Comparative Study of CO2 Capture by Adsorption in Sustainable Date Pits-Derived Porous Activated Carbon and Molecular Sieve
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
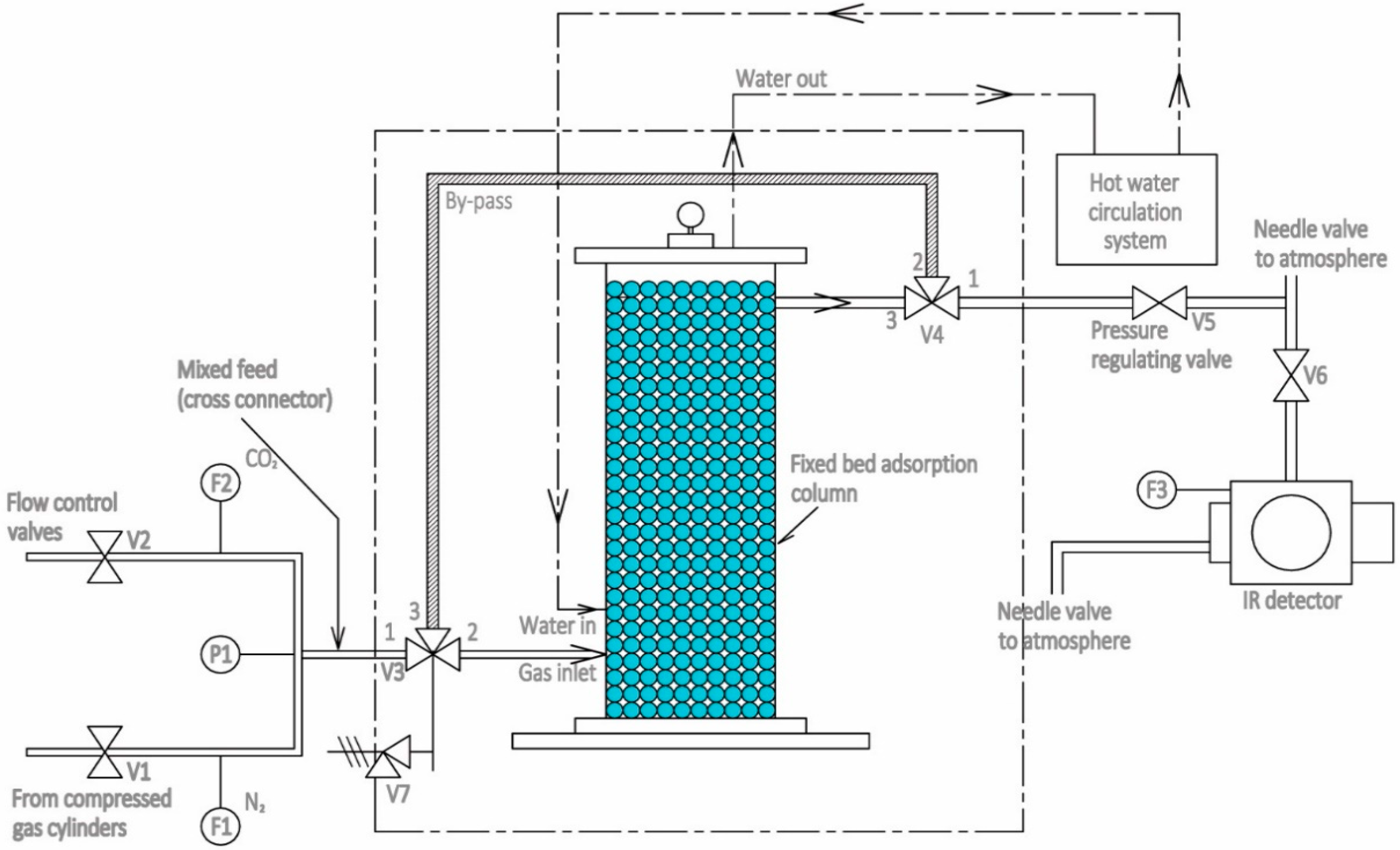
2.2. Experimental Unit
2.3. Procedure
2.4. Physical Activation
3. Results
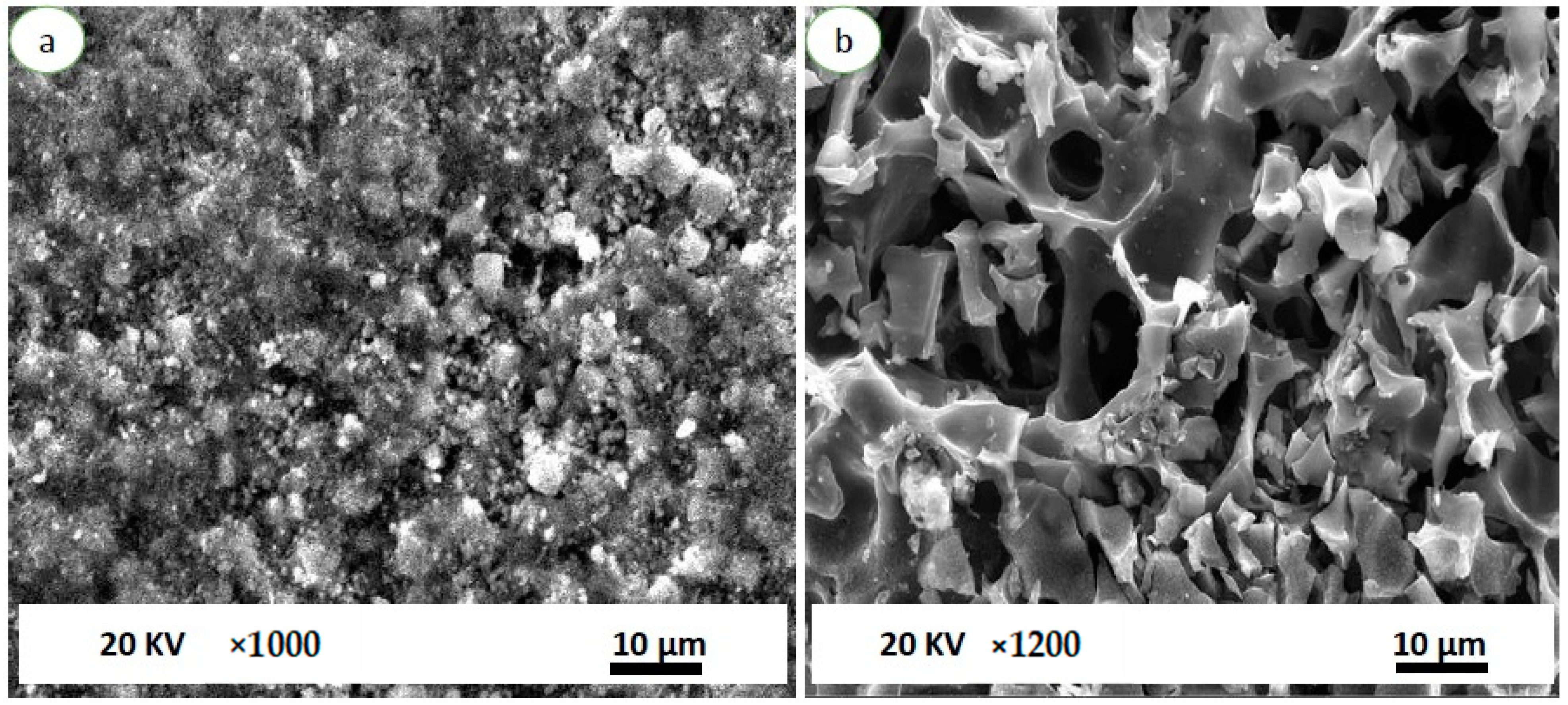
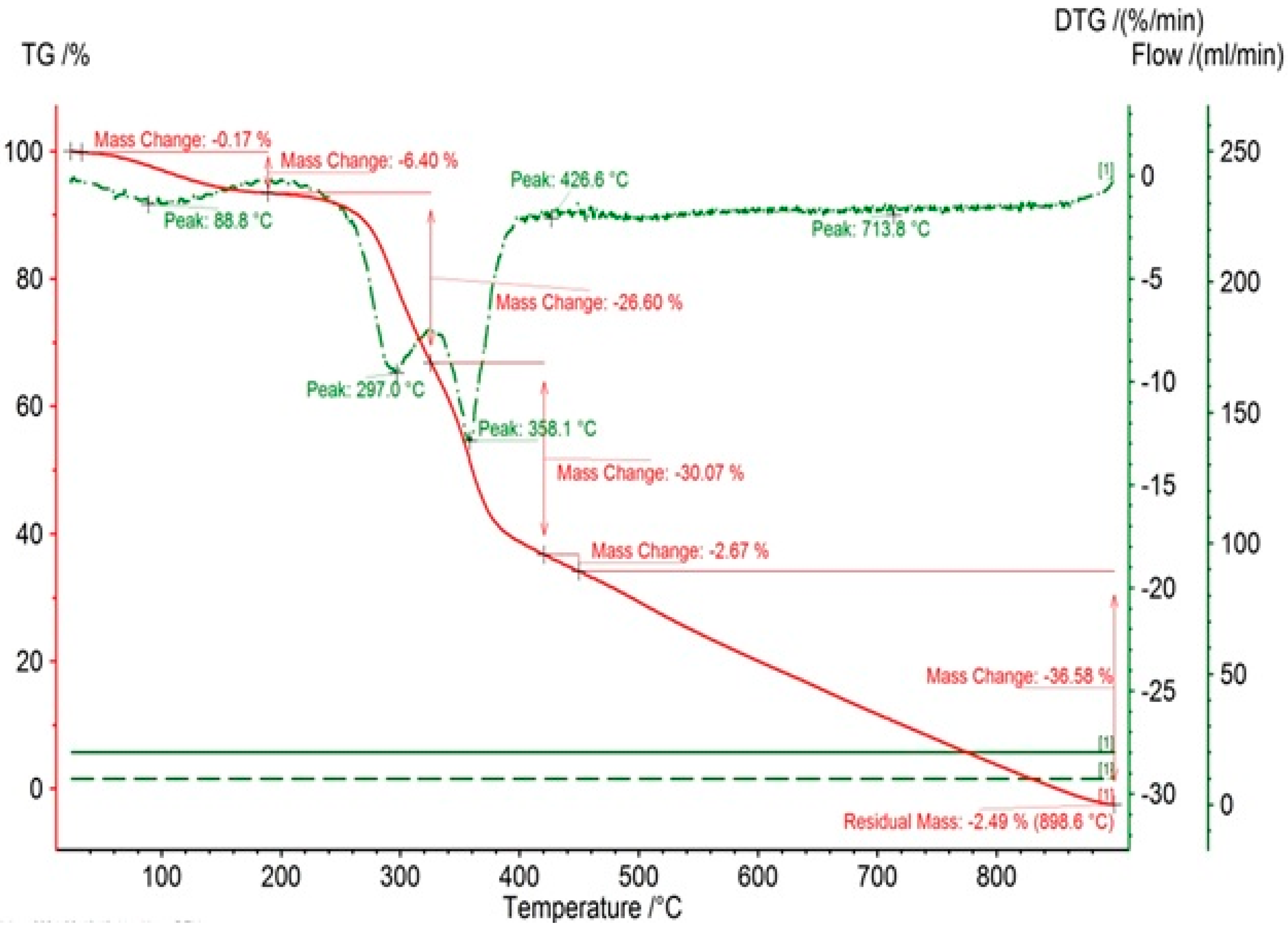
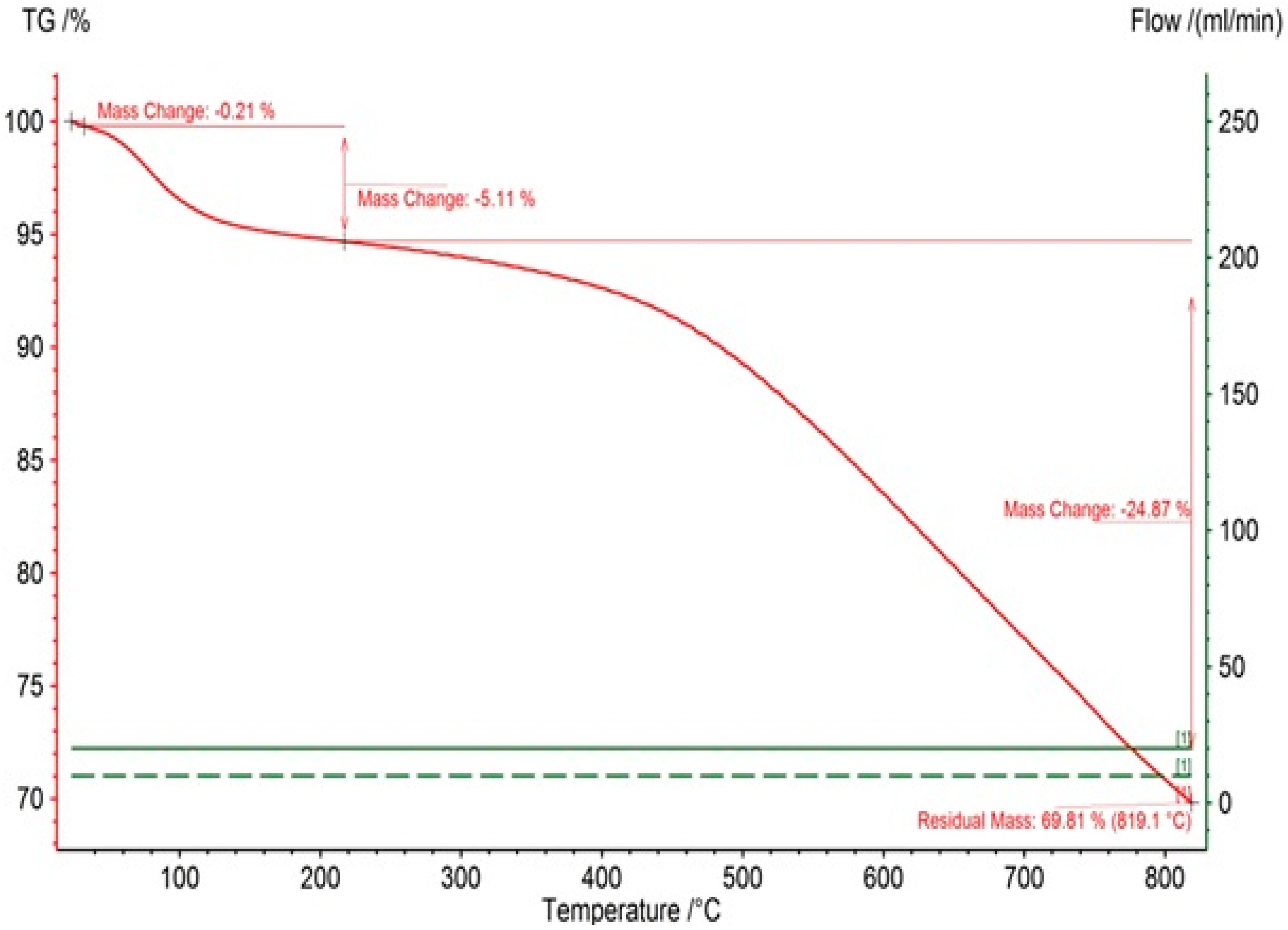
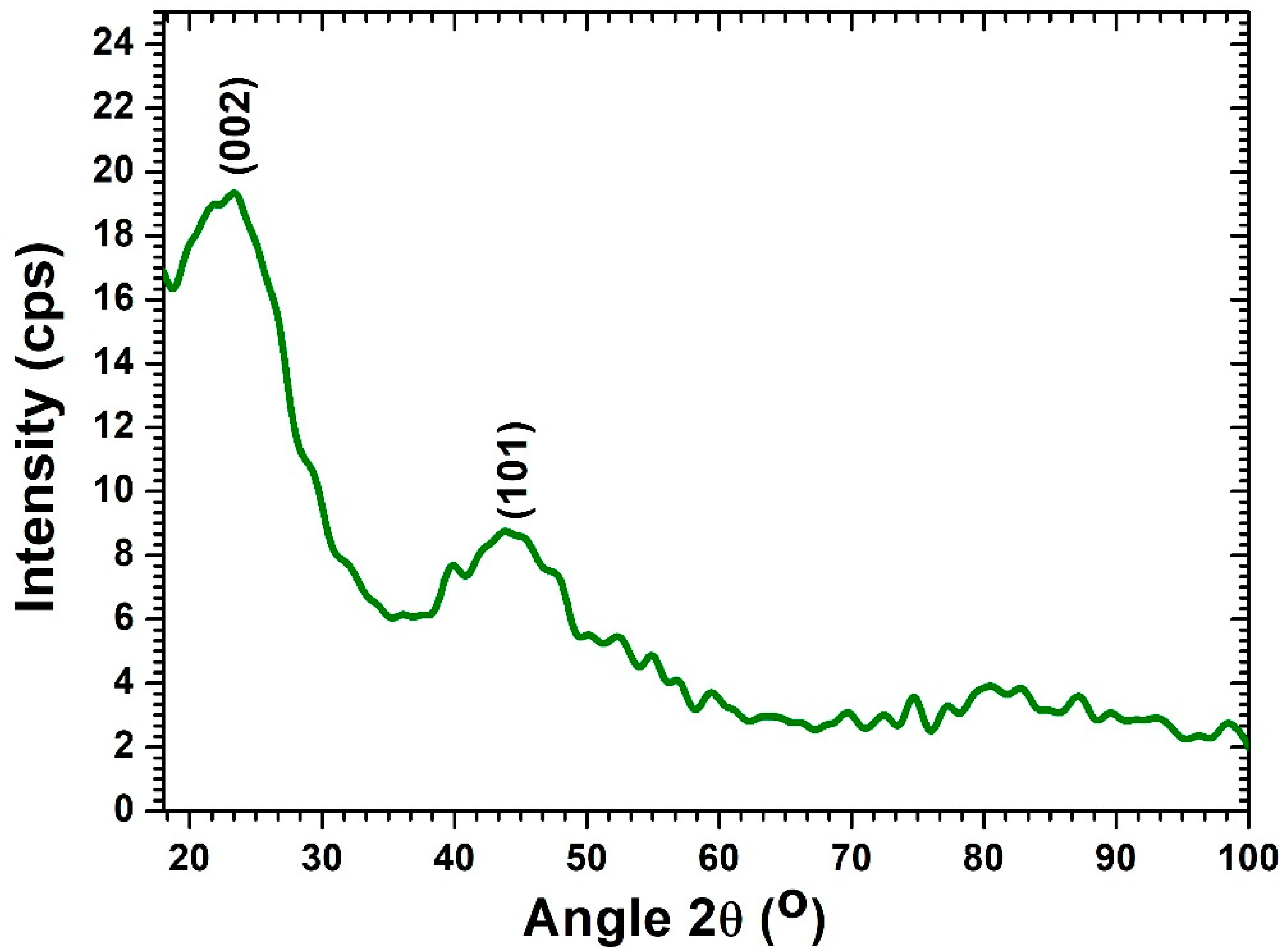
3.1. Morphological and Surface Characteristics
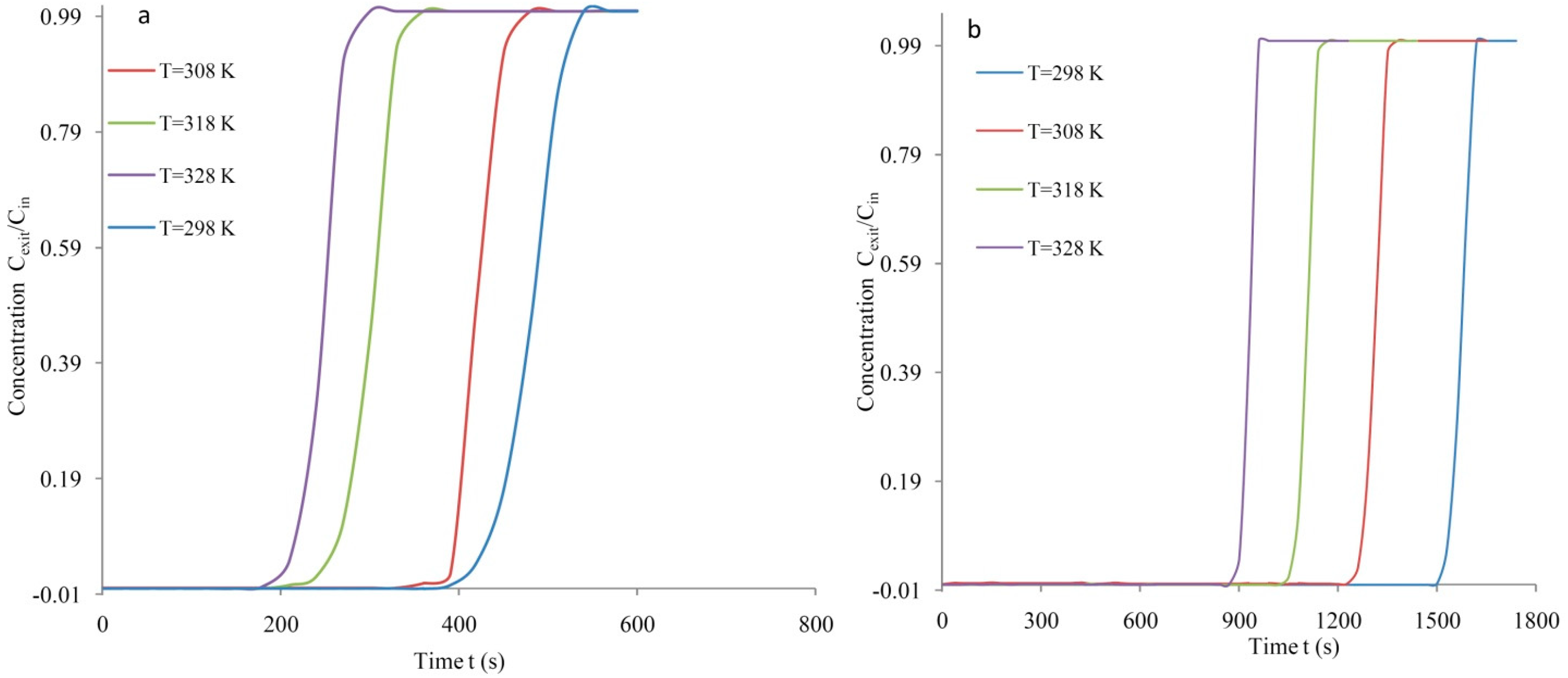
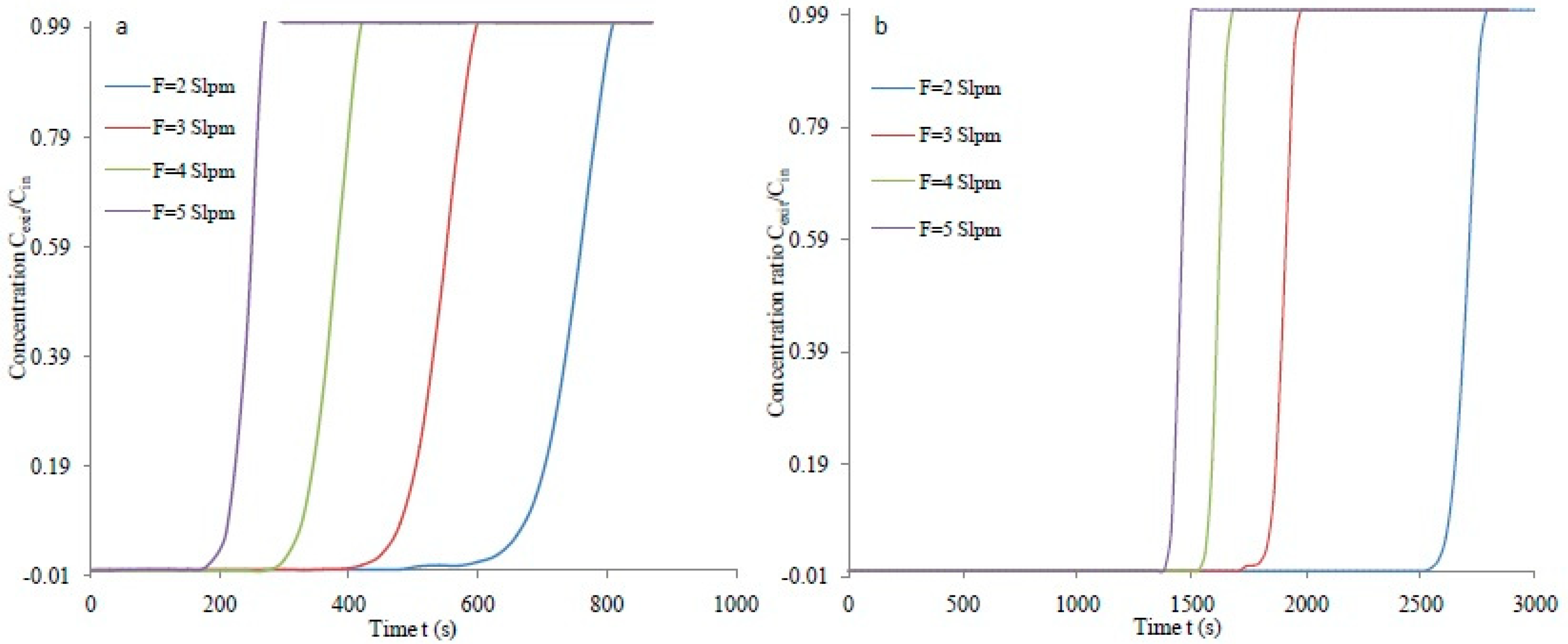
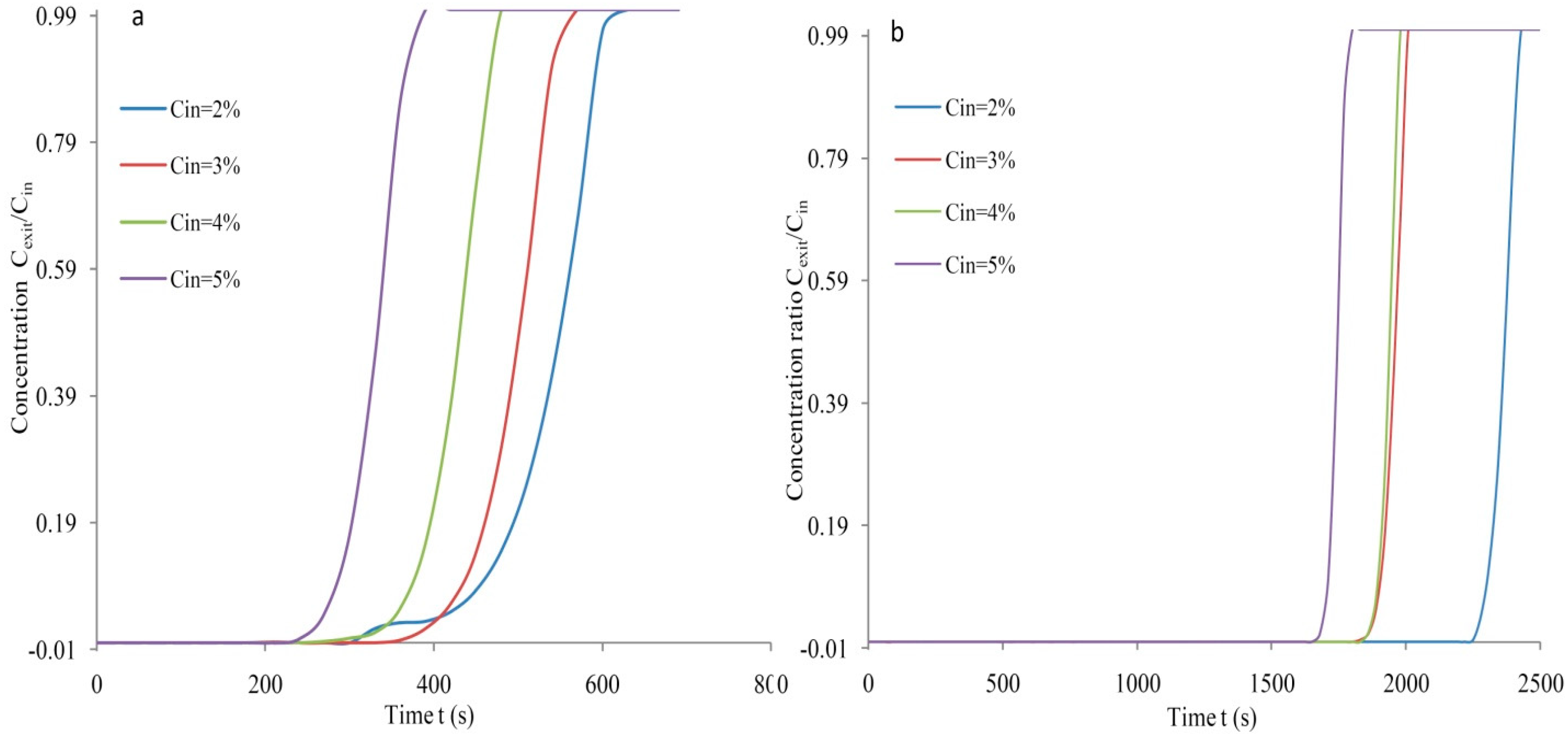
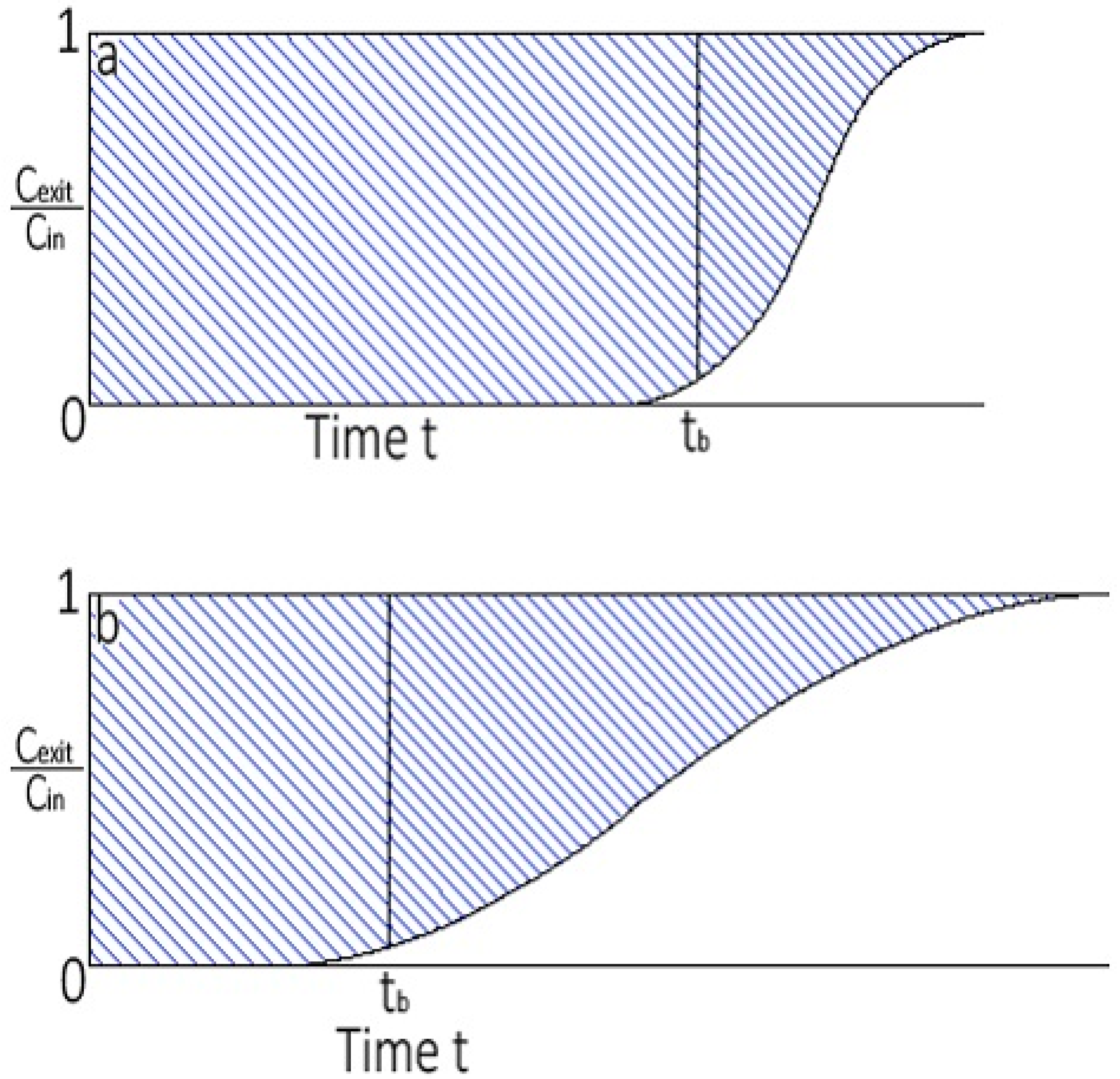
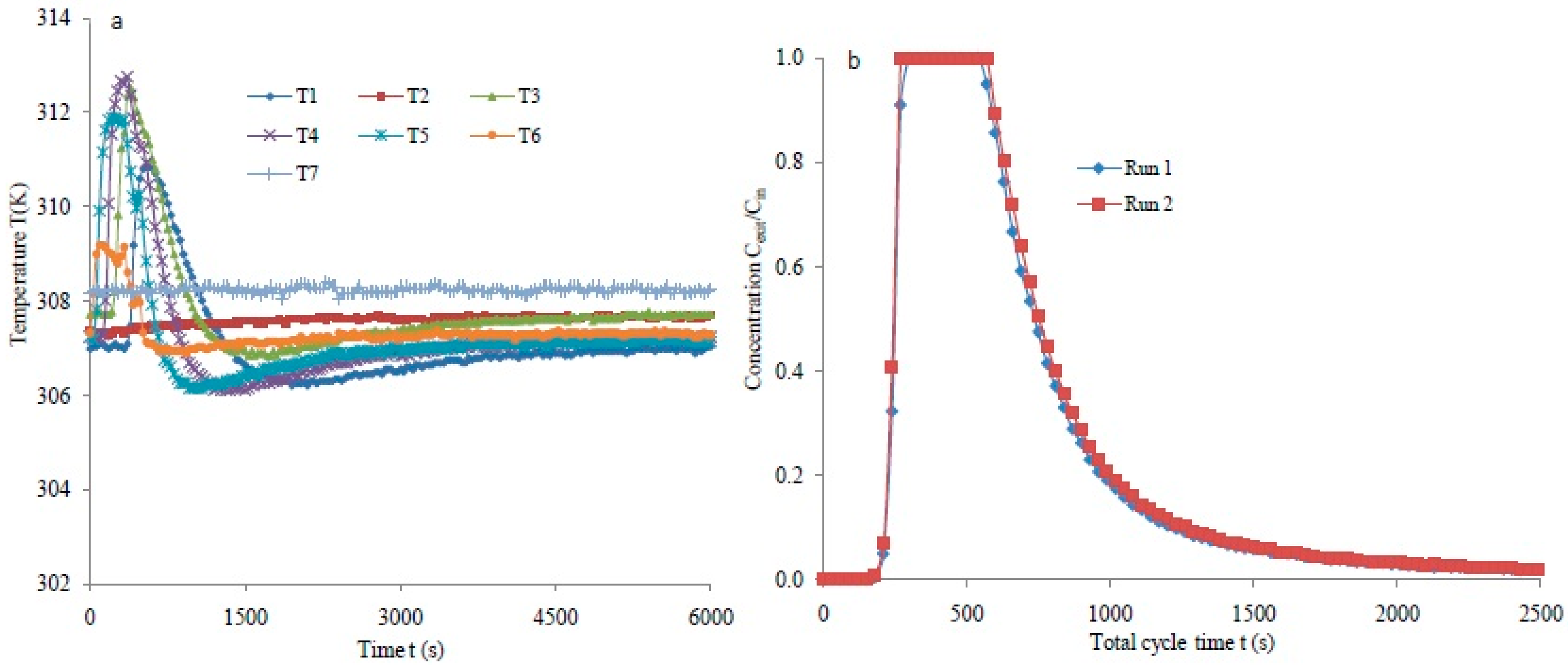
3.2. Breakthrough Analysis
3.3. Adsorption Capacity and Column Efficiency
3.4. Zone of Mass Transfer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rashidi, N.A.; Yusuf, S. An overview of activated carbons utilization for the post combustion carbon dioxide captures. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjman, C.; Williams, C.K.; Shah, N.; Fennel, P. An overview of carbon dioxide captures technologies. Energy. Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Cohen, S.M.; Chalmers, H.L.; Webbe, M.E.; King, C.W. Comparing post-combustion carbon dioxide capture operation at retrofitted coal-fired power plants in the Texas and Great Britain electric grids. Environ. Res. Lett. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Valverde, J.M.; Pontiga, F.; Soria-Hoyo, C.; Quintanlla, M.A.S.; Moreno, H.; Duran, F.J.; Espin, M.J. Improving the gas solids contact efficiency in a fluidized bed of carbon dioxide adsorbent fine particles. Phys. Chem. Chem. Phys. 2011, 13, 14906–14909. [Google Scholar] [CrossRef]
