Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- -
- Primary dysmenorrhea (PD), which is menstrual pain associated with normal ovulatory cycles, without pelvic pathology, and a clear physiological etiology [2]. It is most common in adolescents and young adults.
- -
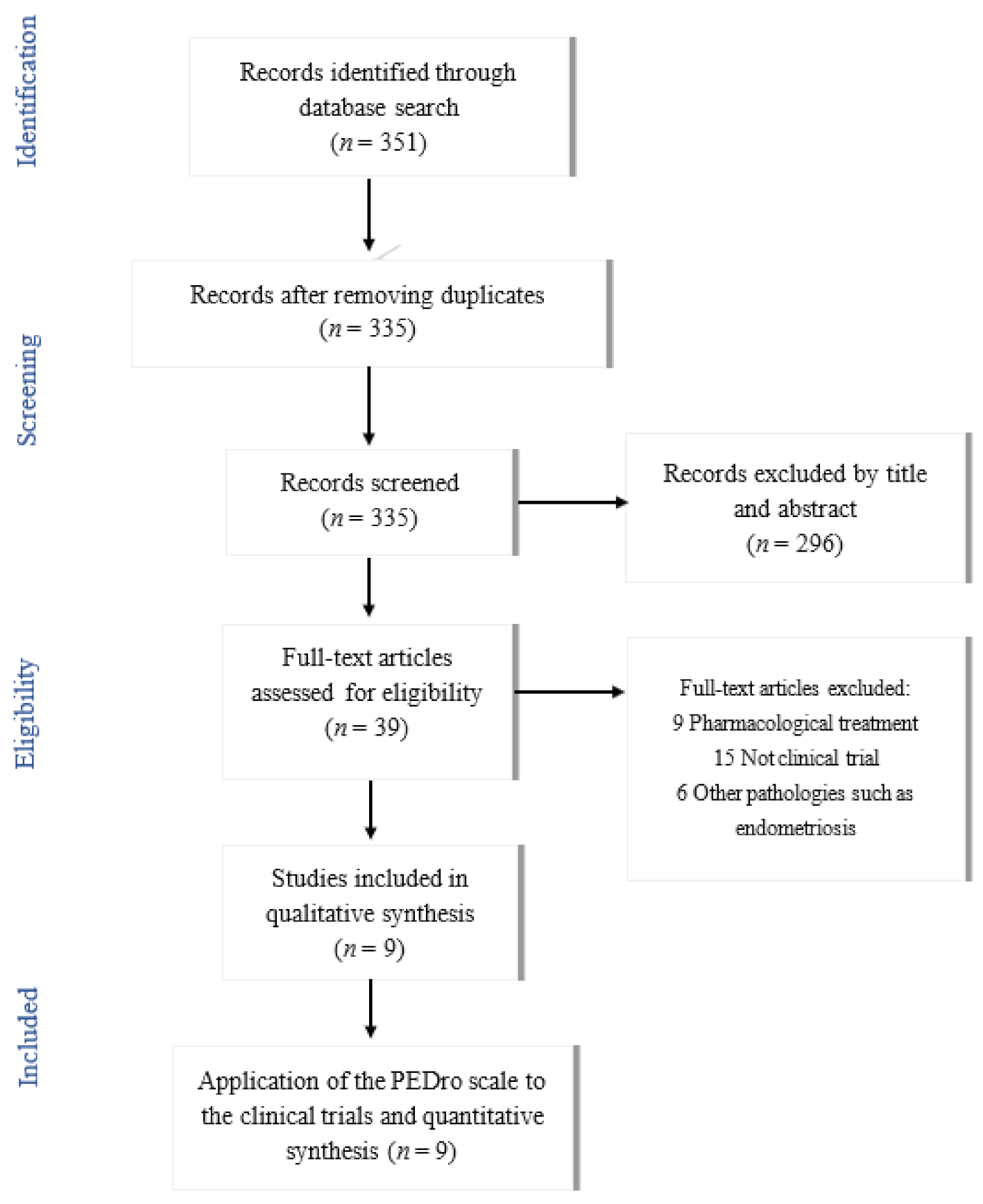
2. Materials and Methods
- Contained the following keywords in English “dysmenorrhea”, “physical therapy”, “physiotherapy” and/or “manual therapy. The search strategy used for procuring articles from the different databases is shown in Table 1.
- Randomized controlled trials.
- Physical therapy or conservative treatment techniques listed as the method of intervention.
- Published from 2015 onwards, until 1 February 2021.
- Treatment of other pathologies such as endometriosis or dyspareunia.
- Pharmacology as a method of treatment.
