Applying Heart Rate Variability to Monitor Health and Performance in Tactical Personnel: A Narrative Review
Abstract
1. Introduction
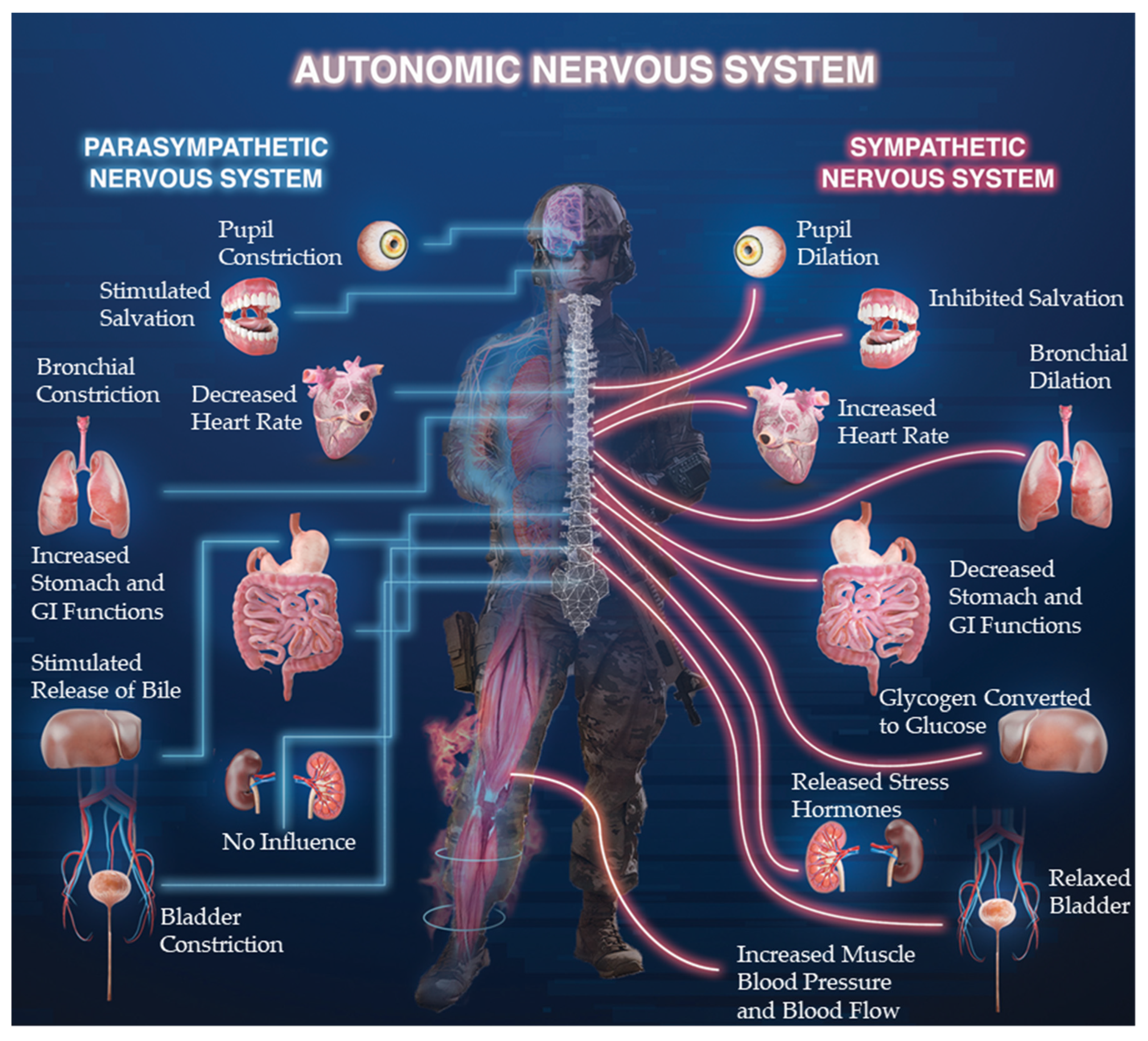
2. Autonomic Nervous System (ANS)
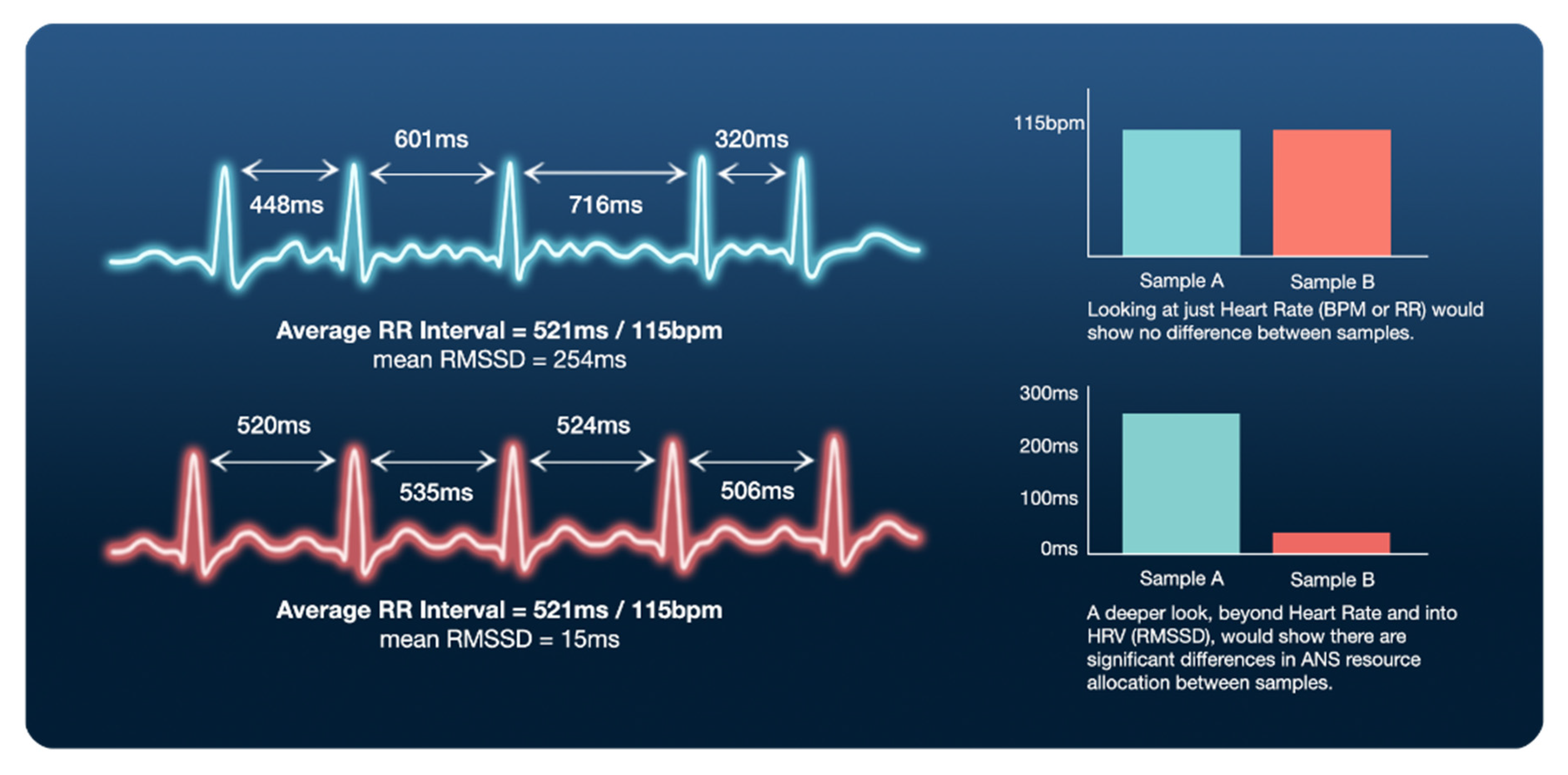
3. Defining Heart Rate Variability (HRV) and Respective Metrics
4. Implementation and Analysis of Heart Rate Variability (HRV) Measures
4.1. How to Measure HRV
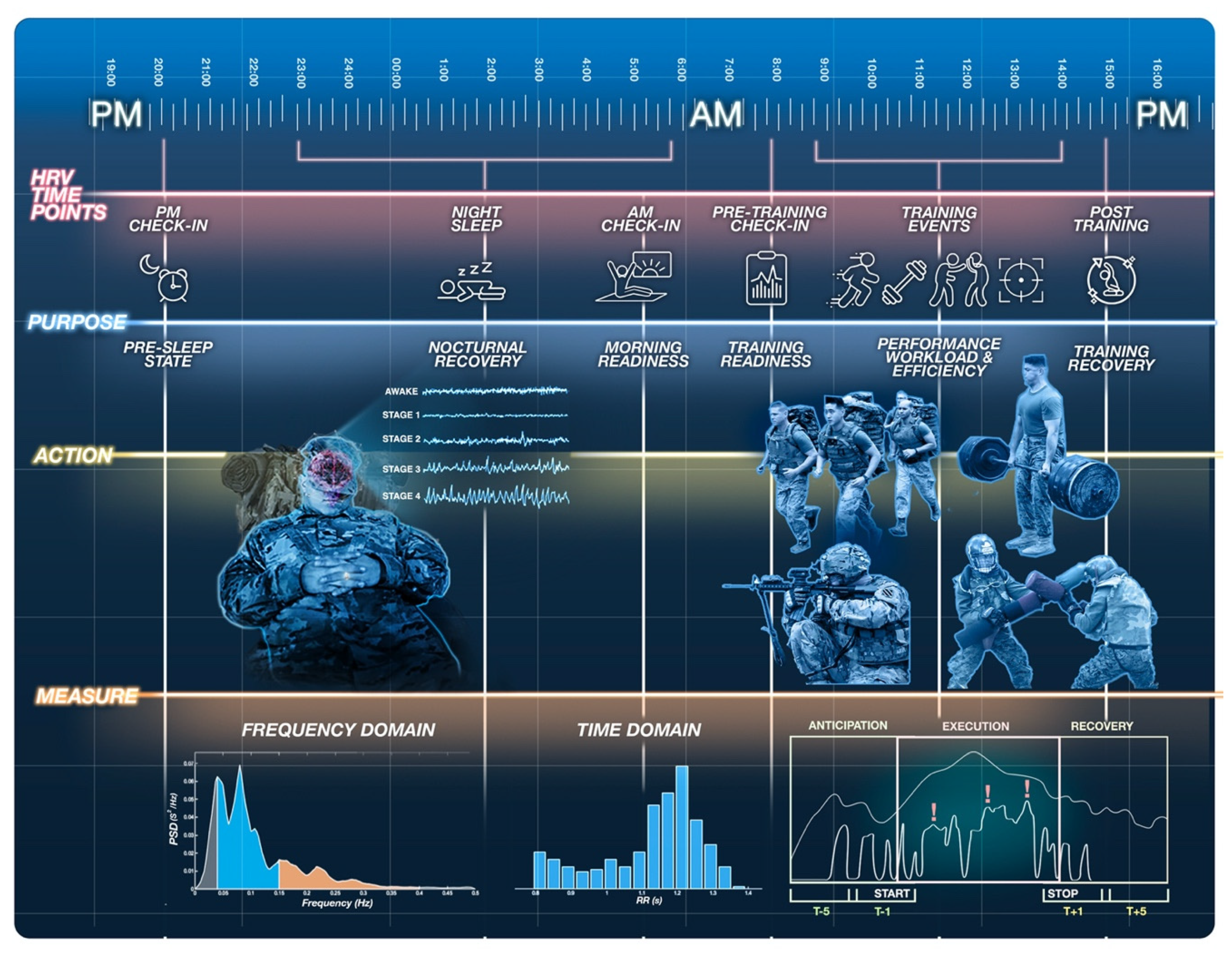
4.2. When to Measure HRV
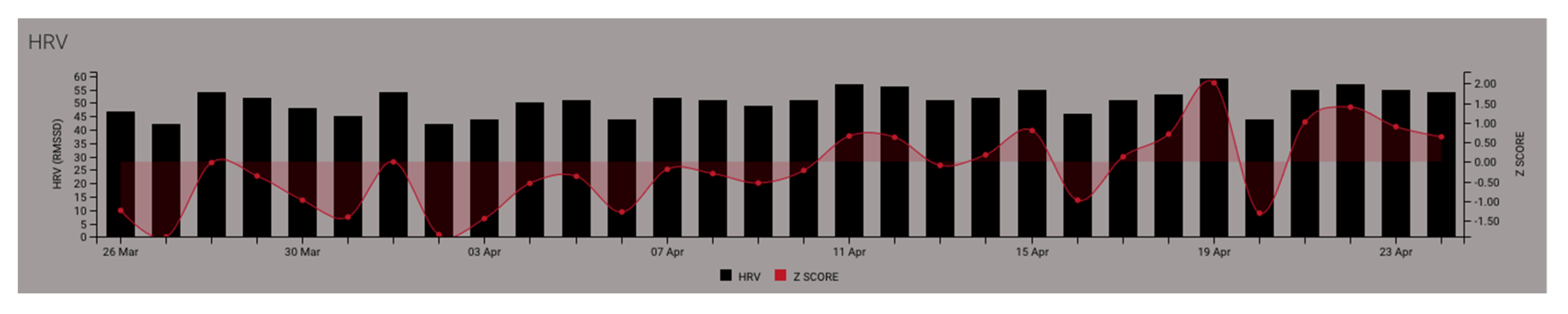
4.2.1. Establishing Baseline HRV Measures
4.2.2. Nocturnal and Morning HRV Measures
4.2.3. Pre-, During-, and Post-Event HRV Measures
4.2.4. Recording 24 h HRV
5. Monitoring Heart Rate Variability (HRV) in Tactical Populations
5.1. Relations between HRV and Stress
5.2. Relations between HRV and Physical Occupational Performance and Training Loads
5.3. Relations between HRV and Cognitive/Motor Skill Performance
5.4. Recovering to Restore HRV
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jonas, W.B.; O’Connor, F.G.; Deuster, P.; Peck, J.; Shake, C.; Frost, S.S. Why total force fitness? Mil. Med. 2010, 175, 6–13. [Google Scholar] [CrossRef]
- Huovinen, J.; Kyrolainen, H.; Linnamo, V.; Tanskanen, M.; Kinnunen, H.; Hakkinen, K.; Tulppo, M. Cardiac autonomic function reveals adaptation to military training. Eur. J. Sport Sci. 2011, 11, 231–240. [Google Scholar] [CrossRef]
- Sporiš, G.; Harasin, D.; Bok, D.; Matika, D.; Vuleta, D. Effects of a training program for special operations battalion on soldiers’ fitness characteristics. J. Strength Cond. Res. 2012, 26, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R. Physical preparation methods for combat operations: A narrative review of the literature. J. Aust. Strength Cond. 2014, 22, 44–49. [Google Scholar]
- Makivić, B.; Nikić Djordjević, M.; Willis, M.S. Heart Rate Variability (HRV) as a tool for diagnostic and monitoring performance in sport and physical activities. J. Exerc. Physiol. Online 2013, 16, 103–131. [Google Scholar]
- Jouanin, J.; Dussault, C.; Pérès, M.; Satabin, P.; Piérard, C.; Guézennec, C.Y.; Jouanin, J.-C.; Dussault, C.; Pérès, M.; Satabin, P.; et al. Analysis of heart rate variability after a ranger training course. Mil. Med. 2004, 169, 583–587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maupin, D.; Schram, B.; Orr, R. Tracking training load and its implementation in tactical populations: A narrative review. Strength Cond. J. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Deus, L.A.; Sousa, C.V.; Rosa, T.S.; Souto Filho, J.M.; Santos, P.A.; Barbosa, L.D.; Aguiar, S.S.; Souza, L.H.R.; Simões, H.G. Heart rate variability in middle-aged sprint and endurance athletes. Physiol. Behav. 2019, 205, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.J.; Mora, J.A.M. Effect of heart rate variability biofeedback on sport performance, a systematic review. Appl. Psychophys. Biofeedback 2017, 42, 235–245. [Google Scholar] [CrossRef]
- Wheat, A.L.; Larkin, K.T. Biofeedback of heart rate variability and related physiology: A critical review. Appl. Psychophys. Biofeedback 2010, 35, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Faw, B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: A tutorial review. Conscious. Cogn. 2003, 12, 83–139. [Google Scholar] [CrossRef]
- Bourdon, P.C.; Cardinale, M.; Murray, A.; Gastin, P.; Kellmann, M.; Varley, M.C.; Gabbett, T.J.; Coutts, A.J.; Burgess, D.J.; Gregson, W.; et al. Monitoring athlete training loads: Consensus statement. Int. J. Sports Physiol. Perform. 2017, 12, S2161–S2170. [Google Scholar] [CrossRef] [PubMed]