- Garcia, S.; Gil, M.V.; Martin, C.F.; Pis, J.J.; Rubiera, F.; Pevida, C. Breakthrough adsorption study of a commercial activated carbon for pre-combustion carbon dioxide capture. Chem. Eng. J. 2011, 171, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.; Rodriguez, N.; Gonzalez, B.; Grasa, G.; Murillo, R.; Abanades, J.C. Carbon dioxide capture from combustion flue gases with a calcium oxide chemical loop: Experimental results and process developments. Int. J. Greenh. Gas Control 2010, 4, 167–173. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Danish, M. Breakthrough adsorption study of activated carbons for CO2 separation from flue gas. J. Environ. Chem. Eng. 2018, 6, 4514–4524. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Danish, M.; Fahmy, M.Y.; Rashid, M.M. Post combustion CO2 capture with activated carbons using fixed bed adsorption. Heat Mass Transf. 2018, 54, 2715–2724. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Vakili, R.; Kalumpasut, P.; Gorgojo, P.; Siperstein, F.R.; Fan, X.; McCloskey, P. Velocity variation effect in fixed bed columns: A case study of CO2 captures using porous solid adsorbents. AIChE J. 2018, 64, 2189–2197. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Grande, C.A.; Li, P.; Yu, J.; Rodrigues, A.E. Adsorption equilibria and kinetics of CO2 and N2 on activated carbon beads. Chem. Eng. J. 2010, 160, 398–407. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Shen, C.; Yu, J.; Li, P.; Grande, C.A.; Rodrigues, A.E. Capture of CO2 from flue gas by vacuum pressure swing adsorption using activated carbon beads. Adsorption 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Dejang, N.; Somprasit, O.; Chindaruksa, S. Preparations of activated carbon from Macadamia shell by microwave irradiation activation. Energy Procedia 2015, 79, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Munoz, E.M.; Garcia-Mateos, F.J.; Juana, R.; Rodrigues-Mirasol, J.; Cordero, T. Biomass waste carbon materials as adsorbents for CO2 capture under post-combustion conditions. Front. Mater. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Toprak, A.; Kopac, T. Carbon dioxide adsorption using high surface area activated carbons from local coals modified by KOH, NaOH and ZnCl2 agents. Int. J. Chem. React. Eng. 2017, 15, 1–16. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Casal, M.D.; Martin, C.F.; Rubiera, F.; Pis, J.J. Development of low-cost biomass-based adsorbents for postcombustion CO2 capture. Fuel 2019, 88, 2442–2447. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Bjorkman, E.; Lilliestrale, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Shahkarami, S.; Azargohar, R.; Dalai, A.K.; Soltan, J. Breakthrough CO2 adsorption in bio-based activated carbons. J. Environ. Sci. 2015, 34, 68–76. [Google Scholar] [CrossRef]
- Coromina, H.M.; Walsh, D.A.; Mokaya, R. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications. J. Mater. Chem. A 2015, 4, 280–289. [Google Scholar] [CrossRef]
- Zhang, C.; Song, W.; Ma, Q.; Xie, L.; Zhang, X.; Guo, H. Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. Energy Fuels 2016, 305, 4181–4190. [Google Scholar] [CrossRef]
- Hong, S.; Jang, E.; Dysart, D.; Pol, V.G.; Lee, K.B. CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lira-Zúñiga, S.; Sáez-Navarrete, C.; Rodrígues-Cordova, L.; Herrera-Zeppelin, L.; Herrera-Urbina, R. CO2 adsorption on agricultural biomass combustion ashes. Maderas-Cienc Technol. 2016, 18, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Serafin, J.; Narkiewicz, U.; Moraeski, A.W.; Wrobel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Liu, S.-H.; Huang, Y.-Y. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Clean. Prod. 2018, 175, 354–360. [Google Scholar] [CrossRef]