- Not written in English or Spanish.
3. Results
3.1. Evaluation or Questionnaires
3.2. Interventions or Treatment
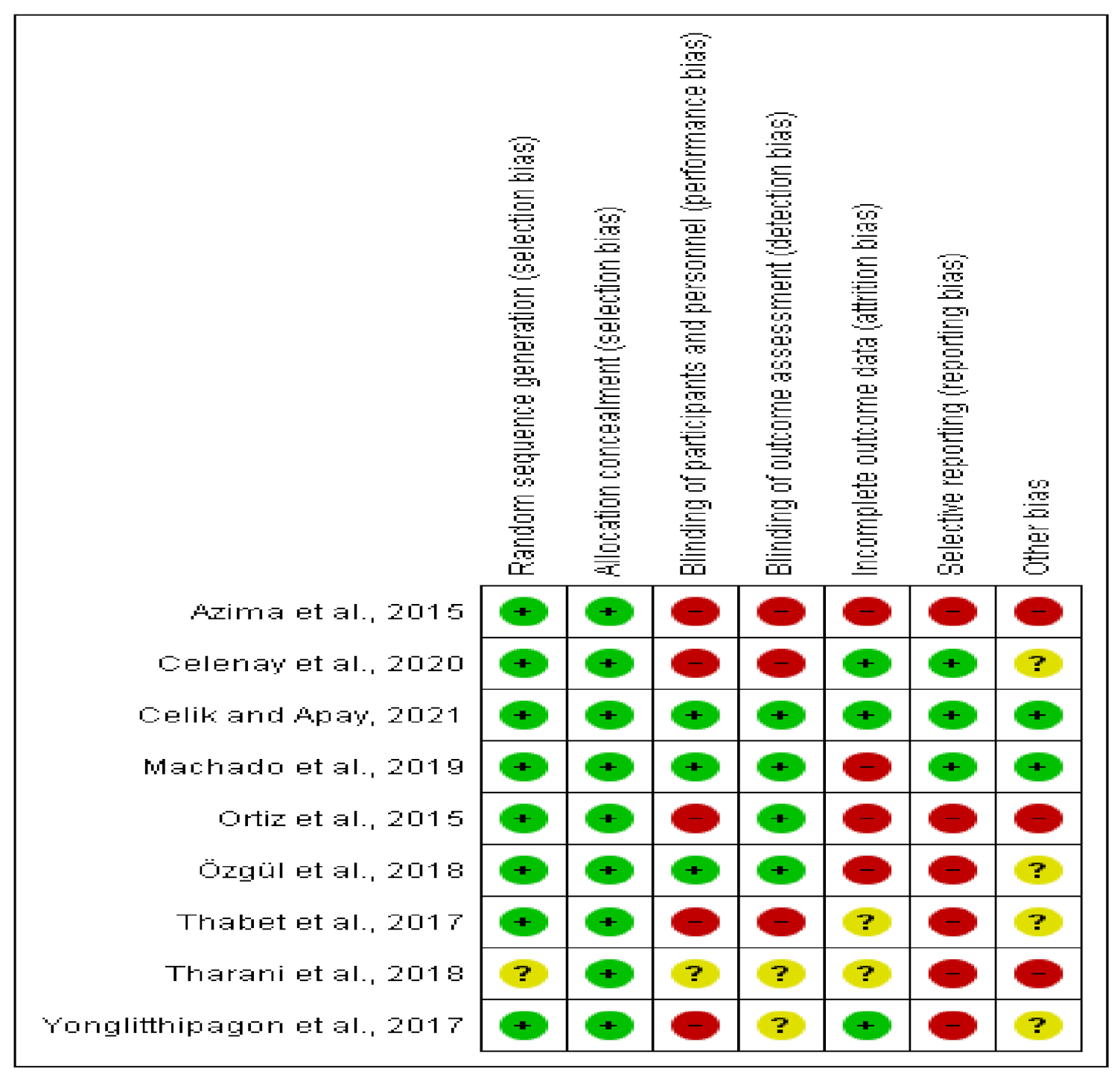
3.3. Methodological Quality of the Included Articles
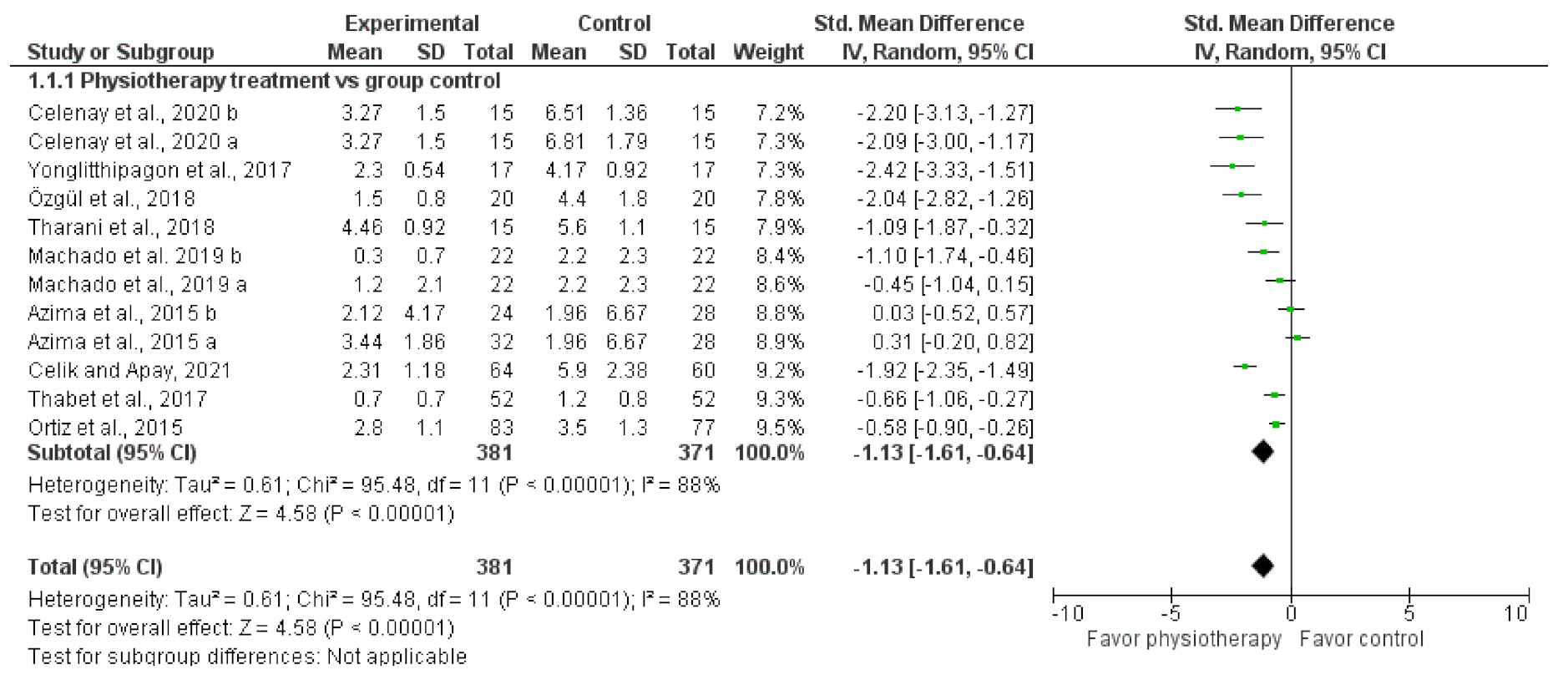
3.4. Meta-Analysis or Quantitative Analysis of the Included Articles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A



References
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and related disorders. F1000Research 2017, 6, 1645. [Google Scholar] [CrossRef] [PubMed]
- Harel, Z. Dysmenorrhea in Adolescents and Young Adults: Etiology and Management. J. Pediatr. Adolesc. Gynecol. 2006, 19, 363–371. [Google Scholar] [CrossRef] [PubMed]
- García Hurtado, B.; Chillón Martínez, R.; Rebollo Roldán, J.; Orta Pérez, M.A. Dismenorrea primaria y fisioterapia. Fisioterapia 2005, 27, 327–342. [Google Scholar] [CrossRef]
- Proctor, M.; Farquhar, C. Diagnosis and management of dysmenorrhoea. BMJ 2006, 332, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Parry, K.; Manohar, N.; Holmes, K.; Ferfolja, T.; Curry, C. The Prevalence and Academic Impact of Dysmenorrhea in 21,573 Young Women: A Systematic Review and Meta-Analysis. J. Women’s Health 2019, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pichardo, J.D.; Ortega-Galán, Á.M.; Iglesias-López, M.T.; Abreu-Sánchez, A.; Fernández-Martínez, E. Why Do Some Spanish Nursing Students with Menstrual Pain Fail to Consult Healthcare Professionals? Int. J. Environ. Res. Public Health 2020, 17, 8173. [Google Scholar] [CrossRef] [PubMed]
- Orhan, C.; Celenay, S.T.; Demirtuerk, F.; Ozgul, S.; Uzelpasaci, E.; Akbayrak, T. Effects of menstrual pain on the academic performance and participation in sports and social activities in Turkish university students with primary dysmenorrhea: A case control study. J. Obstet. Gynaecol. Res. 2018, 44, 2101–2109. [Google Scholar] [CrossRef]
- Dawood, M.Y. Primary Dysmenorrhea. Obs. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef]