- Canino, M.C.; Foulis, S.A.; Cohen, B.S.; Walker, L.A.; Taylor, K.M.; Redmond, J.E.; Sharp, M.A. Quantifying training load during physically demanding tasks in US Army Soldiers: A comparison of physiological and psychological measurements. Mil. Med. 2020, 185, e847–e852. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, F.; Nakamura, F.Y.; Dos-Santos, J.W.; Batista, D.R.; Meneghel, V.; Nogueira, W.J.; Brigatto, F.A.; Germano, M.D.; Sindorf, M.A.G.; Moreno, M.A.; et al. Daily monitoring of the internal training load by the heart rate variability: A case study. J. Exerc. Physiol. Online 2017, 20, 151–163. [Google Scholar]
- Fields, J.B.; Esco, M.R.; Merrigan, J.J.; White, J.B.; Jones, M.T. Internal training load measures during a competitive season in collegiate women lacrosse athletes. Int. J. Exerc. Sci. 2020, 13, 778–788. [Google Scholar] [PubMed]
- Fields, J.B.; Lameira, D.M.; Short, J.L.; Merrigan, J.M.; Gallo, S.; White, J.B.; Jones, M.T. Relationship between external load and self-reported wellness measures across a men’s collegiate soccer preseason. J. Strength Cond. Res. 2021, 35, 1182–1186. [Google Scholar] [CrossRef]
- Schneider, C.; Wiewelhove, T.; Raeder, C.; Flatt, A.A.; Hoos, O.; Hottenrott, L.; Schumbera, O.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; et al. Heart rate variability monitoring during strength and high-intensity interval training overload microcycles. Front. Physiol. 2019, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Pumprla, J.; Howorka, K.; Groves, D.; Chester, M.; Nolan, J. Functional assessment of heart rate variability: Physiological basis and practical applications. Int. J. Cardiol. 2002, 84, 1–14. [Google Scholar] [CrossRef]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.E.; Siri, L.N.; Cardinali, D.P. Heart Rate Variability: A tool to explore autonomic nervous system activity in health and disease. In Psychiatry and Neuroscience Update; Springer: Berlin/Heidelberg, Germany, 2019; pp. 113–126. [Google Scholar]
- Hoareau, V.; Godin, C.; Dutheil, F.; Trousselard, M. The Effect of stress management programs on physiological and psychological components of stress: The influence of baseline physiological state. Appl. Psychophys. Biofeedback 2021, 1–8. [Google Scholar] [CrossRef]
- Fogt, D.L.; Cooper, P.J.; Freeman, C.N.; Kalns, J.E.; Cooke, W.H. Heart rate variability to assess combat readiness. Mil. Med. 2009, 174, 491–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huovinen, J.; Tulppo, M.; Nissilä, J.; Linnamo, V.; Häkkinen, K.; Kyrolainen, H. Relationship between heart rate variability and the serum testosterone-to-cortisol ratio during military service. Eur. J. Sport Sci. 2009, 9, 277–284. [Google Scholar] [CrossRef]
- Taylor, M.K.; Gould, D.R.; Adams, B.D.; Potterat, E.G.; Dial Ward, M.D.; Padilla, G.A.; Evans, K.E.; Markham, A.E. Age-Matched Comparison of Elite and Non-Elite Military Performers during Free Living and Intense Operational Stress; Naval Health Research Center: San Diego, CA, USA, 2009; Available online: https://apps.dtic.mil/sti/citations/ADA499987 (accessed on 3 February 2021).
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5. [Google Scholar] [CrossRef]
- Bota, A.; Urzeală, C.; Courteix, D. Heart rate variability—A tool for analysing the autonomic regulation of the cardiac function in sports. Discobolul 2019, 57, 23. [Google Scholar]
- Souza, R.A.; Beltran, O.A.B.; Zapata, D.M.; Silva, E.; Freitas, W.Z.; Junior, R.V.; da Silva, F.F.; Higino, W.P. Heart rate variability, salivary cortisol and competitive state anxiety responses during pre-competition and pre-training moments. Biol. Sport 2019, 36, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tomes, C.; Schram, B.; Orr, R. Relationships between Heart Rate Variability, Occupational Performance, and Fitness for Tactical Personnel: A Systematic Review. Front. Public Health 2020, 8, 583336. [Google Scholar] [CrossRef]