- Kim, M.-J.; Choi, S.W.; Kim, H.; Mun, S.; Lee, K.B. Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture. Chem. Eng. J. 2020, 397, 125404. [Google Scholar] [CrossRef]
- Sarwar, A.; Ali, M.; Khoja, A.H.; Nawar, A.; Waqas, A.; Liaquat, R.; Naqvi, S.R.; Asjid, M. Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J. CO2 Util. 2021, 46, 101476. [Google Scholar] [CrossRef]
- Lewicka, K. Activated carbons prepared from hazelnut shells, walnut shells and peanut shells for high CO2 adsorption. Pol. J. Chem. Technol. 2017, 19, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Peredo-Mancilla, D.; Hort, C.; Ghimbeu, C.M.; Jeguirim, M.; Bessieres, D. CO2 and CH4 adsorption behaviour of Biomass-based activated carbons. Energies 2018, 11, 3136. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Lou, S.; Ying, H.; Wu, X.; Dou, X.; Ai, N.; Wang, J. Preparation of microporous carbon from sargassumhorneri by hydrothermal carbonization and KOH activation for CO2 capture. J. Chem. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.; Kopac, J. Activated carbons derived from residual biomass pyrolysis and their CO2 adsorption capacity. J Anal. Appl. Pyrolysis 2014, 110, 322–332. [Google Scholar]
- Jahangiri, M.; Shahtaheri, S.J.; Adl, J.; Rashidi, A.; Kakooei, H.; Forushani, A.R.; Nasiri, G.; Ghorbanali, A. Preparation of activated carbon from walnut shell and its utilization for manufacturing organic-vapor respirator cartridge. Fresenius Environ. Bull. 2012, 21, 1508–1514. [Google Scholar]
- Patil, P.; Singh, S.; Kumar, Y.M. Preparation and study of properties of activated carbons produced from agricultural and industrial waste shells. Res. J. Chem. Sci. 2013, 3, 12–15. [Google Scholar]
- Xia, H.; Cheng, S.; Zhang, L.; Peng, J. Utilization of walnut shell as a feedstock for preparing high surface area activated carbon by microwave induced activation: Effect of activation agents. Green Process Synth. 2016, 5, 7–14. [Google Scholar] [CrossRef]
