Early Risk Factors for Obesity in the First 1000 Days—Relationship with Body Fat and BMI at 2 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Population
2.3. Sample
2.4. Study Variables
2.5. Data Collection
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Descriptive Statistics
3.2. Relationship between Independent/Dependent Variables
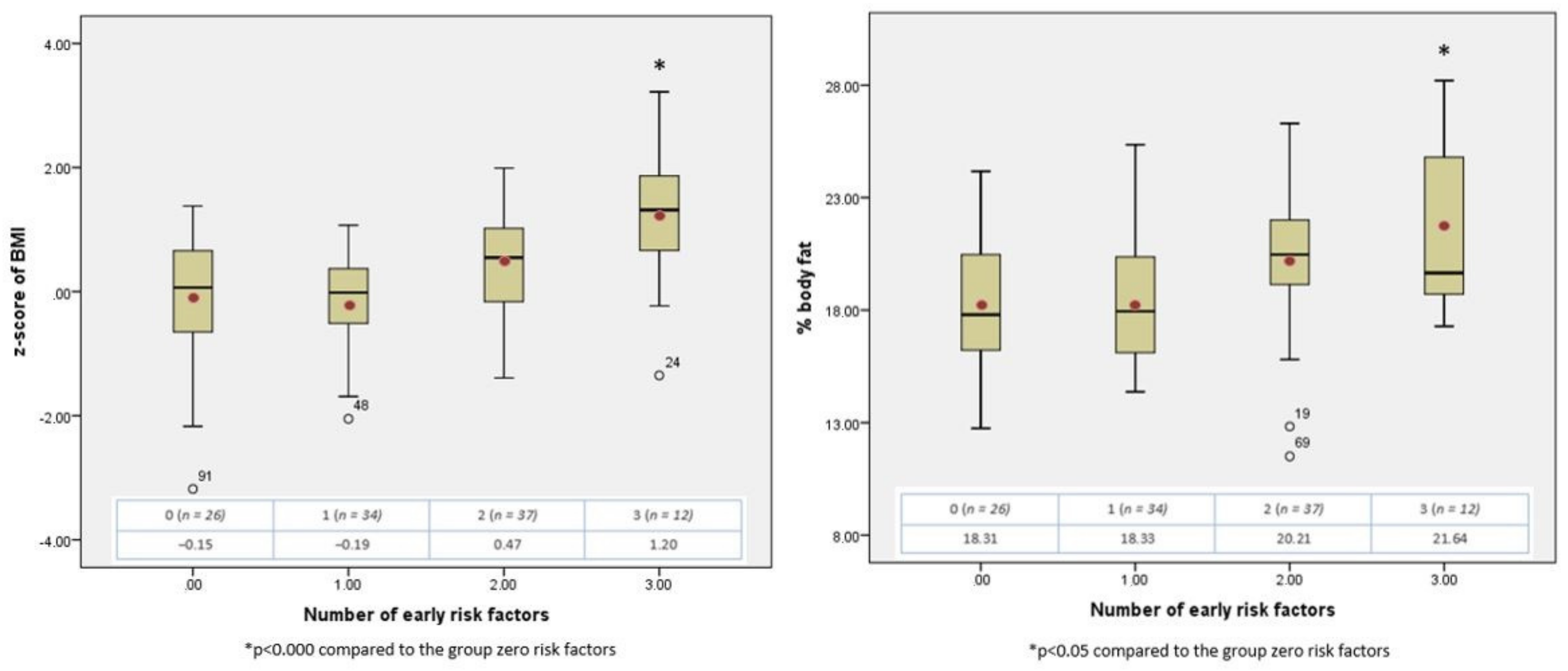
3.3. The Cumulative Effect of Early Risk Factors
4. Discussion
5. Conclusions
6. Strength, Weakness and Limitation of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- WHO. What Are Overweight and Obesity? Available online: https://www.who.int/dietphysicalactivity/childhood_what/es/ (accessed on 1 December 2020).
