Cocaine-Induced Midline Destructive Lesions (CIMDL): A Real Challenge in Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
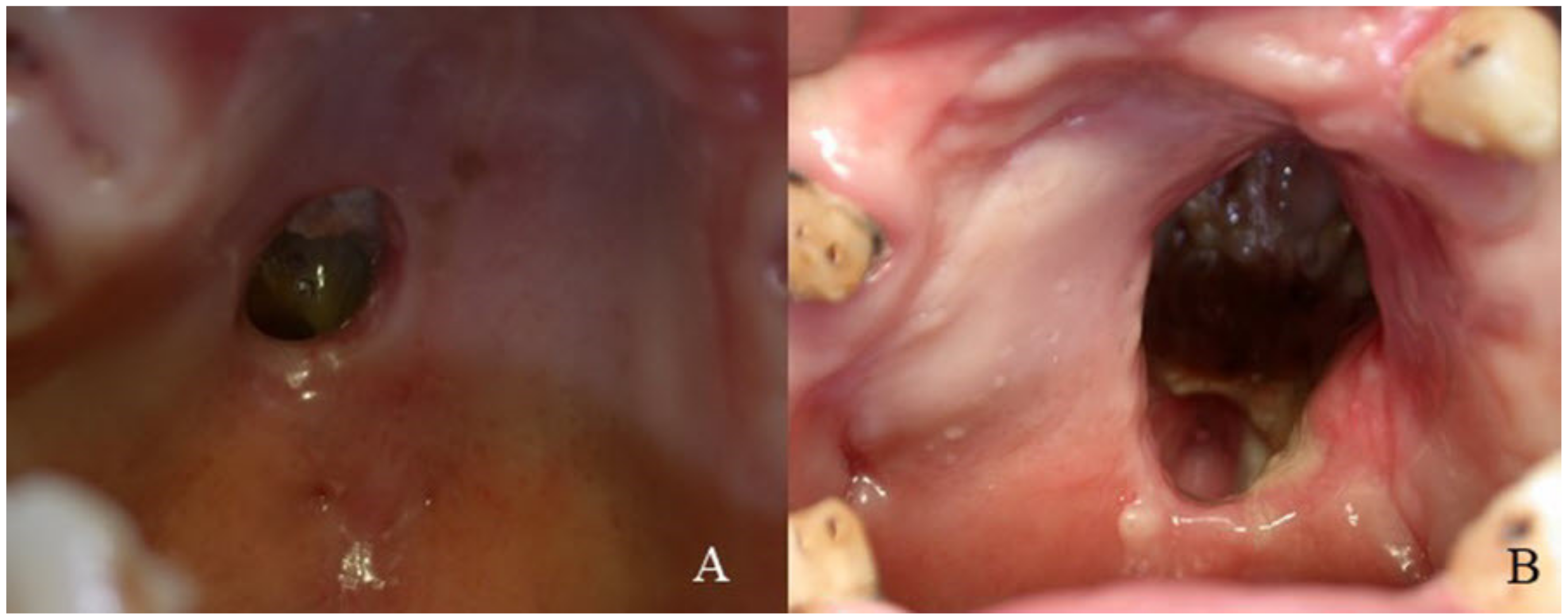
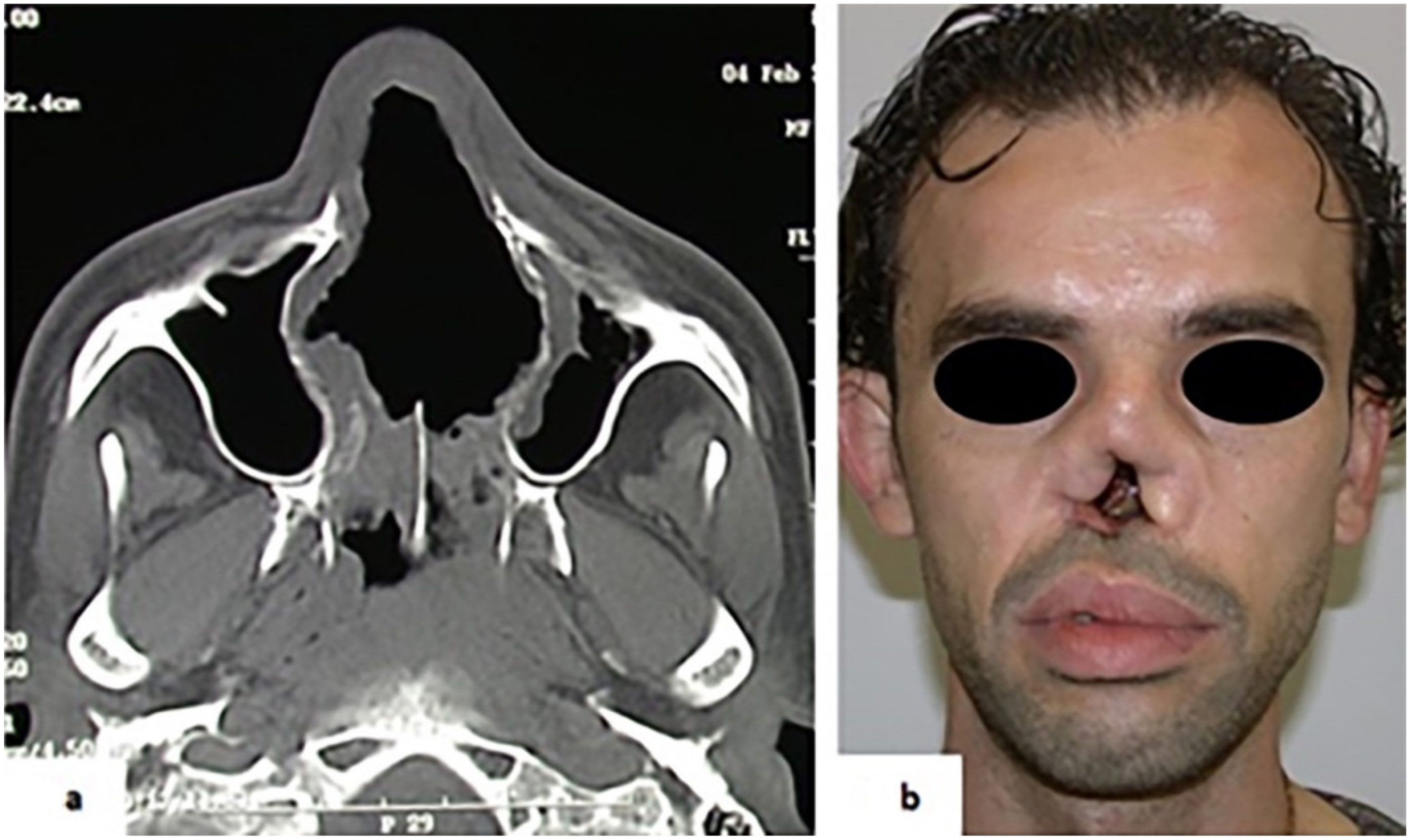
3. Results
4. Discussion
- Detailed medical history to find out the cocaine inhalation abuse;
- Accurate extra- and intra-oral examination to evaluate the tissue damages;
- Histological examination on mucosal biopsies to keep out diseases with typical features;
- CT and MRI examination;
- Laboratory findings:
- ◦
- Complete blood count and sedimentation rate;
- ◦
- ANA test to exclude systemic lupus erythematous;
- ◦
- VDRL test to exclude tertiary syphilis;
- ◦
- Leishmaniasis and fungal serology;
- ◦
- ANCA test to exclude WG;
- ◦
- Chest X-ray to exclude tuberculosis or WG.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyer, B.A.; Grist, W.; Muller, S. Aggressive destructive midfacial lesion from cocaine abuse. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 465–470. [Google Scholar] [CrossRef]
- Kuriloff, D.B.; Kimmelman, C.P. Osteocartilaginous necrosis of the sinonasal tract following cocaine abuse. Laryngoscope 1989, 99, 918–924. [Google Scholar] [CrossRef]
- Gendeh, B.S.; Ferguson, B.J.; Johnson, J.T.; Kapadia, S. Progressive septal and palatal perforation secondary to intranasal cocaine abuse. Med. J. Malays. 1998, 53, 435–438. [Google Scholar]
- Smith, J.C.; Kacker, A.; Anand, V.K. Midline nasal and hard palate destruction in cocaine abusers and cocaine’s role in rhinologic practice. Ear Nose Throat J. 2002, 81, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Rubin, K. The manifestation of cocaine-induced midline destructive lesion in bone tissue and its identification in human skeletal remains. Forensic. Sci. Int. 2013, 231, 408. [Google Scholar] [CrossRef]
- Trimarchi, M.; Bussi, M.; Sinico, R.A.; Meroni, P.; Specks, U. Cocaine-induced midline destructive lesionsan autoimmune disease? Autoimmun. Rev. 2013, 12, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Morassi, M.L.; Trimarchi, M.; Nicolai, P.; Gregorini, G.; Maroldi, R.; Specks, U.; Facchetti, F. Cocaine, ANCA, and Wegener’s granulomatosis. Pathologica 2001, 93, 581–583. [Google Scholar]