- Mataji, M.; Khoshandam, B. Benzene adsorption on activated carbon from walnut shell. Chem. Eng. Commun. 2014, 201, 1294–1313. [Google Scholar] [CrossRef]
- Al Mesfer, M.K. Synthesis and characterization of high-performance activated carbon from walnut shell biomass for CO2 capture. Environ. Sci. Pollut. Res. 2020, 27, 15020–15028. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Makhadmeh, L. Preparation and examination of activated carbons from date pits impregnated with potassium hydroxide for the removal of methylene blue from aqueous solutions. Adsorpt. Sci. Technol. 2003, 21, 597–606. [Google Scholar] [CrossRef]
- Belhachemi, M.; Rios, R.; Addoun, F.; Silvestre-Albero, J.; Sepulveda-Escribano, A.; Rodriguez-Reinoso, F. Preparation of activated carbon from date pits: Effects of agents and liquid phase oxidation. J. Anal. Appl. Pyrolysis 2009, 86, 168–172. [Google Scholar] [CrossRef]
- Daniel, V.V.; Gulyani, B.B.; Kumar, B.G.P. Usage of date stones as adsorbents: A review. J. Disper. Sci. Technol. 2012, 33, 1321–1331. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.; Abu-Zahra, M.R.M. Activated carbon from date seeds for CO2 capture applications. Energy Procedia 2017, 114, 2313–2321. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.; Vega, L.F.; Abu-Zahar, M.R.P. Physical synthesis and characterization of activated carbon from date seed for CO2 capture. J. Environ. Chem. Eng. 2018, 6, 4245–4252. [Google Scholar] [CrossRef]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem. Eng. J. 2019, 360, 250–259. [Google Scholar] [CrossRef]
