Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study
Abstract
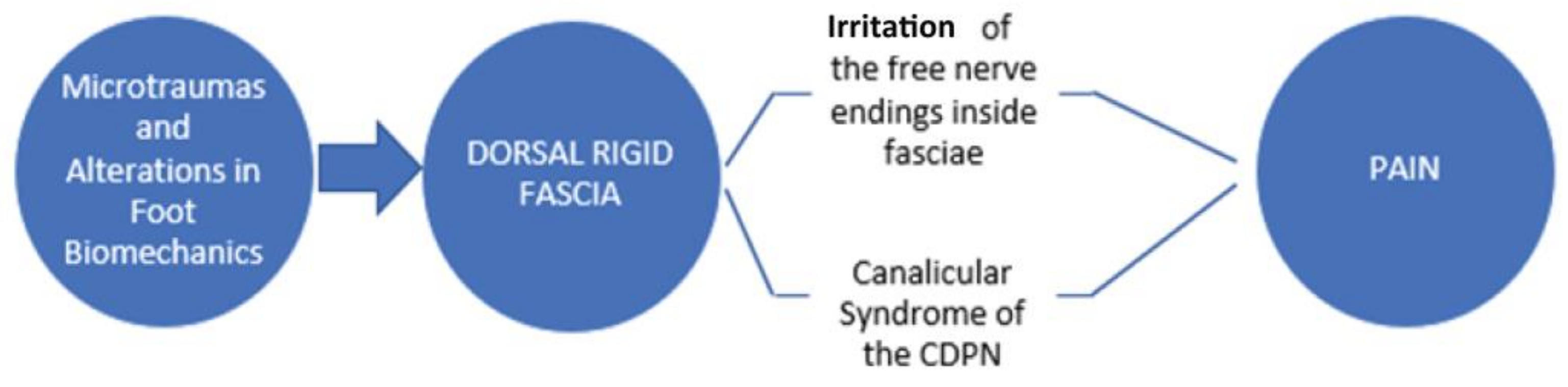
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Fascial Manipulation
2.3. Treatment Protocol
2.4. Patient Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, B.G.; Hurn, S.E.; Harding, M.P.; Henry, R.A.; Ware, R.S. The Effectiveness of Non-Surgical Interventions for Common Plantar Digital Compressive Neuropathy (Morton’s Neuroma): A Systematic Review and Meta-Analysis. J. Foot Ankle Res. 2019, 12, 12. [Google Scholar] [CrossRef]
- Di Caprio, F.; Meringolo, R.; Shehab Eddine, M.; Ponziani, L. Morton’s Interdigital Neuroma of the Foot: A Literature Review. Foot Ankle Surg. 2018, 24, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Morton, T.G. A Peculiar and Painful Affection of the Fourth Metatarso-Phalangeal Articulation. Am. J. Med. Sci. 1876, 71, 37–45. [Google Scholar] [CrossRef]
- Larson, E.E.; Barrett, S.L.; Battiston, B.; Maloney, C.T.; Dellon, A.L. Accurate Nomenclature for Forefoot Nerve Entrapment: A Historical Perspective. J. Am. Podiatr. Med. Assoc. 2005, 95, 298–306. [Google Scholar] [CrossRef]
- Pisani, G. Is It Morton’s or Civinini’s Syndrome? Foot Ankle Surg. 2010, 16, 105–106. [Google Scholar] [CrossRef]
- Lu, V.M.; Spinner, R.J. Morton’s Neuroma as a Surgical Example of Entrapment Syndrome. J. Plast. Reconstr. Aesth. Surg. JPRAS 2020, 73, 1105–1106. [Google Scholar] [CrossRef]
- Bhatia, M.; Thomson, L. Morton’s Neuroma-Current Concepts Review. J. Clin. Orthop. Trauma 2020, 406–409. [Google Scholar] [CrossRef]
- Nijs, J.; Van Houdenhove, B. From Acute Musculoskeletal Pain to Chronic Widespread Pain and Fibromyalgia: Application of Pain Neurophysiology in Manual Therapy Practice. Man. Ther. 2009, 14, 3–12. [Google Scholar] [CrossRef]
- Rajput, K.; Reddy, S.; Shankar, H. Painful Neuromas. Clin. J. Pain 2012, 28, 639–645. [Google Scholar] [CrossRef]
- Pastides, P.; El-Sallakh, S.; Charalambides, C. Morton’s Neuroma: A Clinical versus Radiological Diagnosis. Foot Ankle Surg. 2012, 18, 22–24. [Google Scholar] [CrossRef]