- Board, E.M.; Ispoglou, T.; Ingle, L. Validity of telemetric-derived measures of heart rate variability: A systematic review. J. Exerc. Physiol. Online 2016, 19, 64–84. [Google Scholar]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.; Koo, B.H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Sánchez, Á.; Tornero-Aguilera, J.F.; Fernández-Elías, V.E.; Hormeño-Holgado, A.J.; Dalamitros, A.A.; Clemente-Suárez, V.J. Effect of stress on autonomic and cardiovascular systems in military population: A systematic review. Cardiol. Res. Pr. 2020, 2020, 7986249. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Sollers, J.J.; Stenvik, K.; Thayer, J.F. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur. J. Appl. Physiol. 2004, 93, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Elliot, A.J.; Payen, V.; Brisswalter, J.; Cury, F.; Thayer, J.F. A subtle threat cue, heart rate variability, and cognitive performance. Psychophysiology 2011, 48, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.G.; Swain, D.P.; Branch, J.D.; Spina, R.J.; Grieco, C.R. Autonomic response to tactical pistol performance measured by heart rate variability. J. Strength Cond. Res. 2015, 29, 926–933. [Google Scholar] [CrossRef] [PubMed]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 7, 178. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef]
- Vitale, J.A.; Bonato, M.; La Torre, A.; Banfi, G. Heart rate variability in sport performance: Do time of day and chronotype play a role? J. Clin. Med. 2019, 8, 723. [Google Scholar] [CrossRef]
- Aubert, A.E.; Seps, B.; Beckers, F. Heart rate variability in athletes. Sports Med. 2003, 33, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Sztajzel, J. Heart rate variability: A noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med. Wkly. 2004, 134, 514–522. [Google Scholar]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology 1995, 32, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Gancitano, G.; Baldassarre, A.; Lecca, L.I.; Mucci, N.; Petranelli, M.; Nicolia, M.; Brancazio, A.; Tessarolo, A.; Arcangeli, G. HRV in active-duty special forces and public order military personnel. Sustainability 2021, 13, 3867. [Google Scholar] [CrossRef]
- Park, G.; Vasey, M.W.; Van Bavel, J.J.; Thayer, J.F. When tonic cardiac vagal tone predicts changes in phasic vagal tone: The role of fear and perceptual load. Psychophysiology 2014, 51, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes: Opening the door to effective monitoring. Sports Med. 2013, 43, 773–781. [Google Scholar] [CrossRef]
- Williams, S.; West, S.; Howells, D.; Kemp, S.P.; Flatt, A.A.; Stokes, K. Modelling the HRV response to training loads in elite rugby sevens players. J. Sports Sci. Med. 2018, 17, 402. [Google Scholar]
- Minassian, A.; Geyer, M.A.; Baker, D.G.; Nievergelt, C.M.; O’Connor, D.T.; Risbrough, V.B.; Team, M. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom. Med. 2014, 76, 292. [Google Scholar] [CrossRef] [PubMed]
- Massaro, S.; Pecchia, L. Heart rate variability (HRV) analysis: A methodology for organizational neuroscience. Organ. Res. Methods 2019, 22, 354–393. [Google Scholar] [CrossRef]
- Ernst, G. Heart-rate variability—More than heart beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Aynés, O.; López-Plaza, D.; Alacid, F.; Naranjo-Orellana, J.; Manonelles, P. Recovery of heart rate variability after exercise under hot conditions: The effect of relative humidity. Wilderness Environ. Med. 2019, 30, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Udayanga, M.S. Heart Rate Variability (HRV) for sports and exercise training. Sri Lankan J. Sports Exerc. Med. 2018, 1, 13–18. [Google Scholar] [CrossRef]
- Baevsky, R.M.; Chernikova, A.G. Heart rate variability analysis: Physiological foundations and main methods. Cardiometry 2017, 10, 66–76. [Google Scholar] [CrossRef]
- Zygmunt, A.; Stanczyk, J. Methods of evaluation of autonomic nervous system function. Arch. Med. Sci. 2010, 6, 11–18. [Google Scholar] [CrossRef]