- Ryan, S.A. The Treatment of Dysmenorrhea. Pediatr. Clin. N. Am. 2017, 64, 331–342. [Google Scholar] [CrossRef]
- Monterrosa Castro, A. Dismenorrea primaria: Visión actual. Rev. Colomb. Obs. Ginecol. 2001, 52, 342–354. [Google Scholar] [CrossRef]
- Burnett, M.; Lemyre, M. No. 345-Primary Dysmenorrhea Consensus Guideline. J. Obstet. Gynaecol. Can. 2017, 39, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Daley, A.J. Exercise and Primary Dysmenorrhoea. Sport Med. 2008, 38, 659–670. [Google Scholar] [CrossRef]
- Yacubovich, Y.; Cohen, N.; Tene, L.; Kalichman, L. The prevalence of primary dysmenorrhea among students and its association with musculoskeletal and myofascial pain. J. Bodyw. Mov. Ther. 2019, 23, 785–791. [Google Scholar] [CrossRef] [PubMed]
- French, L. Dysmenorrhea in Adolescents. Pediatr. Drugs 2008, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Parra-Fernández, M.L.; Onieva-Zafra, M.D.; Abreu-Sánchez, A.; Ramos-Pichardo, J.D.; Iglesias-López, M.T.; Fernández-Martínez, E. Management of primary dysmenorrhea among university students in the South of Spain and family influence. Int. J. Environ. Res. Public Health 2020, 17, 5570. [Google Scholar] [CrossRef]
- Zahradnik, H.-P.; Hanjalic-Beck, A.; Groth, K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: A review. Contraception 2010, 81, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Collins, J.; Crosignani, P.G.; Glasier, A.; Jacobs, H.S.; La Vecchia, C. Noncontraceptive health benefits of combined oral contraception. Hum. Reprod. Update 2005, 11, 513–525. [Google Scholar]
- Kannan, P.; Claydon, L.S. Some physiotherapy treatments may relieve menstrual pain in women with primary dysmenorrhea: A systematic review. J. Physiother. 2014, 60, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, E.; Onieva-Zafra, M.D.; Parra-Fernández, M.L. The impact of dysmenorrhea on quality of life among spanish female university students. Int. J. Environ. Res. Public Health 2019, 16, 713. [Google Scholar] [CrossRef] [PubMed]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef]