- Thomson, C.E.; Gibson, J.A.; Martin, D. Interventions for the Treatment of Morton’s Neuroma. Cochrane Database Syst. Rev. 2004, 3. [Google Scholar] [CrossRef]
- Davis, F. Therapeutic Massage Provides Pain Relief to a Client with Morton’s Neuroma: A Case Report. Int. J. Ther. Massage Bodyw. 2012, 5, 12–19. [Google Scholar]
- Jain, S.; Mannan, K. The Diagnosis and Management of Morton’s Neuroma: A Literature Review. Foot Ankle Spec. 2013, 6, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, N.; Lampridis, V.; Sakellariou, A. Morton’s Interdigital Neuroma: Instructional Review. EFORT Open Rev. 2019, 4, 14–24. [Google Scholar] [CrossRef]
- Bignotti, B.; Signori, A.; Sormani, M.P.; Molfetta, L.; Martinoli, C.; Tagliafico, A. Ultrasound versus Magnetic Resonance Imaging for Morton Neuroma: Systematic Review and Meta-Analysis. Eur. Radiol. 2015, 25, 2254–2262. [Google Scholar] [CrossRef]
- Xu, Z.; Duan, X.; Yu, X.; Wang, H.; Dong, X.; Xiang, Z. The Accuracy of Ultrasonography and Magnetic Resonance Imaging for the Diagnosis of Morton’s Neuroma: A Systematic Review. Clin. Radiol. 2015, 70, 351–358. [Google Scholar] [CrossRef] [PubMed]
- DiPreta, J.A. Metatarsalgia, Lesser Toe Deformities, and Associated Disorders of the Forefoot. Med. Clin. N. Am. 2014, 98, 233–251. [Google Scholar] [CrossRef]
- Elghazy, M.A.; Whitelaw, K.C.; Waryasz, G.R.; Guss, D.; Johnson, A.H.; DiGiovanni, C.W. Isolated Intermetatarsal Ligament Release as Primary Operative Management for Morton’s Neuroma: Short-Term Results. Foot Ankle Spec. 2020, 1938640020957851. [Google Scholar] [CrossRef] [PubMed]
- Munir, U.; Tafti, D.; Morgan, S. Morton neuroma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Park, Y.H.; Jeong, S.M.; Choi, G.W.; Kim, H.J. The Role of the Width of the Forefoot in the Development of Morton’s Neuroma. Bone Jt. J. 2017, 99-B, 365–368. [Google Scholar] [CrossRef]