- Chung, S. Body mass index and body composition scaling to height in children and adolescent. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 125. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.P.; Going, S.B.; Lohman, T.G.; Harsha, D.W.; Srinivasan, S.R.; Webber, L.S.; Berenson, G.S. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am. J. Public Health 1992, 82, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, I.; Herrero, M.; Castell, M.; López, E.; Galera, R.; Moráis, A.; GETNI Group. Systematized assessment of nutritional status. Acta Pediatr. Esp. 2011, 69, 165–172. [Google Scholar]
- Casanova Román, M.; Rodríguez Ruiz, I.; Rico de Cos, S.; Casanova Bellido, M. Analysis of body composition by anthropometric and bioelectric parameters. An. Pediat. 2004, 61, 23–31. [Google Scholar]
- NAOS Strategy: Reversing the Obesity Trend Strategy for Nutrition, Physical Activity and Obesity Prevention. Available online: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/nutricion/estrategianaos.pdf (accessed on 5 December 2020).
- Spanish Agency for Food Safety and Nutrition. ALADINO Study 2019: Surveillance Study on Growth, Diet, Physical Activity, Child Development and Obesity in Spain 2019; Ministry of Consumer Affairs: Madrid, Spain, 2020. [Google Scholar]
- Paul, I.M.; Savage, J.S.; Frasca, A.; Birch, L. Obesity Prevention During Infancy: A Change of Focus. 2012. Available online: http://www.child-encyclopedia.com/sites/default/files/textes-experts/en/789/obesity-prevention-during-infancy-a-change-of-focus.pdf (accessed on 5 December 2020).
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Bammann, K.; Peplies, J.; De Henauw, S.; Hunsberger, M.; Molnar, D.; Moreno, L.A.; Tornaritis, M.; Veidebaum, T.; Ahrens, W.; Siani, A.; et al. Early Life Course Risk Factors for Childhood Obesity: The IDEFICS Case-Control Study. PLoS ONE 2014, 9, e86914. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Zhang, Y.; Jiang, Y.; Sun, W.; Zhu, Q.; Liu, S.; Chen, C.; Zhang, Z.; Huang, B.; Jiang, F.; et al. Cesarean section and risks of overweight and obesity in school-aged children: A population-based study. Int. J. Med. 2018, 111, 859–865. [Google Scholar] [CrossRef]
- Harder, T.; Bergman, R.; Kallischnigg, G.; Plagemann, A. Duration of breastfeeding and risk of overweight: A meta-analysis. Am. J. Epidemiol. 2005, 162, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. European Childhood Obesity Trial Study Group. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomised trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef]
- Iguacel, I.; Escartín, L.; Fernández-Alvira, J.M.; Iglesia, I.; Labayen, I.; Moreno, L.A.; Samper, M.P.; Rodríguez, G.; On Behalf of the CALINA Study Group. Early life risk factors and their cumulative effects as predictors of overweight in Spanish children. Int. J. Public Health 2018, 63, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Gillman, M.W.; Ludwig, D.S. How Early Should Obesity Prevention Start? N. Engl. J. Med. 2013, 369, 2173–2175. [Google Scholar] [CrossRef]