- Armour, M.; Ee, C.C.; Naidoo, D.; Ayati, Z.; Chalmers, K.J.; Steel, K.A. Exercise for dysmenorrhoea. Cochrane Database Syst. Rev. 2019, 9, CD004142. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D. Yoga for menstrual pain in primary dysmenorrhea: A meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract. 2019, 36, 94–99. [Google Scholar] [CrossRef]
- Matthewman, G.; Lee, A.; Kaur, J.G.; Daley, A.J. Physical activity for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2018, 219, 255.e1–255.e20. [Google Scholar] [CrossRef]
- Arik, M.I.; Kiloatar, H.; Aslan, B.; Icelli, M. The effect of tens for pain relief in women with primary dysmenorrhea: A systematic review and meta-analysis. Explore 2020, 29, 2541. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, M.; Mansurkhani, S.M.; Larky, D.A.; Kooti, W.; Niksefat, M.; Firoozbakht, M.; Behzadifar, M.; Azami, M.; Servatyari, K.; Jouybari, L. An update and systematic review on the treatment of primary dysmenorrhea. JBRA Assist. Reprod. 2019, 23, 51–57. [Google Scholar] [CrossRef]
- Abaraogu, U.O.; Igwe, S.E.; Tabansi-Ochiogu, C.S. Effectiveness of SP6 (Sanyinjiao) acupressure for relief of primary dysmenorrhea symptoms: A systematic review with meta- and sensitivity analyses. Complement. Ther. Clin. Pract. 2016, 25, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Gerzson, L.; Falcão Padilha, J.; Medeiros Braz, M.; Gasparetto, A. Physiotherapy in primary dysmenorrhea: Literature review. Rev. Dor São Paulo 2014, 15, 290–295. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; Wiley: Chichester, UK, 2008; pp. 187–241. [Google Scholar]
- Herbert, R.; Moseley, A.; Sherrington, C.; Maher, C. Physiotherapy Evidence Database. Physiotherapy 2000, 86, 55. [Google Scholar] [CrossRef]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version. Hamilton (ON): GRADE Working Group, McMaster University. 2015. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 17 June 2021).