- Stecco, A.; Pirri, C.; Stecco, C. Fascial Entrapment Neuropathy. Clin. Anat. 2019, 32, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Fantoni, I.; Macchi, V.; Del Borrello, M.; Porzionato, A.; Biz, C.; De Caro, R. The Role of Fasciae in Civinini-Morton’s Syndrome. J. Anat. 2015, 227, 654–664. [Google Scholar] [CrossRef]
- Thomson, C.E.; Beggs, I.; Martin, D.J.; McMillan, D.; Edwards, R.T.; Russell, D.; Yeo, S.T.; Russell, I.T.; Gibson, J.N.A. Methylprednisolone Injections for the Treatment of Morton Neuroma: A Patient-Blinded Randomized Trial. J. Bone Jt. Surg. Am. 2013, 95 (Suppl. S1), 790–798. [Google Scholar] [CrossRef]
- Lizano-Díez, X.; Ginés-Cespedosa, A.; Alentorn-Geli, E.; Pérez-Prieto, D.; González-Lucena, G.; Gamba, C.; de Zabala, S.; Solano-López, A.; Rigol-Ramón, P. Corticosteroid Injection for the Treatment of Morton’s Neuroma: A Prospective, Double-Blinded, Randomized, Placebo-Controlled Trial. Foot Ankle Int. 2017, 38, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.; Aujla, R.S.; Divall, P.; Bhatia, M. Non-Surgical Treatments for Morton’s Neuroma: A Systematic Review. Foot Ankle Surg. 2020, 26, 736–743. [Google Scholar] [CrossRef]
- Govender, N.; Kretzmann, H.; Price, J.; Brantingham, J.; Globe, G. A Single-Blinded Randomized Placebo-Controlled Clinical Trial of Manipulation and Mobilization in the Treatment of Morton’s Neuroma. J. Am. Chiropr. Assoc. 2007, 44, 8–18. [Google Scholar]
- Cashley, D.G.; Cochrane, L. Manipulation in the Treatment of Plantar Digital Neuralgia: A Retrospective Study of 38 Cases. J. Chiropr. Med. 2015, 14, 90–98. [Google Scholar] [CrossRef][Green Version]
- Sault, J.D.; Morris, M.V.; Jayaseelan, D.J.; Emerson-Kavchak, A.J. Manual Therapy in the Management of a Patient with a Symptomatic Morton’s Neuroma: A Case Report. Man. Ther. 2016, 21, 307–310. [Google Scholar] [CrossRef]
- Pérez-Domínguez, B.; Casaña-Granell, J. The Effects of a Combined Physical Therapy Approach on Morton’s Neuroma. An N-of-1 Case Report. Foot 2020, 44, 101684. [Google Scholar] [CrossRef]
