COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects
Abstract
:1. Introduction
Objectives
- (a)
- To estimate the prevalence of both local and systemic side effects following each of the COVID-19 vaccines among healthcare workers (HCWs), teachers and academics (TAs), senior adults ≥65 years old (SAs), and minors ≤18 years old (MIs);
- (b)
- To evaluate the potential demographic and medical risk factors for the frequency and intensity of side effects;
- (c)
- To evaluate the long-term safety of COVID-19 vaccines.
- (a)
- To evaluate the relative effectiveness and safety of COVID-19 vaccines in relation to one another;
- (b)
- To evaluate the impact of palliative medications used by the vaccinated individuals for short-term side effect resolution.
2. Materials and Methods
2.1. Design
2.1.1. Phase A
2.1.2. Phase B
2.1.3. Phase C
2.2. Population
2.2.1. Inclusion Criteria
- HCWs, TAs, SAs, and MIs who received a COVID-19 vaccine in the post-authorization phase;
- The recently vaccinated individuals who received their vaccine dose within the previous 30 days will be prioritized to be invited for the study, even though the study will not be limited to the recently vaccinated individuals;
- Participating subjects should be at least 18 years old in order to give their informed consent independently, or in case of the minors (below 18 years old), their caregivers will be asked to give their informed consent.
2.2.2. Exclusion Criteria
- HCWs, TAs, SAs, and MIs who received the COVID-19 vaccines as part of phase III clinical trials.
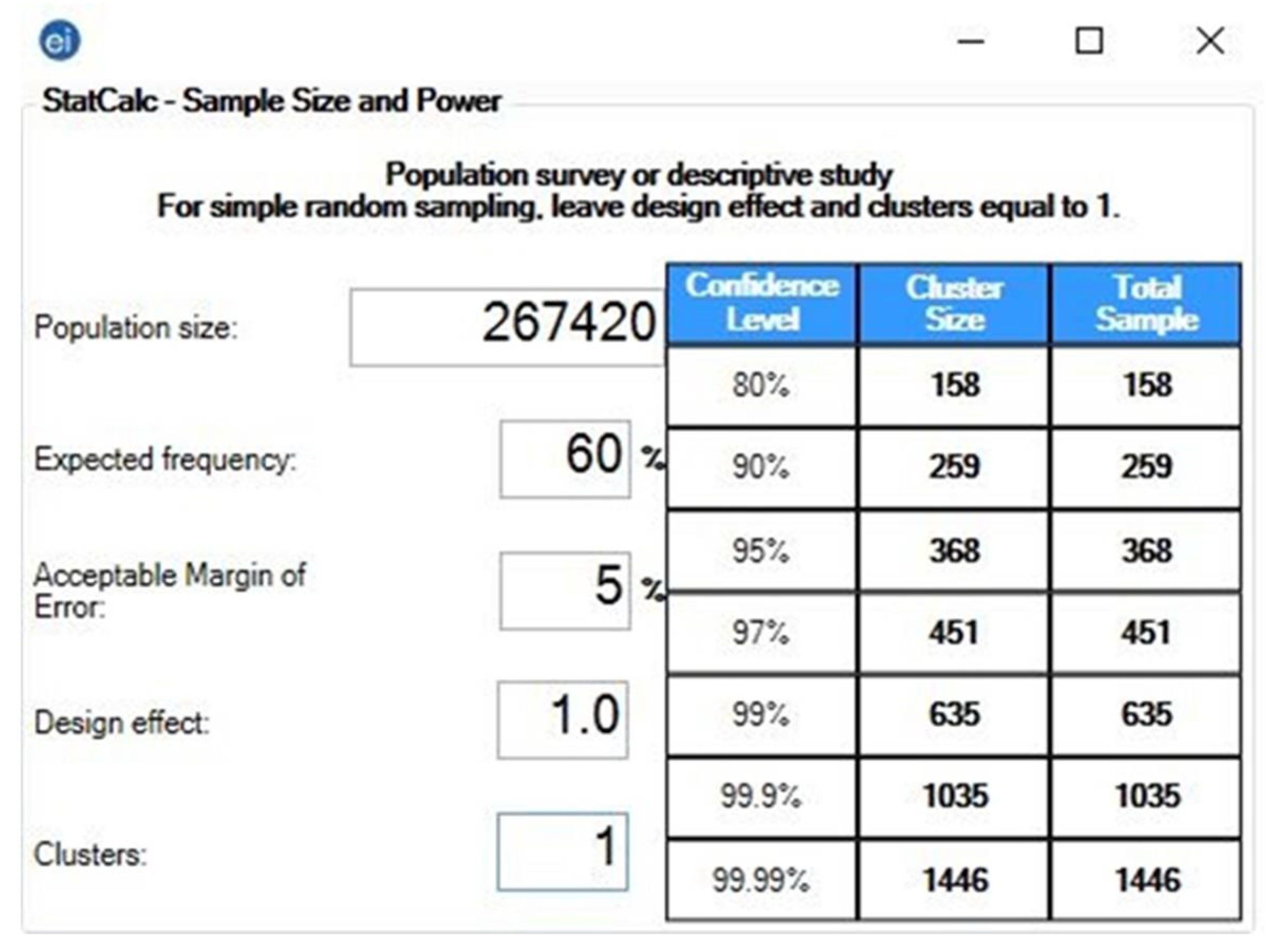
2.2.3. Sample Size
2.3. Instrument
2.4. Recruitment
2.4.1. Phase A
2.4.2. Phase B and C
2.5. Timeline
2.6. Ethics
2.7. Analysis
3. Registration and Dissemination
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). COVID-19 Vaccine Country Readiness and Delivery. COVAX. Available online: https://www.who.int/initiatives/act-accelerator/covax/covid-19-vaccine-country-readiness-and-delivery (accessed on 7 May 2021).