- Bahamon, D.; Ogungbenro, A.E.; Khaleel, M.; Quang, D.V.; Abu-Zahra, M.R.P.; Vega, L.F. Performance of activated carbon from date seeds in CO2 swing adsorption determined by combining experimental and molecular simulation data. Ind. Eng. Chem. Res. 2010, 59, 7161–7173. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Muhtaseb, A.H.; Naushad, M.; Baawain, M.; Al-Mamun, A.; Saxena, S.K.; Viswanadham, N. Evaluation of synthesized green carbon catalyst from waste date pits for tertiary butalyation of phenol. Arab. J. Chem. 2020, 13, 298–307. [Google Scholar] [CrossRef]
- Monazam, E.R.; Spenik, J.; Shadle, L.J. Fluid bed adsorption of carbon dioxide on immobilized polyethylenimine (PEI): Kinetic analysis and breakthrough behaviour. Chem. Eng. J. 2013, 223, 795–805. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Modeling adsorption of CO2 on amine functionalized mesoporous silica. 2: Kinetics and breakthrough curve. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Pota, A.A.; Mathews, A.P. Effect of particle stratification on fixed bed adsorber performance. J. Environ. Eng. 1999, 8, 705–711. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of amine-containing CO2 adsorbents: Dramatic effect of water vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Da’na, E. CO2 deactivation of supported amines: Does the nature of amine matter? Langmuir 2012, 28, 4241–4247. [Google Scholar] [CrossRef]

| Variable (u) | Sensor Type | Uncertainty (δu) |
|---|---|---|
| N2 flow rate (slpm) | F1 | ±0.04 |
| CO2 flow rate (slpm) | F2 | ±0.02 |
| IR flow rate (slpm) | F3 | ±0.01 |
| Temperature (K) | Thermocouple type-K | ±0.15 |
| Adsorbent Characteristics | MS-3Å | AC-SY |
|---|---|---|
| Single point surface (m2/g) | 26.42 | 149.11 |
| Multiple point surface area | 26.87 | 463.80 |
| Langmuir surface area (m2/g) | 46.21 | 1444.0 |
| DFT Pore volume (cm3/g) | 0.02 | 0.46 |
| DFT Pore radius (Å) | 8.44 | 9.23 |
| MS-3Å | AC-SY | |||||
|---|---|---|---|---|---|---|
| T(K) | q (mg/g) | η (fr.) | Lb (cm) | q (mg/g) | η (fr.) | Lb (cm) |