- Bucknall, V.; Rutherford, D.; MacDonald, D.; Shalaby, H.; McKinley, J.; Breusch, S.J. Outcomes Following Excision of Morton’s Interdigital Neuroma: A Prospective Study. Bone Jt. J. 2016, 98-B, 1376–1381. [Google Scholar] [CrossRef]
- Bauer, T.; Gaumetou, E.; Klouche, S.; Hardy, P.; Maffulli, N. Metatarsalgia and Morton’s Disease: Comparison of Outcomes Between Open Procedure and Neurectomy Versus Percutaneous Metatarsal Osteotomies and Ligament Release with a Minimum of 2 Years of Follow-Up. J. Foot Ankle Surg. 2015, 54, 373–377. [Google Scholar] [CrossRef]
- Valisena, S.; Petri, G.J.; Ferrero, A. Treatment of Morton’s Neuroma: A Systematic Review. Foot Ankle Surg. 2018, 24, 271–281. [Google Scholar] [CrossRef]
- Lee, K.T.; Kim, J.B.; Young, K.W.; Park, Y.U.; Kim, J.S.; Jegal, H. Long-Term Results of Neurectomy in the Treatment of Morton’s Neuroma: More than 10 Years’ Follow-Up. Foot Ankle Spec. 2011, 4, 349–353. [Google Scholar] [CrossRef]
- Casato, G.; Stecco, C.; Busin, R. Role of Fasciae in Nonspecific Low Back Pain. Eur. J. Transl. Myol. 2019, 29, 8330. [Google Scholar] [CrossRef] [PubMed]
- Branchini, M.; Lopopolo, F.; Andreoli, E.; Loreti, I.; Marchand, A.M.; Stecco, A. Fascial Manipulation® for Chronic Aspecific Low Back Pain: A Single Blinded Randomized Controlled Trial. F1000Research 2015, 4, 1208. [Google Scholar] [CrossRef]
- Harper, B.; Steinbeck, L.; Aron, A. Fascial Manipulation vs. Standard Physical Therapy Practice for Low Back Pain Diagnoses: A Pragmatic Study. J. Bodyw. Mov. Ther. 2019, 23, 115–121. [Google Scholar] [CrossRef]
- Pintucci, M.; Imamura, M.; Thibaut, A.; de Exel Nunes, L.M.; Mayumi Nagato, M.; Kaziyama, H.H.; Tomikawa Imamura, S.; Stecco, A.; Fregni, F.; Rizzo Battistella, L. Evaluation of Fascial Manipulation in Carpal Tunnel Syndrome: A Pilot Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 630–631. [Google Scholar] [CrossRef]
- Day, J.A.; Stecco, C.; Stecco, A. Application of Fascial Manipulation Technique in Chronic Shoulder Pain—Anatomical Basis and Clinical Implications. J. Bodyw. Mov. Ther. 2009, 13, 128–135. [Google Scholar] [CrossRef]