- Robinson, S.; Crozier, S.; Harvey, N.; Barton, B.D.; Law, C.M.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Modifiable early-life risk factors for childhood adiposity and overweight: An analysis of their combined impact and potential for prevention. Am. J. Clin. Nutr. 2015, 101, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.F.; Harrall, K.K.; Sauder, K.A.; Glueck, D.H.; Dabelea, D. Neonatal Adiposity and Childhood Obesity. Pediatrics 2020, 146, e20200737. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, M.; Pérez-Muñoz, C.; Lendínez-de la Cruz, J.M.; Fernández-Gutiérrez, M.; Bas-Sarmiento, P.; Ferriz-Mas, B.C. Effectiveness of a Multifactorial Intervention in the First 1000 Days of Life to Prevent Obesity and Overweight in Childhood: Study Protocol. Int. J. Environ. Res. Public Health 2020, 17, 2239. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.M.; Yaktine, A.L. (Eds.) Institute of Medicine (US) and National Research Council(US) Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- World Health Organization. Indicators for assessing infant and young child feeding practices. Part 1. In Definitions: Conclusions of the Consensus Meeting Held 6–8 November 2007 in Washington, DC, USA; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- WHO. Software for assessing the growth and development of the world’s children. In Anthro for Personal Computers, Version 3; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Ong, K.K.; Loos, R.J. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006, 95, 904–908. [Google Scholar] [CrossRef]
- Siri, W.B. The gross composition of the body. In Advances in Biological and Medical Physis; Tobias, C.A., Lawrence, J.H., Eds.; Academic Press: New York, NY, USA, 1956; pp. 239–280. [Google Scholar]
- Brook, C.G.D. Determination of body composition of children from skinfolds measurements. Arch. Dis. Child. 1972, 46, 182. [Google Scholar] [CrossRef] [Green Version]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; p. 312. [Google Scholar]
- Expert Committee on Physical Status. Physical Status: The Use and Interpretation of Anthropometry; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Fomon, S.J.; Haschke, F.; Ziegler, E.E.; Nelson, S.E. Body composition of reference children from birth to age 10 years. Am. J. Clin. Nutr. 1982, 35, 1169–1175. [Google Scholar] [CrossRef]
- Ayerza Casas, A.; Rodríguez Martínez, G.; Samper Villagrasa, M.P.; Arnal, P.M.; Sauras, M.L.A.; Aznar, L.A.M.; López, J.L.O.; Grupo Colaborativo CALINA. Nutritional characteristics of newborns born to overweight and obese mothers. An. Pediatr. 2011, 75, 175–181. [Google Scholar] [CrossRef]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef] [Green Version]
- Catalano, P.M.; Ehrenberg, H.M. The short-and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Steegers, E.A.; Duijts, L.; Felix, J.F.; Hofman, A.; Franco, O.H.; Jaddoe, V.W. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: The Generation R Study. Hypertension 2014, 63, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamun, A.A.; Mannan, M.; Doi, S.A. Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2014, 15, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Steegers, E.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int. J. Obes. 2015, 39, 677–685. [Google Scholar] [CrossRef]
- Hivert, M.F.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity 2016, 24, 1546–1553. [Google Scholar] [CrossRef] [Green Version]
- Oken, E.; Taveras, E.M.; Kleinman, K.P.; Rich-Edwards, J.W.; Gillman, M.W. Gestational weight gain and child adiposity at age 3 years. Am. J. Obstet. Gynecol. 2007, 196, 322.e1–322.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oken, E.; Levitan, E.B.; Gillman, M.W. Maternal smoking during pregnancy and child overweight: Systematic review and meta-analysis. Int. J. Obes. 2008, 32, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Sato, M.; Ando, D.; Kondo, N.; Yamagata, Z. Differences in the effect of maternal smoking during pregnancy for childhood overweight before and after 5 years of age. J. Obstet. Gynaecol. Res. 2013, 39, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Thomas, A.; Huston-Presley, L.; Amini, S.B. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am. J. Obstet. Gynecol. 2003, 189, 1698–1704. [Google Scholar] [CrossRef]