- Talbott, J.F.; Gorti, G.K.; Koch, R.J. Midfacial osteomyelitis in a chronic cocaine abuser: A case report. Ear Nose Throat J. 2001, 80, 738–740+742–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimarchi, M.; Nicolai, P.; Lombardi, D.; Facchetti, F.; Morassi, M.L.; Maroldi, R.; Gregorini, G.; Specks, U. Sinonasal osteocartilaginous necrosis in cocaine abusers: Experience in 25 patients. Am. J. Rhinol. 2003, 17, 33–43. [Google Scholar] [CrossRef]
- Melo, C.A.A.; Guimaraes, H.R.G.; Medeiros, R.C.F.; Souza, G.C.A.; Santos, P.; Torres, A. Oral changes in cocaine abusers: An integrative review. Braz. J. Otorhinolaryngol 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.F.; Madeo, M.C.; Martinez, M.; Vazquez, M.E. Case for diagnosis. Palate perforation due to cocaine use. Bras. Derm. 2017, 92, 877–878. [Google Scholar] [CrossRef] [Green Version]
- Perez Alamino, R.; Espinoza, L.R. Vasculitis mimics: Cocaine-induced midline destructive lesions. Am. J. Med. Sci 2013, 346, 430–431. [Google Scholar] [CrossRef]
- Wiesner, O.; Russell, K.A.; Lee, A.S.; Jenne, D.E.; Trimarchi, M.; Gregorini, G.; Specks, U. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004, 50, 2954–2965. [Google Scholar] [CrossRef] [PubMed]
- Rampi, A.; Vinciguerra, A.; Bondi, S.; Policaro, N.S.; Gastaldi, G. Cocaine-Induced Midline Destructive Lesions: A Real Challenge in Oral Rehabilitation. Int. J. Environ. Res. Public Health 2021, 18, 3219. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, J.; Corchero, G.; Soler, F. Surgical treatment of cocaine-induced palatal perforations: Report of three cases and literature review. J. Clin. Exp. Dent. 2021, 13, e201–e206. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Rampi, A.; Vinciguerra, A.; Polizzi, E.; Policaro, N.S.; Gastaldi, G. Palatal prosthetic rehabilitation in patients affected by cocaine-induced midline destructive lesions. J. Biol. Regul. Homeost. Agents 2020, 34, 59–68. [Google Scholar]
- Chou, D.W.; Shih, C. Surgical Reconstruction of Cocaine-Induced Cleft Lip: A Case Report. Perm J. 2020, 24, 24. [Google Scholar] [CrossRef]