- Azima, S.; Bakhshayesh, H.R.; Kaviani, M.; Abbasnia, K.; Sayadi, M. Comparison of the Effect of Massage Therapy and Isometric Exercises on Primary Dysmenorrhea: A Randomized Controlled Clinical Trial. J. Pediatr. Adolesc. Gynecol. 2015, 28, 486–491. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cortés-Márquez, S.K.; Romero-Quezada, L.C.; Murguía-Cánovas, G.; Jaramillo-Díaz, A.P. Effect of a physiotherapy program in women with primary dysmenorrhea. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yonglitthipagon, P.; Muansiangsai, S.; Wongkhumngern, W.; Donpunha, W.; Chanavirut, R.; Siritaratiwat, W. Effect of yoga on the menstrual pain, physical fitness, and quality of life of young women with primary dysmenorrhea. J. Bodyw. Mov. Ther. 2017, 21, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.A.E.-M.; Elsodany, A.M.; Battecha, K.H.; Alshehri, M.A.; Refaat, B. High-intensity laser therapy versus pulsed electromagnetic field in the treatment of primary dysmenorrhea. J. Phys. Ther. Sci. 2017, 29, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Özgül, S.; Üzelpasaci, E.; Orhan, C.; Baran, E.; Beksaç, M.S.; Akbayrak, T. Short-term effects of connective tissue manipulation in women with primary dysmenorrhea: A randomized controlled trial. Complement Ther. Clin. Pract. 2018, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tharani, G.; Dharshini, E.; Rajalaxmi, V.; Kamatchi, K.; Vaishnavi, G. To compare the effects of stretching exercise versus aerobic dance in primary dysmenorrhea among collegiates. Drug Invent. Today 2018, 10, 2844–2848. [Google Scholar]
- Machado, A.F.P.; Perracini, M.R.; Rampazo, É.P.; Driusso, P.; Liebano, R.E. Effects of thermotherapy and transcutaneous electrical nerve stimulation on patients with primary dysmenorrhea: A randomized, placebo-controlled, double-blind clinical trial. Complement. Ther. Med. 2019, 47, 102188. [Google Scholar] [CrossRef]
- Celenay, S.T.; Kavalci, B.; Karakus, A.; Alkan, A. Effects of kinesio tape application on pain, anxiety, and menstrual complaints in women with primary dysmenorrhea: A randomized sham-controlled trial. Complement. Ther. Clin. Pract. 2020, 39, 101148. [Google Scholar] [CrossRef]
- Çelik, A.S.; Apay, S.E. Effect of progressive relaxation exercises on primary dysmenorrhea in Turkish students: A randomized prospective controlled trial. Complement. Ther. Clin. Pract. 2021, 42, 101280. [Google Scholar] [CrossRef]
- Torres-Pascual, C. Alternativas al tratamiento farmacológico de las alteraciones menstruales en adolescentes y jovenes adultas. Med. Natur. 2016, 10, 15–20. [Google Scholar]
- Corral-Moreno, V.; Munuera-Jiménez, F.J.; Cascos-Vicente, L.; Juárez-Díaz, E.; Rodríguez-Almagro, D.; Obrero-Gaitán, E.; Ibáñez-Vera, A.J. Tratamiento fisioterapéutico para la dismenorrea primaria: Una revisión sistemática. Fisioterapia 2021. [Google Scholar] [CrossRef]
- Zhai, S.; Ruan, Y.; Liu, Y.; Lin, Z.; Xia, C.; Fang, F.F.; Zhou, Q.H. Time-effective analgesic effect of acupressure ankle strip pressing wrist and ankle acupuncture point on primary dysmenorrhea: Study protocol clinical trial (SPIRIT compliant). Medicine 2020, 99, e19496. [Google Scholar] [CrossRef]
- Boguszewski, D.; Borowska, J.; Szymańska, A.; Adamczyk, J.G.; Lewandowska, M.; Białoszewski, D. Effectiveness of kinesiotaping for the treatment of menstrual pain. Physiother. Quart. 2020, 28, 20–24. [Google Scholar] [CrossRef]
- Doğan, H.; Eroğlu, S.; Akbayrak, T. The effect of kinesio taping and life style changes on pain, body awareness and quality of life in primary dysmenorrhea. Complement. Ther. Clin. Pract. 2020, 39, 101120. [Google Scholar] [CrossRef] [PubMed]
- Gotpagar, M.T.; Davi, P. Effect of Bosu Pilates on Primary Dysmenorrhea in Adolescent Girls. Indian J. Forensic Med. Toxicol. 2020, 14, 2039–2044. [Google Scholar]