- Attia, S.; Howaldt, H.-P. Impact of COVID-19 on the Dental Community: Part I before Vaccine (BV). J. Clin. Med. 2021, 10, 288. [Google Scholar] [CrossRef]
- Butler, R.; MacDonald, N.E. Diagnosing the determinants of vaccine hesitancy in specific subgroups: The Guide to Tailoring Immunization Programmes (TIP). Vaccine 2015, 33, 4176–4179. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.A.; Wu, J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020, 35, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Ten Threats to Global Health in 2019. Newsroom. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 7 May 2021).
- Luyten, J.; Bruyneel, L.; van Hoek, A.J. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine 2019, 37, 2494–2501. [Google Scholar] [CrossRef]
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J. Strategies for addressing vaccine hesitancy-A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef] [Green Version]
- Strategic Advisory Group of Experts on Immunization (SAGE). Vaccine Hesitancy Survey Questions Related to SAGE Vaccine Hesitancy Matrix. Available online: https://www.who.int/immunization/programmes_systems/Survey_Questions_Hesitancy.pdf (accessed on 14 March 2021).
- Greenberg, J.; Dubé, E.; Driedger, M. Vaccine Hesitancy: In Search of the Risk Communication Comfort Zone. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Dinga, J.N.; Sinda, L.K.; Titanji, V.P.K. Assessment of vaccine hesitancy to a covid-19 vaccine in cameroonian adults and its global implication. Vaccines 2021, 9, 175. [Google Scholar] [CrossRef]
- Dıaz Crescitelli, M.E.; Ghirotto, L.; Sisson, H.; Sarli, L.; Artioli, G.; Bassi, M.C. A meta-synthesis study of the key elements involved in childhood vaccine hesitancy. Public Health 2020, 180, 38–45. [Google Scholar] [CrossRef]
- Six Ways to Better Understand COVID-19 Vaccine Hesitancy by Surgo Ventures Medium. Available online: https://surgoventures.medium.com/six-ways-to-better-understand-covid-19-vaccine-hesitancy-3689dfd65b86 (accessed on 24 March 2021).
- Mahase, E. Covid-19: AstraZeneca vaccine is not linked to increased risk of blood clots, finds European Medicine Agency. BMJ 2021, 372, n774. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J. European Healthcare Workers Are Refusing AstraZeneca Vaccine Over Efficacy Concerns. Forbes. Available online: https://www.forbes.com/sites/jemimamcevoy/2021/02/21/european-healthcare-workers-are-refusing-astrazeneca-vaccine-over-efficacy-concerns/?sh=547867806520 (accessed on 19 May 2021).
- European Medicines Agency (EMA). COVID-19 Vaccine AstraZeneca: Benefits Still Outweigh the Risks Despite Possible Link to Rare Blood Clots with Low Blood Platelets. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots (accessed on 20 March 2021).
- Institute for Health Metrics and Evaluation (IHME). COVID-19 model FAQs. COVID-19 Resources. Available online: http://www.healthdata.org/covid/faqs (accessed on 18 May 2021).
- Pfizer. Pfizer and BioNTech Initiate a Study as Part of Broad Development Plan to Evaluate COVID-19 Booster and New Vaccine Variants. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-part-broad-development (accessed on 18 May 2021).
- World-First COVID-19 Vaccine Booster Study Launches in UK-GOV.UK. Available online: https://www.gov.uk/government/news/world-first-covid-19-vaccine-booster-study-launches-in-uk (accessed on 20 May 2021).
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study Among Healthcare Workers in Turkey. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.; Almukadi, H.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N.; et al. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-reported real-world safety and reactogenicity of covid-19 vaccines: A vaccine recipient survey. Life 2021, 11, 249. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Djanas, D.; Martini, R.D.; Putra, H.; Zanir, A.; Nindrea, R.D. Survey Data of COVID-19 Vaccine Side Effects among Hospital Staff in a National Referral Hospital in Indonesia. Data Br. 2021, 36, 107098. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Adverse effects of COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 1–10. [Google Scholar] [CrossRef]
- Pagotto, V.; Ferloni, A.; Soriano, M.M.; Diaz, M.; Braguinsky, N.; Asprea, V.; Vidal, G.G.; Silveira, M.G.; Zingoni, P.; Aliperti, V.; et al. Active Surveillance of the SPUTNIK V vaccine in health workers. medRxiv 2021. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Population Survey or Descriptive Study. StatCalc User Guide. Available online: https://www.cdc.gov/epiinfo/user-guide/statcalc/samplesize.html (accessed on 19 May 2021).