- Saboul, D.; Hautier, C. A new algorithm to reduce and individualize HRV recording time. J. Med. Syst. 2019, 43, 45. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Goldstein, D.S.; Eldadah, B.A.; Saleem, A.; Holmes, C.; Pechnik, S.; Sharabi, Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 2007, 4, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Napadow, V.; Dhond, R.; Conti, G.; Makris, N.; Brown, E.N.; Barbieri, R. Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. Neuroimage 2008, 42, 169–177. [Google Scholar] [CrossRef]
- Forte, G.; Casagrande, M. Heart rate variability and cognitive function: A systematic review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A.; Aikins, D.E.; Steffian, G.; Coric, V.; Southwick, S. Relation between cardiac vagal tone and performance in male military personnel exposed to high stress: Three prospective studies. Psychophysiology 2007, 44, 120–127. [Google Scholar] [CrossRef]
- Scherer, M.; Martinek, J.; Mayr, W. HRV (Heart Rate Variability) as a non-invasive measurement method for performance diagnostics and training control. Curr. Dir. Biomed. Eng. 2019, 5, 97–100. [Google Scholar] [CrossRef]
- Ako, M.; Kawara, T.; Uchida, S.; Miyazaki, S.; Nishihara, K.; Mukai, J.; Hirao, K.; Ako, J.; Okubo, Y. Correlation between electroencephalography and heart rate variability during sleep. Psychiatry Clin. Neurosci. 2003, 57, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, M.T.; Artoni, F.; Faraguna, U. Heart rate variability at bedtime predicts subsequent sleep features. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6784–6788. [Google Scholar]
- von Rosenberg, W.; Chanwimalueang, T.; Adjei, T.; Jaffer, U.; Goverdovsky, V.; Mandic, D.P. Resolving ambiguities in the LF/HF Ratio: LF-HF scatter plots for the categorization of mental and physical stress from HRV. Front. Physiol. 2017, 8, 360. [Google Scholar] [CrossRef]
- Mendonca, G.V.; Fernhall, B.; Heffernan, K.S.; Pereira, F.D. Spectral methods of heart rate variability analysis during dynamic exercise. Clin. Auton. Res. 2009, 19, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Lombardi, F.; Pagani, M. Power spectrum analysis of heart rate variability: A tool to explore neural regulatory mechanisms. Br. Heart J. 1994, 71, 1. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Valkama, J.O.; Airaksinen, K.; Seppänen, T.; Kessler, K.M.; Takkunen, J.T.; Myerburg, R.J. Frequency domain measures of heart rate variability before the onset of nonsustained and sustained ventricular tachycardia in patients with coronary artery disease. Circulation 1993, 87, 1220–1228. [Google Scholar] [CrossRef]
- Kinnunen, H.O.; Rantanen, A.; Kenttä, T.V.; Koskimäki, H. Feasible assessment of recovery and cardiovascular health: Accuracy of nocturnal HR and HRV assessed via ring PPG in comparison to medical grade ECG. Physiol. Meas. 2020, 41, 04NT01. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Stone, J.D.; Ulman, H.K.; Tran, K.; Thompson, A.G.; Halter, M.D.; Ramadan, J.H.; Stephenson, M.; Finomore, V.S.; Galster, S.M.; Rezai, A.R.; et al. Assessing the accuracy of popular commercial technologies that measure resting heart rate and heart rate variability. Front. Sports Act. Living 2021, 3, 37. [Google Scholar] [CrossRef]
- Hinde, K.; White, G.; Armstrong, N. Wearable devices suitable for monitoring twenty four hour heart rate variability in military populations. Sensors 2021, 21, 1061. [Google Scholar] [CrossRef]