- Arumugam, K.; Harikesavan, K. Effectiveness of Fascial Manipulation on Pain and Disability in Musculoskeletal Conditions. A Systematic Review. J. Bodyw. Mov. Ther. 2021, 25, 230–239. [Google Scholar] [CrossRef]
- Bertoldo, D.; Pirri, C.; Roviaro, B.; Stecco, L.; Day, J.A.; Fede, C.; Guidolin, D.; Stecco, C. Pilot Study of Sacroiliac Joint Dysfunction Treated with a Single Session of Fascial Manipulation® Method: Clinical Implications for Effective Pain Reduction. Medicina 2021, 57, 691. [Google Scholar] [CrossRef]
- Schleip, R.; Findley, T.W.; Chaitow, L.; Huijing, P.A. Fascia: The Tensional Network of the Human Body; Churchill Livingstone: London, UK, 2012. [Google Scholar]
- Stecco, C.; Day, J.A. The Fascial Manipulation Technique and Its Biomechanical Model: A Guide to the Human Fascial System. Int. J. Ther. Massage Bodyw. 2010, 3, 38–40. [Google Scholar]
- Fan, C.; Guidolin, D.; Ragazzo, S.; Fede, C.; Pirri, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Medicina 2020, 56, 260. [Google Scholar] [CrossRef]
- Pirri, C.; Fede, C.; Stecco, A.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Ultrasound Imaging of Crural Fascia and Epimysial Fascia Thicknesses in Basketball Players with Previous Ankle Sprains Versus Healthy Subjects. Diagnostics 2021, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Stern, R.; Fantoni, I.; De Caro, R.; Stecco, C. Fascial Disorders: Implications for Treatment. PMR 2016, 8, 161–168. [Google Scholar] [CrossRef]
- Stecco, L. Fascial Manipulation for Musculoskeletal Pain; Piccin Nuova Libraria S.p.A.: Padova, Italy, 2004. [Google Scholar]
- Stecco, A.; Stecco, C.; Macchi, V.; Porzionato, A.; Ferraro, C.; Masiero, S.; De Caro, R. RMI Study and Clinical Correlations of Ankle Retinacula Damage and Outcomes of Ankle Sprain. Surg. Radiol. Anat. 2011, 33, 881–890. [Google Scholar] [CrossRef]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in