- Institute of Health Information and Statistics of the Czech Republic (UZIS). Health Yearbook of the Czech Republic 2017; UZIS Publications: Prague, Czech Republic, 2018; Available online: https://www.uzis.cz/index-en.php?pg=record&id=8166 (accessed on 3 March 2021).
- World Health Organization (WHO). Process of Translation and Adaptation of Instruments. Available online: https://www.who.int/substance_abuse/research_tools/translation/en/ (accessed on 25 December 2020).
- SPSS Inc. IBM SPSS Statistics 27. Available online: https://www.ibm.com/support/pages/node/3006603 (accessed on 14 March 2021).
- Klugar, M.; Riad, A. COVID-19 Vaccines Safety Tracking (CoVaST). ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04834869 (accessed on 9 May 2021).
- Huang, Y.L.; Moon, J.; Segal, J.B. A comparison of active adverse event surveillance systems worldwide. Drug Saf. 2014, 37, 581–596. [Google Scholar] [CrossRef] [Green Version]
- Farrington, P.; Rush, M.; Miller, E.; Pugh, S.; Colville, A.; Flower, A.; Nash, J.; Morgan-Capner, P. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet 1995, 345, 567–569. [Google Scholar] [CrossRef]
- Medicines and Healthcare products Regulatory Agency (MHRA). Report of the Commission on Human Medicines Expert Working Group on COVID-19 Vaccine Safety Surveillance. Vigilance, Safety Alerts and Guidance. Available online: https://www.gov.uk/government/publications/report-of-the-commission-on-human-medicines-expert-working-group-on-covid-19-vaccine-safety-surveillance/report-of-the-commission-on-human-medicines-expert-working-group-on-covid-19-vaccine-safety-surveillance (accessed on 9 May 2021).
- DataLab. OpenSAFELY. University of Oxford for the The DataLab 2021. Available online: https://www.opensafely.org/ (accessed on 9 May 2021).
- Centres for Diseases Control and Prevention (CDC). REACTIONS and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 7 March 2021).
- Centres for Diseases Control and Prevention (CDC). Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html (accessed on 2 April 2021).
| Phase | Stage | Population | Schedule |
|---|---|---|---|
| Phase A | Stage A.1. | HCWs | May–August 2021 |
| Stage A.2. | SAs | June–December 2021 | |
| Stage A.3. | TAs | June–December 2021 | |
| Stage A.4. | MIs | June–December 2021 | |
| Phase B | Stage B.1. | HCWs | October 2021–February 2021 |
| Stage B.2. | SAs | November 2021–April 2022 | |
| Stage B.3. | TAs | November 2021–April 2022 | |
| Stage B.4. | MIs | November 2021–April 2022 | |
| Stage C | Stage C.1. | HCWs, SAs, TAs, MIs | January–December 2022 |
| Stage C.2. | HCWs, SAs, TAs, MIs | January–December 2023 | |
| Stage C.3. | HCWs, SAs, TAs, MIs | January–December 2024 | |
| Stage C.4. | HCWs, SAs, TAs, MIs | January–December 2025 | |
| Stage C.5. | HCWs, SAs, TAs, MIs | January–December 2026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riad, A.; Schünemann, H.; Attia, S.; Peričić, T.P.; Žuljević, M.F.; Jürisson, M.; Kalda, R.; Lang, K.; Morankar, S.; Yesuf, E.A.; et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int. J. Environ. Res. Public Health 2021, 18, 7859. https://doi.org/10.3390/ijerph18157859
Riad A, Schünemann H, Attia S, Peričić TP, Žuljević MF, Jürisson M, Kalda R, Lang K, Morankar S, Yesuf EA, et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. International Journal of Environmental Research and Public Health. 2021; 18(15):7859. https://doi.org/10.3390/ijerph18157859
Chicago/Turabian StyleRiad, Abanoub, Holger Schünemann, Sameh Attia, Tina Poklepović Peričić, Marija Franka Žuljević, Mikk Jürisson, Ruth Kalda, Katrin Lang, Sudhakar Morankar, Elias Ali Yesuf, and et al. 2021. "COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects" International Journal of Environmental Research and Public Health 18, no. 15: 7859. https://doi.org/10.3390/ijerph18157859
APA StyleRiad, A., Schünemann, H., Attia, S., Peričić, T. P., Žuljević, M. F., Jürisson, M., Kalda, R., Lang, K., Morankar, S., Yesuf, E. A., Mekhemar, M., Danso-Appiah, A., Sofi-Mahmudi, A., Pérez-Gaxiola, G., Dziedzic, A., Apóstolo, J., Cardoso, D., Marc, J., Moreno-Casbas, M., ... Klugar, M. (2021). COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. International Journal of Environmental Research and Public Health, 18(15), 7859. https://doi.org/10.3390/ijerph18157859
