- Stone, J.D.; Rentz, L.E.; Forsey, J.; Ramadan, J.; Markwald, R.R.; Finomore, V.S. Evaluations of commercial sleep technologies for objective monitoring during routine sleeping conditions. Nat. Sci. Sleep 2020, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.E.; Semple, S.; Naumovski, N.; McKune, A.J. Measuring heart rate variability using commercially available devices in healthy children: A Validity and Reliability Study. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 390–404. [Google Scholar] [CrossRef]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable devices accurately measure heart rate variability? A systematic review. Folia Med. 2018, 60, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Plews, D.; Scott, B.; Altini, M.; Wood, M.; Kilding, A.; Laursen, P. Comparison of heart rate variability recording with smart phone photoplethysmographic, Polar H7 Chest Strap and Electrocardiogram methods. Int. J. Sports Physiol. Perform. 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Young, F.L.; Leicht, A.S. Short-term stability of resting heart rate variability: Influence of position and gender. Appl. Physiol. Nutr. Metab. 2011, 36, 210–218. [Google Scholar] [CrossRef]
- Kahneman, D. Attention and Effort; Prentice-Hall: Upper Saddle River, NJ, USA, 1973. [Google Scholar]
- Sanders, A.F. Towards a model of stress and human performance. Acta Psychol. 1983, 74, 123–167. [Google Scholar] [CrossRef]
- Saboul, D.; Balducci, P.; Millet, G.; Pialoux, V.; Hautier, C. A pilot study on quantification of training load: The use of HRV in training practice. Eur. J. Sport Sci. 2016, 16, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Lambertz, M.; Nelesen, R.; Bardwell, W.; Choi, J.-B.; Dimsdale, J. Effects of stress on heart rate complexity—A comparison between short-term and chronic stress. Biol. Psychol. 2009, 80, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, C.R.; Fuller, J.T.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. Monitoring athletic training status through autonomic heart rate regulation: A systematic review and meta-analysis. Sports Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef] [PubMed]
- Israel, S. Problems of overtraining from an internal medical and performance physiological standpoint. Med. Sport 1976, 16, 1–12. [Google Scholar]
- Kuipers, H. Training and overtraining: An introduction. Med. Sci. Sports Exerc. 1998, 30, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, E.; Vesterinen, V.; Rusko, H.; Nummela, A. Effects of moderate and heavy endurance exercise on nocturnal HRV. Int. J. Sports Med. 2010, 31, 428–432. [Google Scholar] [CrossRef]
- Herzig, D.; Testorelli, M.; Schäfer Olstad, D.; Erlacher, D.; Achermann, P.; Eser, P.; Wilhelm, M. Heart-rate variability during deep sleep in world-class alpine skiers: A time-efficient alternative to morning supine measurements. Int. J. Sports Physiol. Perform. 2017, 12, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Bless, H.; Blomann, F.; Kleinböhl, D. Physiological aspects of flow experiences: Skills-demand-compatibility effects on heart rate variability and salivary cortisol. J. Exp. Soc. Psychol. 2011, 47, 849–852. [Google Scholar] [CrossRef]
- Mullen, R.; Faull, A.; Jones, E.S.; Kingston, K. Attentional focus and performance anxiety: Effects on simulated race-driving performance and heart rate variability. Front. Psychol. 2012, 3, 426. [Google Scholar] [CrossRef]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal influence on working memory and attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef]
- Maupin, D.; Wills, T.; Orr, R.; Schram, B. Fitness profiles in elite tactical units: A critical review. Int. J. Exerc. Sci. 2018, 11, 1041. [Google Scholar] [PubMed]
- de Visser, E.J.; Dorfman, A.; Chartrand, D.; Lamon, J.; Freedy, E.; Weltman, G. Building resilience with the Stress Resilience Training System: Design validation and applications. Work 2016, 54, 351–366. [Google Scholar] [CrossRef]
- Movius, H.L.; Allen, J.J. Cardiac vagal tone, defensiveness, and motivational style. Biol. Psychol. 2005, 68, 147–162. [Google Scholar] [CrossRef]