| 298 | 13.83 | 0.845 | 19.44 | 63.18 | 0.944 | 21.71 |
| 308 | 11.64 | 0.834 | 19.18 | 50.60 | 0.938 | 21.57 |
| 318 | 8.24 | 0.746 | 17.16 | 44.45 | 0.931 | 21.41 |
| 328 | 6.74 | 0.742 | 17.07 | 33.09 | 0.939 | 21.60 |
| MS-3Å | AC-SY | |||||
|---|---|---|---|---|---|---|
| F (lpm) | q (mg/g) | η (fr.) | Lb (cm) | q (mg/g) | η (fr.) | Lb (cm) |
| 2 | 10.33 | 0.890 | 20.47 | 54.30 | 0.940 | 21.62 |
| 3 | 11.52 | 0.776 | 17.80 | 57.55 | 0.940 | 21.62 |
| 4 | 10.73 | 0.749 | 17.23 | 65.43 | 0.939 | 21.60 |
| 5 | 8.54 | 0.746 | 17.16 | 73.08 | 0.938 | 21.57 |
| MS-3Å | AC-SY | |||||
|---|---|---|---|---|---|---|
| Cin (vol.%) | q (mg/g) | η (fr.) | Lb (fr.) | q (mg/g) | η (fr.) | Lb (cm) |
| 2 | 7.94 | 0.702 | 16.15 | 37.93 | 0.941 | 21.65 |
| 3 | 10.93 | 0.760 | 17.48 | 46.95 | 0.940 | 19.74 |
| 4 | 12.12 | 0.758 | 17.43 | 61.82 | 0.949 | 21.83 |
| 5 | 12.31 | 0.711 | 16.35 | 70.13 | 0.948 | 21.84 |
| Adsorbent | Temperature (°C) | Pressure (bar) | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Date pits-AC | 40 | 0.14 | 118.8 | [37] |
| Date pits-AC | 20 | 1 | 154.0 | [41] |
| Date sheet-AC | 0 | 1 | 281.6 | [42] |
| Date sheet-AC | 25 | 1 | 193.6 | [42] |
| Date sheet-AC | 25 | 40 | 968.0 | [42] |
| Date pits-AC | 25 | 1.3 | 73.08 | [Current work] |
| Molecular sieve 3Å | 25 | 1.3 | 13.93 | [Current work] |
| T (K) | F (slpm) | Cin (vol.%) | tb (s) | ts (s) | LMTZ (cm) | f |
|---|---|---|---|---|---|---|
| 298 | 4 | 5 | 417 | 513 | 4.75 | 0.897 |
| 308 | 4 | 5 | 388 | 459 | 3.86 | 0.916 |
| 318 | 4 | 5 | 250 | 335 | 6.67 | 0.855 |
| 328 | 4 | 5 | 210 | 283 | 6.81 | 0.852 |
| 298 | 2 | 5 | 650 | 803 | 4.84 | 0.895 |
| 298 | 3 | 5 | 460 | 593 | 2.28 | 0.950 |
| 298 | 5 | 5 | 200 | 268 | 6.68 | 0.855 |
| 298 | 4 | 2 | 420 | 598 | 8.04 | 0.825 |
| 298 | 4 | 3 | 413 | 543 | 6.26 | 0.864 |