- Kirmizigil, B.; Demiralp, C. Effectiveness of functional exercises on pain and sleep quality in patients with primary dysmenorrhea: A randomized clinical trial. Arch. Gynecol. Obstet. 2020, 302, 153–163. [Google Scholar] [CrossRef]
- Vance, A.R.; Hayes, S.H.; Spielholz, N.I. Microwave Diathermy Treatment for Primary Dysmenorrhea. Phys. Ther. 1996, 76, 1003–1008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.-F.; Lee, J.-P.; Hwa, H.-L. Effect of Transcutaneous Electrical Nerve Stimulation on Primary Dysmenorrhea. Neuromodul. Technol. Neural Interface 2009, 12, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Tugay, N.; Akbayrak, T.; Demirtürk, F.; Karakaya, I.; Kocaacar, Ö.; Tugay, U. Effectiveness of Transcutaneous Electrical Nerve Stimulation and Interferential Current in Primary Dysmenorrhea. Pain Med. 2007, 8, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-I.; Kim, N.-G.; Park, K.-J.; Kim, D.-W.; Hong, G.-Y.; Shin, B.-C. Skin adhesive low-level light therapy for dysmenorrhoea: A randomized, double-blind, placebo-controlled, pilot trial. Arch. Gynecol. Obstet. 2012, 286, 947–952. [Google Scholar] [CrossRef]
- Apay, S.E.; Arslan, S.; Akpinar, R.B.; Celebioglu, A. Effect of Aromatherapy Massage on Dysmenorrhea in Turkish Students. Pain Manag. Nurs. 2012, 13, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.A.; Hardy, E.; Sousa, M.H. The effectiveness of connective tissue massage in the treatment of primary dysmenorrhea among young women. Rev. Bras. Saúde Matern. Infant. 2010, 10, 247–256. [Google Scholar] [CrossRef]
| Databases and Search Terms | Results | Selected Articles |
|---|---|---|
| SCOPUS | ||
| 1. “Physical therapy” AND “dysmenorrhea” | 50 | 16 |
| 2. “Physiotherapy” AND “dysmenorrhea” | 115 | |
| 3. “manual therapy” AND “dysmenorrhea” | 14 | |
| PUBMED | 10 | |
| 1. “Physical therapy” AND “dysmenorrhea” | 38 | |
| 2. “Physiotherapy” AND “dysmenorrhea” | 43 | |
| 3. “manual therapy” AND “dysmenorrhea” | 3 | |
| PEDRO | 5 | |
| 1. “Physical therapy” AND “dysmenorrhea” | 5 | |
| 2. “Physiotherapy” AND “dysmenorrhea” | 11 | |
| 3. “manual therapy” AND “dysmenorrhea” | 2 | |
| WEB OF SCIENCE | 4 | |
| 1. “Physical therapy” AND “dysmenorrhea” | 26 | |
| 2. “Physiotherapy” AND “dysmenorrhea” | 11 | |
| 3. “manual therapy” AND “dysmenorrhea” | 3 | |
| MEDLINE | 4 | |
| 1. “Physical therapy” AND “dysmenorrhea” | 16 | |
| 2. “Physiotherapy” AND “dysmenorrhea” | 11 | |
| 3. “manual therapy” AND “dysmenorrhea” | 3 |
| Author, Year | Type of Study | Sample Size (Participants) | Age | Measured Variables | Ain Results |
|---|---|---|---|---|---|
| Azima et al. [35] 2015 | Randomized clinical trial | 102 participants 34: EG 1 34: EG 2 34: CG | 19–23 years old | Pain (VAS), duration of pain (hours) and anxiety (STAI). | Significant improvement in pain intensity in both EGs (massage EG1 and isometric EG2), but greater in massage therapy group (p < 0.001) in the 2nd and 3rd cycle. |
| Ortiz et al. [36] 2015 | Prospective, parallel-group, randomized clinical trial | 173 participants 89: EG 84: CG | 18–22 years old | VAS, presence and magnitude of symptoms (LS). | Significant reduction of pain in EG according to VAS from the 2nd and 3rd menstrual cycles (p < 0.05) compared with the CG. |
| Yonglitthipagon et al. [37] 2017 | Randomized clinical trial | 34 participants 17: EG 17: CG | 18–22 years old | VAS; quality of life (SF-36); flexibility (SR), and back and leg strength (dynamometer). | Statistically significant differences in yoga EG in terms of pain intensity, flexibility, and muscle strength (p < 0.02). |
| Thabet et al. [38] 2017 | Randomized clinical trial | 52 participants 26: EG 1 26: EG 2 | 18–24 years old | Pain Intensity was measured with PPI (0–4 scale); pain relief scale; and prostaglandin PG2α concentration with blood samples. | Both groups had a decrease in pain, but the effect was more pronounced in the HILT group (p < 0.05). There was a decrease in the PG2α level in both groups (p < 0.001). |