- Grossman, P.; Taylor, E.W. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Regnard, J.; Millet, G.P. Monitoring fatigue status with HRV Measures in elite athletes: An avenue beyond RMSSD? Front. Physiol. 2015, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Ravé, G.; Zouhal, H.; Boullosa, D.; Doyle-Baker, P.K.; Saeidi, A.; Abderrahman, A.B.; Fortrat, J.-O. Heart Rate Variability is Correlated with Perceived Physical Fitness in Elite Soccer Players. J. Hum. Kinet. 2020, 72, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.D.; Verri, S.M.; Nakamura, F.Y.; Machado, F.A. Longitudinal changes in cardiac autonomic function and aerobic fitness indices in endurance runners: A case study with a high-level team. Eur. J. Sport Sci. 2014, 14, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Nuuttila, O.P.; Nikander, A.; Polomoshnov, D.; Laukkanen, J.A.; Häkkinen, K. Effects of HRV-Guided vs. Predetermined Block Training on Performance, HRV and Serum Hormones. Int. J. Sports Med. 2017, 38, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Merrigan, J.J.; Stone, J.D.; Thompson, A.G.; Hornsby, W.G.; Hagen, J.A. Monitoring Neuromuscular Performance in Military Personnel. Int. J. Environ. Res. Public Health 2020, 17, 9147. [Google Scholar] [CrossRef]
- Porges, S.W. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J., III; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.C.; Paulus, M.P.; Simmons, A.N.; Nelesen, R.A.; Dimsdale, J.E. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage 2004, 22, 1151–1156. [Google Scholar] [CrossRef]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability during emotion. Neuroimage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Coping 2009, 22, 77–89. [Google Scholar] [CrossRef]
- Spangler, D.P.; Gamble, K.R.; McGinley, J.J.; Thayer, J.F.; Brooks, J.R. Intra-individual variability in vagal control is associated with response inhibition under stress. Front. Hum. Neurosci. 2018, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Ortega, A.R.; Del Paso, G.A.R. Anxiety, attention, and decision making: The moderating role of heart rate variability. Int. J. Psychophysiol. 2015, 98, 490–496. [Google Scholar] [CrossRef]
- Pojman, N.; Behneman, A.; Kintz, N.; Johnson, R.; Chung, G.; Nagashima, S.; Espinosa, P.; Berka, C. Characterizing the psychophysiological profile of expert and novice marksmen. In Proceedings of the International Conference on Foundations of Augmented Cognition, San Diego, CA, USA, 19–24 July 2009; pp. 524–532. [Google Scholar]
- Nugent, A.C.; Bain, E.E.; Thayer, J.F.; Sollers, J.J., III; Drevets, W.C. Heart rate variability during motor and cognitive tasks in females with major depressive disorder. Psychiatry Res. Neuroimaging 2011, 191, 1–8. [Google Scholar] [CrossRef]
- Prinsloo, G.E.; Rauch, H.L.; Derman, W.E. A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Physician Sportsmed. 2014, 42, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Mogenson, G.J.; Jones, D.L.; Yim, C.Y. From motivation to action: Functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980, 14, 69–97. [Google Scholar] [CrossRef]
- Pavuluri, M.; May, A. I feel, therefore, I am: The insula and its role in human emotion, cognition and the sensory-motor system. AIMS Neurosci. 2015, 2, 18–27. [Google Scholar] [CrossRef]
- Di Nota, P.M.; Huhta, J.-M. Complex motor learning and police training: Applied, cognitive, and clinical perspectives. Front. Psychol. 2019, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Jaquess, K.J.; Lo, L.-C.; Oh, H.; Lu, C.; Ginsberg, A.; Tan, Y.Y.; Lohse, K.R.; Miller, M.W.; Hatfield, B.D.; Gentili, R.J. Changes in mental workload and motor performance throughout multiple practice sessions under various levels of task difficulty. Neuroscience 2018, 393, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.; Bennell, C.; Andersen, J.P.; Semple, T.; Jenkins, B. Stress-activity mapping: Physiological responses during general duty police encounters. Front. Psychol. 2019, 10, 2216. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S.; Di Nota, P.M.; Metz, G.A.S.; Andersen, J.P. The impact of acute stress physiology on skilled motor performance: Implications for policing. Front. Psychol. 2019, 10, 2501. [Google Scholar] [CrossRef] [PubMed]