- Bider-Canfield, Z.; Martinez, M.P.; Wang, X. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr. Obes. 2017, 12, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Crume, T.L.; Ogden, L.; Daniels, S.; Hamman, R.F.; Norris, J.M.; Dabelea, D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: The EPOCH study. J. Pediatr. 2011, 158, 941–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifas-Shiman, S.L.; Gillman, M.W.; Hawkins, S.S.; Oken, E.; Taveras, E.M.; Kleinman, K.P. Association of Cesarean Delivery With Body Mass Index z Score at Age 5 Years. JAMA Pediatr. 2018, 172, 777–779. [Google Scholar] [CrossRef] [Green Version]
- Kuhle, S.; Tong, O.; Woolcott, C. Association between caesarean section and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.; de Castro, N.; Euclydes, V.; Souza, J.M.; Rondó, P.H.C. Birth weight, rapid weight gain in infancy and markers of overweight and obesity in childhood. Eur. J. Clin. Nutr. 2013, 67, 1147–1153. [Google Scholar] [CrossRef]
- Stettler, N.; Zemel, B.; Kumanyika, S.; Stallings, V. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 2002, 109, 194–199. [Google Scholar] [CrossRef]
- Druet, C.; Stettler, N.; Sharp, S.; Simmons, R.K.; Cooper, C.; Davey Smith, G.; Ekelund, U.; Lévy-Marchal, C.; Jarvelin, M.; Kuh, D.; et al. Prediction of childhood obesity by infancy weight gain: An individual-level meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 19–26. [Google Scholar] [CrossRef]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Cu, L.; Villarreal, E.; Rangel, B.; Galicia, L.; Vargas, E.; Martinez, L. Risk factors for overweight and obesity in infants. Rev. Nutr. 2015, 42, 139–144. [Google Scholar] [CrossRef]

| Maternal Variables | Mean | SD | 95% CI | |
|---|---|---|---|---|
| Maternal age (years) | 32.37 | 5.24 | (31.38, 33.35) | |
| Parity | 1.65 | 0.67 | (1.53, 1.78) | |
| Pre-pregnancy BMI | 26.52 | 6.02 | (25.39, 27.66) | |
| Newborn Variables | n | (%) | ||
| Sex | Female | 58 | 53.21 | |
| Male | 51 | 46.79 | ||
| Mean | SD | 95% CI | ||
| Weight (kg) | 3.31 | 0.47 | (3.23, 3.40) | |
| Length (cm) | 49.81 | 2.29 | (49.38, 50.24) | |
| n | (%) | |||
|---|---|---|---|---|
| Pre-pregnancy BMI | Average weight/Low weight | 50 | 45.87 | |
| Overweight | 34 | 31.19 | ||
| Obesity | 25 | 22.94 | ||
| Weight gain * | Adequate | 73 | 66.97 | |
| Excessive | 36 | 33.03 | ||
| Smoking | Yes | 8 | 7.34 | |
| No | 101 | 92.66 | ||
| Gestational diabetes | Yes | 14 | 12.84 | |
| No | 95 | 87.16 | ||
| Caesarean delivery | Yes | 30 | 27.52 | |
| No | 79 | 72.48 | ||
| Exclusive breastfeeding ≥4 months | 50 | 45.87 | ||
| Prevalence of breastfeeding at 6 months | 55 | 50.45 | ||
| Duration of breastfeeding | 0 months | 23 | 21.10 | |
| 1–6 months | 37 | 33.94 | ||
| >6 months | 49 | 44.95 | ||
| Early complementary feeding (<4 m) | 1 | 0.96 | ||
| Accelerated weight gain 0–2 years | 49 | 44.95 | ||
| Mean | SD | 95% CI | ||
| Duration of breastfeeding (months) | 7.56 | 4.33 | (6.64, 8.47) | |
| Increment z-score weight 0–4 m | 0.17 | 1.45 | (−0.11, 0.44) | |
| Increment z-score weight 0–6 m | 0.40 | 1.40 | (0.13, 0.67) | |
| Increment z-score weight 0–12 m | 0.41 | 1.41 | (0.15, 0.68) | |
| Increment z-score weight 0–24 m | 0.49 | 1.30 | (0.24, 0.73) | |
| BMI Z-score 2 years | Girls | 0.25 | 1.03 | (−0.02, 0.52) |