Myofascial Neck Pain: Randomized Clinical Trial for Diagnosis and Follow-Up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Kitaoka, H.B.; Alexander, I.J.; Adelaar, R.S.; Nunley, J.A.; Myerson, M.S.; Sanders, M.; Lutter, L.D. Clinical Rating Systems for the Ankle-Hindfoot, Midfoot, Hallux, and Lesser Toes. Foot Ankle Int. 1997, 18, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of Adult Pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain: A Comprehensive Model. Man Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Brandolini, S.; Lugaresi, G.; Santagata, A.; Ermolao, A.; Zaccaria, M.; Marchand, A.M.; Stecco, A. Sport Injury Prevention in Individuals with Chronic Ankle Instability: Fascial Manipulation® versus Control Group: A Randomized Controlled Trial. J. Bodyw. Mov. Ther. 2019, 23, 316–323. [Google Scholar] [CrossRef]
- Faulkner, A.; Mayne, A.; Davies, P.; Ridley, D.; Harrold, F. Patient-Related Outcome Measures (PROMs) With Nonoperative and Operative Management of Morton’s Neuroma. Foot Ankle Int. 2021, 42, 151–156. [Google Scholar] [CrossRef]
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; Caro, R.D.; Stecco, C. Fascia and Soft Tissues Innervation in the Human Hip and Their Possible Role in Post-Surgical Pain. J. Orthop. Res. 2020, 38, 1646–1654. [Google Scholar] [CrossRef]
- Stecco, C.; Pirri, C.; Fede, C.; Fan, C.; Giordani, F.; Stecco, L.; Foti, C.; De Caro, R. Dermatome and Fasciatome. Clin. Anat. 2019, 32, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Soo, M.J.; Perera, S.D.; Payne, S. The Use of Ultrasound in Diagnosing Morton’s Neuroma and Histological Correlation. Ultrasound 2010, 18, 14–17. [Google Scholar] [CrossRef]
- Mak, M.S.; Chowdhury, R.; Johnson, R. Morton’s Neuroma: Review of Anatomy, Pathomechanism, and Imaging. Clin. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cöster, M.C.; Rosengren, B.E.; Bremander, A.; Brudin, L.; Karlsson, M.K. Comparison of the Self-Reported Foot and Ankle Score (SEFAS) and the American Orthopedic Foot and Ankle Society Score (AOFAS). Foot Ankle Int. 2014, 35, 1031–1036. [Google Scholar] [CrossRef]