| 298 | 4 | 4 | 360 | 475 | 5.53 | 0.880 |
| T (K) | Q (slpm) | Cin (vol.%) | tb (s) | ts (s) | LMTZ (cm) | f |
|---|---|---|---|---|---|---|
| 298 | 4 | 5 | 1524 | 1615 | 1.33 | 0.971 |
| 308 | 4 | 5 | 1265 | 1348 | 1.46 | 0.968 |
| 318 | 4 | 5 | 1059 | 1138 | 1.65 | 0.964 |
| 328 | 4 | 5 | 900 | 958 | 1.44 | 0.969 |
| 298 | 2 | 5 | 2603 | 2769 | 1.42 | 0.969 |
| 298 | 3 | 5 | 1833 | 1951 | 1.43 | 0.969 |
| 298 | 5 | 5 | 1401 | 1494 | 1.48 | 0.968 |
| 298 | 4 | 2 | 2280 | 2422 | 1.39 | 0.970 |
| 298 | 4 | 3 | 1884 | 2005 | 1.43 | 0.969 |
| 298 | 4 | 4 | 1875 | 1975 | 1.20 | 0.974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danish, M.; Parthasarthy, V.; Al Mesfer, M.K. Comparative Study of CO2 Capture by Adsorption in Sustainable Date Pits-Derived Porous Activated Carbon and Molecular Sieve. Int. J. Environ. Res. Public Health 2021, 18, 8497. https://doi.org/10.3390/ijerph18168497
Danish M, Parthasarthy V, Al Mesfer MK. Comparative Study of CO2 Capture by Adsorption in Sustainable Date Pits-Derived Porous Activated Carbon and Molecular Sieve. International Journal of Environmental Research and Public Health. 2021; 18(16):8497. https://doi.org/10.3390/ijerph18168497
Chicago/Turabian StyleDanish, Mohd, Vijay Parthasarthy, and Mohammed K. Al Mesfer. 2021. "Comparative Study of CO2 Capture by Adsorption in Sustainable Date Pits-Derived Porous Activated Carbon and Molecular Sieve" International Journal of Environmental Research and Public Health 18, no. 16: 8497. https://doi.org/10.3390/ijerph18168497
APA StyleDanish, M., Parthasarthy, V., & Al Mesfer, M. K. (2021). Comparative Study of CO2 Capture by Adsorption in Sustainable Date Pits-Derived Porous Activated Carbon and Molecular Sieve. International Journal of Environmental Research and Public Health, 18(16), 8497. https://doi.org/10.3390/ijerph18168497






