijerph18168527
Mianowska B, Fedorczak A, Michalak A, Pokora W, Barańska-Nowicka I, Wilczyńska M, Szadkowska A. Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020. International Journal of Environmental Research and Public Health. 2021; 18(16):8527. https://doi.org/10.3390/ijerph18168527
Chicago/Turabian StyleMianowska, Beata, Anna Fedorczak, Arkadiusz Michalak, Weronika Pokora, Inga Barańska-Nowicka, Monika Wilczyńska, and Agnieszka Szadkowska. 2021. "Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020" International Journal of Environmental Research and Public Health 18, no. 16: 8527. https://doi.org/10.3390/ijerph18168527
APA StyleMianowska, B., Fedorczak, A., Michalak, A., Pokora, W., Barańska-Nowicka, I., Wilczyńska, M., & Szadkowska, A. (2021). Diabetes Related Distress in Children with Type 1 Diabetes before and during the COVID-19 Lockdown in Spring 2020. International Journal of Environmental Research and Public Health, 18(16), 8527. https://doi.org/10.3390/ijerph18168527