| Boys | 0.13 | 1.05 | (−0.16, 0.43) | |
| Total | 0.20 | 1.04 | (−0.00, 0.39) | |
| % body fat 2 years | Girls ** | 18.43 | 3.38 | (17.56, 19.30) |
| Boys ** | 20.35 | 2.81 | (19.58, 21.12) | |
| Total | 19.33 | 3.26 | (18.72, 19.94) | |
| VD1: BMI at 2 Years (z-Score) | No Risk (±SD) | At Risk (±SD) | β | t | p-value |
|---|---|---|---|---|---|
| Pre-gestational BMI (</≥25) | 0.03 ± 0.97 | 0.34 ± 1.08 | 0.094 | 1.031 | 0.305 |
| Accelerated weight gain during pregnancy (no/yes) | −0.01 ± 0.95 | 0.40 ± 1.16 | 0.174 | 2.012 | 0.047 * |
| Gestational diabetes (no/yes) | 0.16 ± 1.06 | 0.41 ± 0.89 | 0.059 | 0.682 | 0.497 |
| Caesarean section (no/yes) | 0.21 ± 1.07 | 0.17 ± 0.97 | −0.042 | −0.480 | 0.633 |
| Smoking (no/yes) | 0.24 ± 1.01 | −0.37 ± 1.30 | −0.116 | −1.341 | 0.183 |
| Breastfeeding ≥6 m (no/yes) | 0.05 ± 1.04 | 0.35 ± 1.02 | 0.081 | 0.929 | 0.355 |
| Exclusive breastfeeding ≥4 m (no/yes) | 0.09 ± 1.15 | 0.28 ± 0.94 | 0.049 | 0.566 | 0.573 |
| Accelerated weight gain 0–24 months (no/yes) | −0.21 ± 0.95 | 0.69 ± 0.93 | 0.417 | 4.826 | 0.000 * |
| VD2: % fat mass at 2 years of age | No risk (±SD) | At risk (±SD) | β | t | p-value |
| Pre-gestational BMI (</≥25) | 18.61 ± 2.99 | 19.94 ± 3.39 | 0.203 | 2.256 | 0.026 * |
| Accelerated weight gain during pregnancy (no/yes) | 18.89 ± 3.19 | 19.61 ± 3.27 | 0.148 | 1.541 | 0.126 |
| Gestational diabetes (no/yes) | 19.46 ± 3.23 | 18.42 ± 3.49 | −0.129 | −1.404 | 0.163 |
| Caesarean section (no/yes) | 19.37 ± 3.29 | 19.21 ± 3.24 | −0.049 | −0.537 | 0.592 |
| Smoking (no/yes) | 19.17 ± 3.22 | 21.36 ± 3.37 | 0.192 | 2.124 | 0.036 * |
| Breastfeeding ≥6 m (no/yes) | 19.34 ± 3.30 | 19.32 ± 3.25 | −0.084 | −0.915 | 0.362 |
| Exclusive breastfeeding ≥4 m (no/yes) | 19.52 ± 3.63 | 19.17 ± 2.94 | 0.081 | 0.929 | 0.355 |
| Accelerated weight gain 0–24 months (no/yes) | 18.55 ± 2.87 | 20.28 ± 3.48 | 0.269 | 2.986 | 0.004 * |
| Factors | Girls | Children | % Total |
|---|---|---|---|
| No factor | 9 | 6 | 13.76 |
| 1 factor | 22 | 5 | 24.77 |
| 2 factors | 15 | 15 | 27.52 |
| 3 factors | 9 | 20 | 26.61 |
| 4 factors | 3 | 5 | 7.34 |
| chi2 = 15.591, p = 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Rodríguez, M.; Pérez-Muñoz, C.; Carretero-Bravo, J.; Ruíz-Ruíz, C.; Serrano-Santamaría, M.; Ferriz-Mas, B.C. Early Risk Factors for Obesity in the First 1000 Days—Relationship with Body Fat and BMI at 2 Years. Int. J. Environ. Res. Public Health 2021, 18, 8179. https://doi.org/10.3390/ijerph18158179
Díaz-Rodríguez M, Pérez-Muñoz C, Carretero-Bravo J, Ruíz-Ruíz C, Serrano-Santamaría M, Ferriz-Mas BC. Early Risk Factors for Obesity in the First 1000 Days—Relationship with Body Fat and BMI at 2 Years. International Journal of Environmental Research and Public Health. 2021; 18(15):8179. https://doi.org/10.3390/ijerph18158179
Chicago/Turabian StyleDíaz-Rodríguez, Mercedes, Celia Pérez-Muñoz, Jesús Carretero-Bravo, Catalina Ruíz-Ruíz, Manuel Serrano-Santamaría, and Bernardo C. Ferriz-Mas. 2021. "Early Risk Factors for Obesity in the First 1000 Days—Relationship with Body Fat and BMI at 2 Years" International Journal of Environmental Research and Public Health 18, no. 15: 8179. https://doi.org/10.3390/ijerph18158179
APA StyleDíaz-Rodríguez, M., Pérez-Muñoz, C., Carretero-Bravo, J., Ruíz-Ruíz, C., Serrano-Santamaría, M., & Ferriz-Mas, B. C. (2021). Early Risk Factors for Obesity in the First 1000 Days—Relationship with Body Fat and BMI at 2 Years. International Journal of Environmental Research and Public Health, 18(15), 8179. https://doi.org/10.3390/ijerph18158179