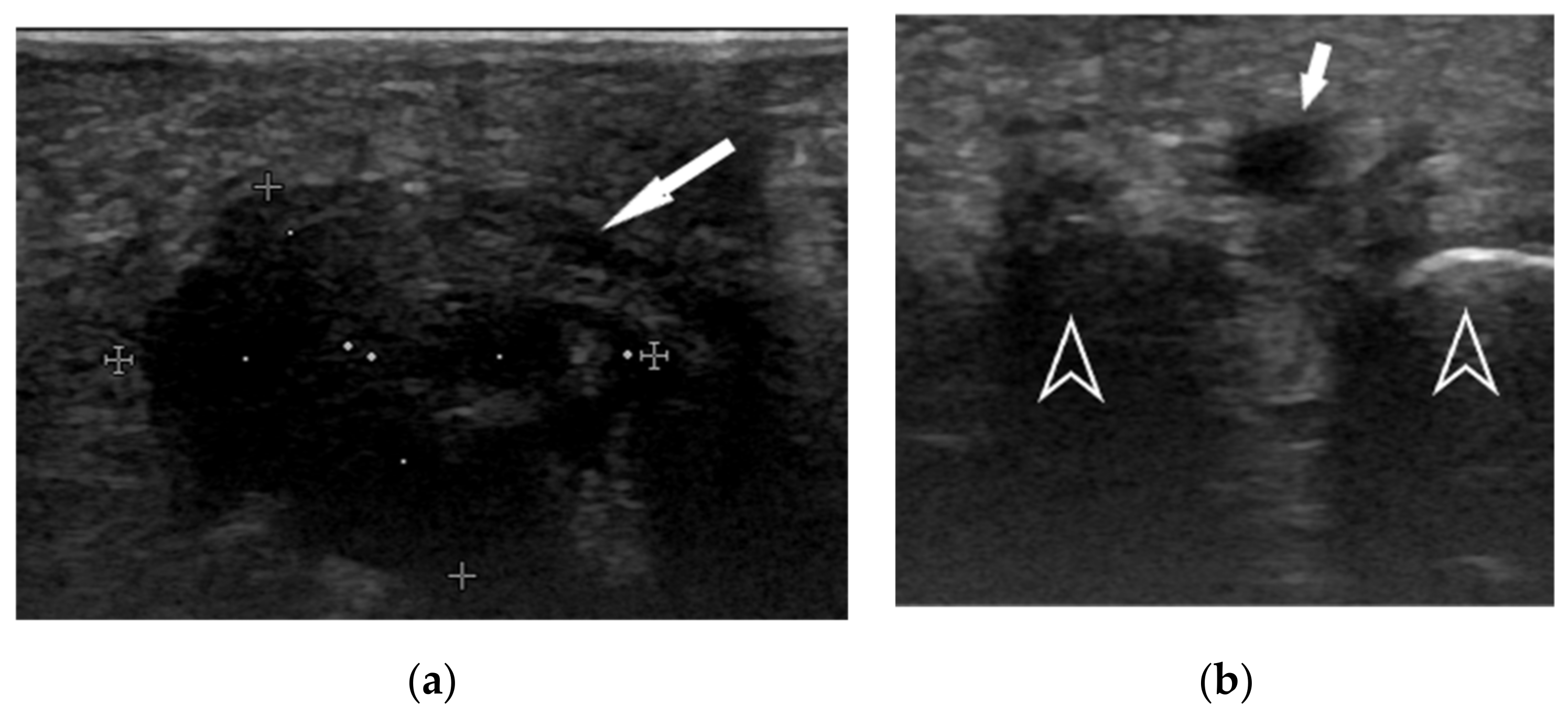
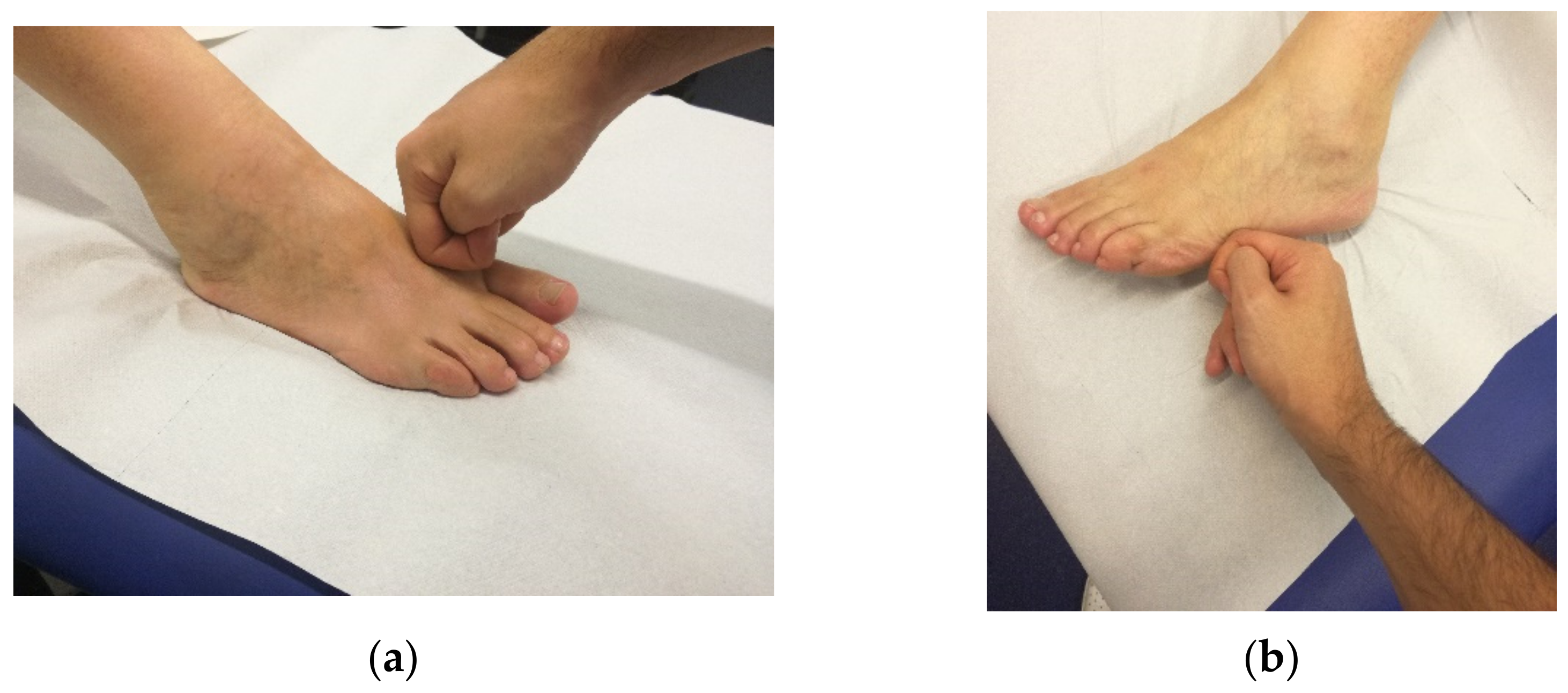

| Treated District | Number of Treated Points |
|---|---|
| Back | 9 |
| Pelvis | 16 |
| Thigh | 10 |
| Knee | 28 |
| Leg | 27 |
| Foot | 53 |
| T0-VAS | T1-VAS | T2-VAS | |
|---|---|---|---|
| Mean | 6.444 | 3.222 | 3.556 |
| Std. Deviation | 1.944 | 1.394 | 0.7265 |
| Std. Error of Mean | 0.6479 | 0.4648 | 0.2422 |
| Lower 95% CI of mean | 4.95 | 2.15 | 2.997 |
| Upper 95% CI of mean | 7.938 | 4.294 | 4.114 |
| Coefficient of variation | 30.16% | 43.28% | 20.43% |
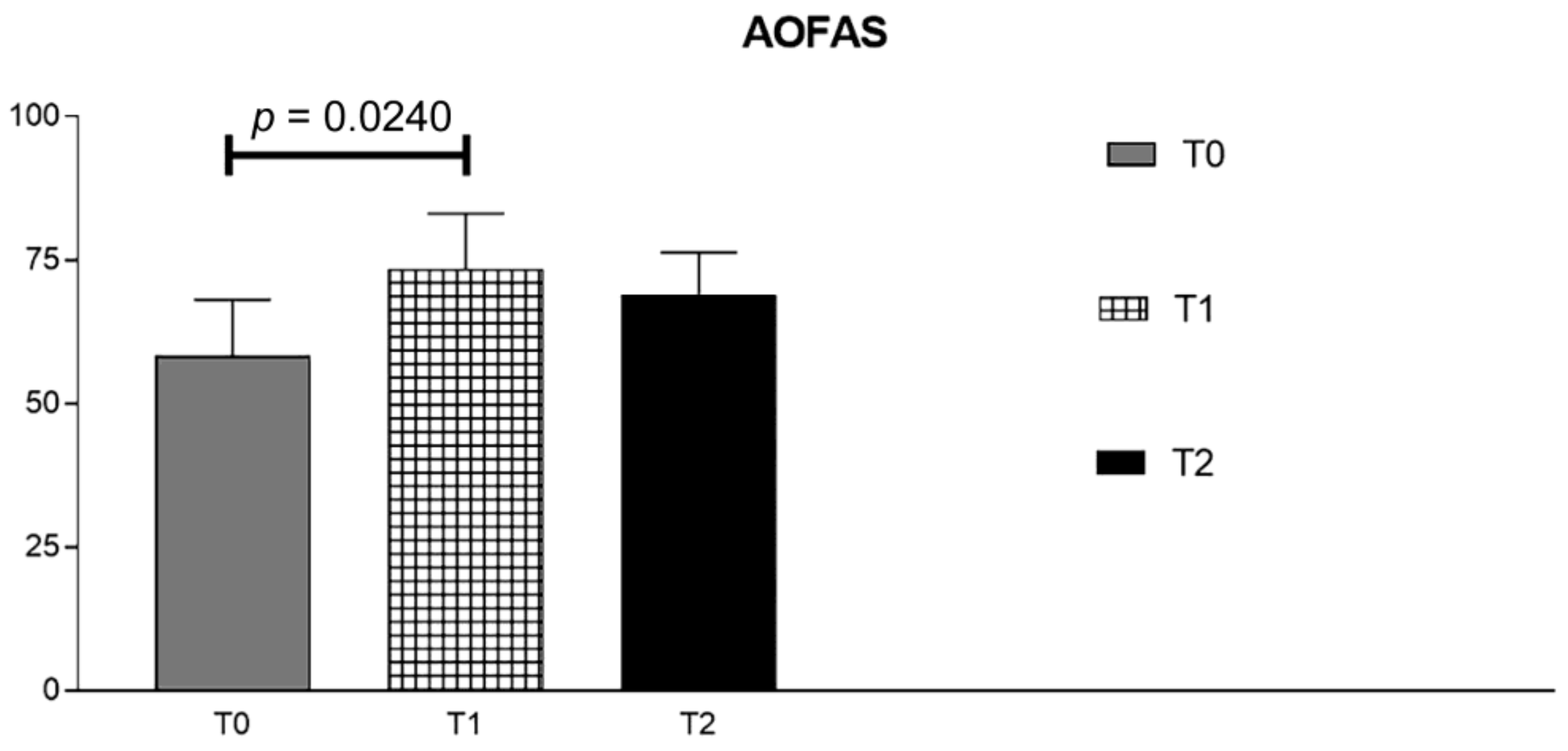
| T0-OFAS | T1-AOFAS | T2-AOFAS | |
|---|---|---|---|
| Mean | 58.29 | 73.43 | 69 |
| Std. Deviation | 9.827 | 9.641 | 7.326 |
| Std. Error of Mean | 3.714 | 3.644 | 2.769 |
| Lower 95% CI of mean | 49.2 | 64.51 | 62.22 |
| Upper 95% CI of mean | 67.37 | 82.35 | 75.78 |
| Coefficient of variation | 16.86% | 13.13% | 10.62% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biz, C.; Stecco, C.; Fantoni, I.; Aprile, G.; Giacomini, S.; Pirri, C.; Ruggieri, P. Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7952. https://doi.org/10.3390/ijerph18157952
Biz C, Stecco C, Fantoni I, Aprile G, Giacomini S, Pirri C, Ruggieri P. Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(15):7952. https://doi.org/10.3390/ijerph18157952
Chicago/Turabian StyleBiz, Carlo, Carla Stecco, Ilaria Fantoni, Gianluca Aprile, Stefano Giacomini, Carmelo Pirri, and Pietro Ruggieri. 2021. "Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 15: 7952. https://doi.org/10.3390/ijerph18157952
APA StyleBiz, C., Stecco, C., Fantoni, I., Aprile, G., Giacomini, S., Pirri, C., & Ruggieri, P. (2021). Fascial Manipulation Technique in the Conservative Management of Morton’s Syndrome: A Pilot Study. International Journal of Environmental Research and Public Health, 18(15), 7952. https://doi.org/10.3390/ijerph18157952