| Özgül et al. [39] 2018 | Randomized controlled clinical trial | 44 participants 21: EG 23: CG | aged over 18 years old | Pain intensity (VAS and PCS), anxiety level (STAI), menstrual symptoms (MSQ) and menstrual attitude (MAQ). | CTM group showed statistically significant improvement in pain, medication use, PCS, MSQ (p = 0.001) and in the perception of menstruation (p = 0.029). |
| Tharani et al. [40] 2018 | Pre- and post-comparative experimental study | 30 participants 15: EG 1 15: EG 2 | 17–23 years old | Stress (DASS-21) and pain (VAS). | Both groups showed a reduction in pain and stress, but aerobic dance was significantly more efficient (p < 0.001). |
| Machado et al. [41] 2019 | Placebo- controlled, double-blind clinical trial | 88 participants 22: EG 1 22: EG 2 22: EG 3 22: CG | 18–44 years old | Pain intensity (NRS, Br-MPQ), pressure pain threshold (PPT) and conditioned pain modulation (CPM). | Thermotherapy reduced pain intensity compared to TENS (p = 0.01) and placebo (p = 0.05) after 20, 110 min, and 24 h. |
| Celenay et al. [42] 2020 | A randomized sham- controlled trial | 45 participants 15: EG1 15: EG2 15: CG | 18–35 years old | VAS, anxiety level (STAI), and menstrual complaints. | The decreases in pain, anxiety levels, and menstrual complaints were higher in the KT group than those in the other two groups (p < 0.05). |
| Çelik and Apay [43] 2021 | A randomized prospective controlled trial | 124 participants 64: EG 60: CG | 18–22 years old | VAS and a dysmenorrhea monitoring form. | Progressive relaxation exercises are an effective method for reducing PD. |
| Author, Year | Description INTERVENTION | Duration of Sessions | Follow-Up | Support |
|---|---|---|---|---|
| Azima et al. [35] 2015 | Effect of massage therapy and isometric exercises on PD. EG 1. Massage therapy: effleurage massage in the pubic symphysis and navel area with lavender oil. EG 2. Isometric exercises. These exercises included 7 stages. CG. No intervention. | EG1: two consecutive cycles of effleurage massage (15 min each). On the first day of onset of menstrual pain and the following day. EG2: from 3rd day of menstruation for 8 weeks, 5 days/week, 2 sessions/ day, and 10 times/session. | Pain intensity and duration were measured in 3 consecutive cycles (at the peak of PD). The level of anxiety was measured 4 and 8 weeks after the treatment. | Exercises modified and confirmed by a specialized rehabilitation consultant. |
| Ortiz et al. [36] 2015 | Effectiveness of physical therapy treatment in relieving PD. EG. Physiotherapy program: Stretching, specific stretches, Kegel exercises, jogging, and relaxation exercises, performed 5–10 repetitions. CG. No intervention. | 5-phases of the physiotherapy program, for 50 min, 3 times/week, for 3 menstrual cycles (three months). | Measurements within 5 days after the last day of menstruation. | The program was led and monitored by three trained researchers. |
| Yonglitthipagon et al. [37] 2017 | Effect of a yoga program designed for non-athletic women with PD. EG Yoga. Poses of Shavasana, Surya Namaskar, Supta Vajrasana, Janu Sirsasana and Pashimottanasana GC. No intervention. | EG yoga for 30 min. per day, twice a week, for 12 weeks at home. | Evaluation at the beginning and at 12 weeks. | The booklet “Yoga for PD” was given to participants. |
| Thabet et al. [38] 2017 | Efficacy HILT compared to PEMF. EG 1. High Intensity Laser Therapy group. 15 min./ session EG 2. Pulsed Electromagnetic Field group. 30 min./ session. | 3 sessions, 3 consecutive menstrual cycles, when pain was intolerable (1st and 2nd day of menstrual flow). | Pain was assessed before and after the treatment, along with its relief after each treatment, and blood samples were collected before and after 3 months. | The devices were calibrated at the Department of physical therapy. |