- Vincze, J.; Vincze-Tiszay, G. The physiological aspects of the stress. J. Med. Biomed. Appl. Sci. 2020, 8, 529–534. [Google Scholar]
- Mosley, E.; Laborde, S.; Kavanagh, E. Coping related variables, cardiac vagal activity and working memory performance under pressure. Acta Psychol. 2018, 191, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J. Recovery and performance in sport: Consensus statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Soligard, T.; Schwellnus, M.; Alonso, J.-M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R. How much is too much? (Part 1) International Olympic Committee consensus statement on load in sport and risk of injury. Br. J. Sports Med. 2016, 50, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Dancy, C.; Goldberg, B.; Sottilare, R. A cognitive modeling approach-does tactical breathing in a psychomotor task influence skill development during adaptive instruction? In International Conference on Augmented Cognition; Springer: Cham, Switzerland, 2017; pp. 162–174. [Google Scholar]
- Almeida, A.C.; Machado, A.F.; Albuquerque, M.C.; Netto, L.M.; Vanderlei, F.M.; Vanderlei, L.C.M.; Junior, J.N.; Pastre, C.M. The effects of cold water immersion with different dosages (duration and temperature variations) on heart rate variability post-exercise recovery: A randomized controlled trial. J. Sci. Med. Sport 2016, 19, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, M.; Watson, G.; Abbiss, C.R. What are the physiological mechanisms for post-exercise cold water immersion in the recovery from prolonged endurance and intermittent exercise? Sports Med. 2016, 46, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, A.; Norell-Clarke, A.; Jonsson, K.; Tillfors, M. Does flotation-rest (restricted environmental stimulation technique) have an effect on sleep? Eur. J. Integr. Med. 2020, 33, 101047. [Google Scholar] [CrossRef]
- Feinstein, J.; Khalsa, S.; Yeh, H.-W.; Wohlrab, C.; Simmons, W.; Stein, M.; Paulus, M. Examining the short-term anxiolytic and antidepressant effect of Floatation-REST. PLoS ONE 2018, 13, e0190292. [Google Scholar] [CrossRef]
- Louis, J.; Theurot, D.; Filliard, J.R.; Volondat, M.; Dugué, B.; Dupuy, O. The use of whole-body cryotherapy: Time-and dose-response investigation on circulating blood catecholamines and heart rate variability. Eur. J. Appl. Physiol. 2020, 120, 1733–1743. [Google Scholar] [CrossRef]
- Bouzigon, R.; Grappe, F.; Ravier, G.; Dugue, B. Whole-and partial-body cryostimulation/cryotherapy: Current technologies and practical applications. J. Therm. Biol. 2016, 61, 67–81. [Google Scholar] [CrossRef]
- Zalewski, P.; Bitner, A.; Słomko, J.; Szrajda, J.; Klawe, J.J.; Tafil-Klawe, M.; Newton, J.L. Whole-body cryostimulation increases parasympathetic outflow and decreases core body temperature. J. Therm. Biol. 2014, 45, 75–80. [Google Scholar] [CrossRef]
- Douzi, W.; Dupuy, O.; Tanneau, M.; Boucard, G.; Bouzigon, R.; Dugué, B. 3-min whole body cryotherapy/cryostimulation after training in the evening improves sleep quality in physically active men. Eur. J. Sport Sci. 2019, 19, 860–867. [Google Scholar] [CrossRef]
- Douzi, W.; Dupuy, O.; Theurot, D.; Boucard, G.; Dugué, B. Partial-body cryostimulation after training improves sleep quality in professional soccer players. BMC Res. Notes 2019, 12, 141. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Arena, R.; Dutra, D.B.; de Cassia Marqueti Durigan, R.; de Araujo, H.S.; de Souza, H.C.D.; Parizotto, N.A.; Cipriano Jr, G.; Chiappa, G.; Borghi-Silva, A. Low-level laser therapy associated with high intensity resistance training on cardiac autonomic control of heart rate and skeletal muscle remodeling in wistar rats. Lasers Surg. Med. 2014, 46, 796–803. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Huang, Y.Y.; Hamblin, M.R. Photobiomodulation in human muscle tissue: An advantage in sports performance? J. Biophotonics 2016, 9, 1273–1299. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, Y.; Nie, J.; Xu, J.; Liu, D. Red light and the sleep quality and endurance performance of Chinese female basketball players. J. Athl. Train. 2012, 47, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-D.; Rau, C.-L.; Liou, T.-H.; Tsauo, J.-Y.; Lin, L.-F. Effects of linearly polarized near-infrared irradiation near the stellate ganglion region on pain and heart rate variability in patients with neuropathic pain. Pain Med. 2016, 18, 488–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortega, E.; Wang, C. Pre-performance physiological state: Heart rate variability as a predictor of shooting performance. Appl. Psychophys. Biofeedback 2018, 43, 75–85. [Google Scholar] [CrossRef]
- Reardon, M.; Malik, M. Changes in heart rate variability with age. Pacing Clin. Electrophysiol. 1996, 19, 1863–1866. [Google Scholar] [CrossRef] [PubMed]
- Antelmi, I.; De Paula, R.S.; Shinzato, A.R.; Peres, C.A.; Mansur, A.J.; Grupi, C.J. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am. J. Cardiol. 2004, 93, 381–385. [Google Scholar] [CrossRef] [PubMed]
| Time Domains in Short-Term Recordings | |||
| Index | Definition | Interpretation | Correlates |
| SDNN (ms) | Standard deviation of all R-R intervals | Global quantification of HRV | Total Power |
| rMSSD (ms) | Root-mean square of successive differences between R-R intervals in a specified time segment | Vagal tone | High Frequency, Parasympathetic activity |
| Basic Frequency Domains | |||
| Index | Definition | Interpretation | Correlates |
| VLF (ms2) | Power in the very-low frequency range (<0.04 Hz) | Hormonal factors and peripheral thermoregulation origination | Parasympathetic activity |
| LF (ms2) | Power in the low frequency range (0.04–0.15 Hz) | Baroreflex, arousal | Sympathetic activity, Parasympathetic activity |
| HF (ms2) | Power in the high frequency range (0.15–0.4 Hz) | Cardiopulmonary reflex, cognitive regulatory state, dependent on resource availability and interpretation of environmental demands | Parasympathetic activity |
| LF/HF | Low frequency/high frequency ratio | Sympathetic-parasympathetic balance (assuming known LF) | Sympathetic activity, Parasympathetic activity |
| Total Power | Total power in the entire frequency range (<0.4 Hz) | General autonomic resource allocation | Sympathetic activity. Parasympathetic activity |
| HF/Total Power | High frequency/total power ratio | Proportion of parasympathetic to total autonomic resources | Parasympathetic activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephenson, M.D.; Thompson, A.G.; Merrigan, J.J.; Stone, J.D.; Hagen, J.A. Applying Heart Rate Variability to Monitor Health and Performance in Tactical Personnel: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 8143. https://doi.org/10.3390/ijerph18158143
Stephenson MD, Thompson AG, Merrigan JJ, Stone JD, Hagen JA. Applying Heart Rate Variability to Monitor Health and Performance in Tactical Personnel: A Narrative Review. International Journal of Environmental Research and Public Health. 2021; 18(15):8143. https://doi.org/10.3390/ijerph18158143
Chicago/Turabian StyleStephenson, Mark D., Andrew G. Thompson, Justin J. Merrigan, Jason D. Stone, and Joshua A. Hagen. 2021. "Applying Heart Rate Variability to Monitor Health and Performance in Tactical Personnel: A Narrative Review" International Journal of Environmental Research and Public Health 18, no. 15: 8143. https://doi.org/10.3390/ijerph18158143
APA StyleStephenson, M. D., Thompson, A. G., Merrigan, J. J., Stone, J. D., & Hagen, J. A. (2021). Applying Heart Rate Variability to Monitor Health and Performance in Tactical Personnel: A Narrative Review. International Journal of Environmental Research and Public Health, 18(15), 8143. https://doi.org/10.3390/ijerph18158143