| Özgül et al. [39] 2018 | Short-term effectiveness of Connective Tissue Manipulation (CTM). EG. CTM in pelvic zones (sacral, lumbar, lower thoracic) and anterior pelvic regions.CG. Lifestyle tips and stretching exercises. | The EG received CTM for 5 days/week, from ovulation until the beginning of the next period. | Measurements were taken at the beginning and immediately after the first menstruation in postintervention period. | Treatment was performed by a trained physiotherapist. |
| Tharani et al. [40] 2018 | Effects of stretching exercises in PD compared to aerobic dance. EG 1. Stretching and advice. EG 2. Aerobic dance. Both avoided exercise during the menstrual cycle. | Stretching EG1 and aerobic dance EG2, 45 min. for 3 days/week, for 8 weeks. | The evaluation was performed before and after the treatment. | Carried out in the Faculty of Physiotherapy. |
| Machado et al. [41] 2019 | Effects of thermotherapy and TENS, PPT and CTM in women with PD. EG1. Thermotherapy and TENS. EG2. Thermotherapy (microwave diathermy, 20 min.) EG3. TENS (200 μs, 100 Hz, 30 mi) CG. Placebo | One session Thermotherapy 20 min. TENS 30 min. | The evaluation was performed: at the beginning, after 20, 50 and 110 min., and 24 h. after the intervention. | Physiotherapy Department |
| Celenay et al. [42] 2020 | Effects of kinesio tape (KT) application on pain, anxiety, and menstrual complaints in women with PD. EG1. Kinesio Tape EG2. Sham Tape CG. No tape application | KT 2 days a week, from the ovulation until the next period begins. | Before and after the applications. | Applied by an experienced physical therapist (Kenzo Kase’s Kinesio Taping Method). |
| Çelik and Apay [43] 2021 | Effects of progressive relaxation exercises on pain in PD. EG. Progressive relaxation exercises CG. No intervention | Exercises, average of 30 min. for two months, every day or at least three times a week. | Values measured at the first, second, and third cycles. | Exercises were self-administered via compact disc. |
| Item (PEDro Scale) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azima et al. [35] 2015 | X | X | N | N | N | N | N | X | N | X | X | 5 |
| Ortiz et al. [36] 2015 | X | X | X | X | N | N | X | N | X | X | N | 7 |
| Yonglitthipagon et al. [37] 2017 | N | X | X | X | N | N | N | N | N | X | X | 5 |
| Thabet et al. [38] 2017 | N | X | N | X | N | N | N | N | N | X | X | 4 |
| Özgül et al. [39] 2018 | X | X | X | X | N | N | X | X | N | X | X | 8 |
| Tharani et al. [40] 2018 | N | X | N | X | N | N | N | N | N | X | X | 4 |
| Machado et al. [41] 2019 | N | X | X | X | X | N | X | N | N | X | X | 7 |
| Celenay et al. [42] 2020 | N | X | N | X | N | N | N | X | N | X | X | 5 |
| Çelik and Apay [43] 2021 | X | X | X | X | X | N | N | N | N | X | X | 7 |
| Certainty Assessment | No of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Physiotherapy Treatment | Control Group | Relative (95% CI) | Absolute (95% CI) | ||
| 9 | randomised trials | serious a | serious b | not serious | not serious | none | 381 | 371 | - | MD 1.13 lower (1.61 lower to 0.64 lower) | ⨁⨁◯◯ LOW | IMPORTANT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. https://doi.org/10.3390/ijerph18157832
López-Liria R, Torres-Álamo L, Vega-Ramírez FA, García-Luengo AV, Aguilar-Parra JM, Trigueros-Ramos R, Rocamora-Pérez P. Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(15):7832. https://doi.org/10.3390/ijerph18157832
Chicago/Turabian StyleLópez-Liria, Remedios, Lucía Torres-Álamo, Francisco A. Vega-Ramírez, Amelia V. García-Luengo, José M. Aguilar-Parra, Rubén Trigueros-Ramos, and Patricia Rocamora-Pérez. 2021. "Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 15: 7832. https://doi.org/10.3390/ijerph18157832
APA StyleLópez-Liria, R., Torres-Álamo, L., Vega-Ramírez, F. A., García-Luengo, A. V., Aguilar-Parra, J. M., Trigueros-Ramos, R., & Rocamora-Pérez, P. (2021). Efficacy of Physiotherapy Treatment in Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(15), 7832. https://doi.org/10.3390/ijerph18157832