- Trimarchi, M.; Sykopetrites, V.; Bussi, M. Management of a cocaine-induced palatal perforation with a nasal septal button. Ear Nose Throat J. 2016, 95, E36–E38. [Google Scholar] [CrossRef]
- Colletti, G.; Autelitano, L.; Chiapasco, M.; Biglioli, F.; Giovanditto, F.; Mandala, M.; Allevi, F. Comprehensive surgical management of cocaine-induced midline destructive lesions. J. Oral Maxillofac. Surg. 2014, 72, 1395. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Steinbacher, D.M. Repair of the cocaine-induced cleft palate using the modified double-opposing z-plasty. Cleft Palate Craniofac. J. 2013, 50, 494–497. [Google Scholar] [CrossRef]
- Fuchs, H.A. Granulomatous disorders of the nose and paranasal sinuses. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Zabihiyeganeh, M.; Haqiqi, A. Differentiation of Cocaine-Induced Midline Destructive Lesions from ANCA-Associated Vasculitis. Iran. J. Otorhinolaryngol. 2018, 30, 309–313. [Google Scholar]
- Peikert, T.; Hummel, A.M.; McKenney, M.E.; Gregorini, G.; Trimarchi, M. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum. 2008, 58, 1546–1551. [Google Scholar] [CrossRef]
| Patients characteristics | Data | % |
|---|---|---|
| Males | 5 | 62.5% |
| Females | 3 | 37.5% |
| Males-to-females ratio | 1.67:1 | |
| Age range | 25–46 years | |
| Mean age | 36.37 ± 7.13 SD | |
| Mean duration of cocaine abuse | 13 years | |
| Mean daily dose | 3.5 grams |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cosola, M.; Ambrosino, M.; Limongelli, L.; Favia, G.; Santarelli, A.; Cortelazzi, R.; Lo Muzio, L. Cocaine-Induced Midline Destructive Lesions (CIMDL): A Real Challenge in Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 7831. https://doi.org/10.3390/ijerph18157831
Di Cosola M, Ambrosino M, Limongelli L, Favia G, Santarelli A, Cortelazzi R, Lo Muzio L. Cocaine-Induced Midline Destructive Lesions (CIMDL): A Real Challenge in Diagnosis. International Journal of Environmental Research and Public Health. 2021; 18(15):7831. https://doi.org/10.3390/ijerph18157831
Chicago/Turabian StyleDi Cosola, Michele, Mariateresa Ambrosino, Luisa Limongelli, Gianfranco Favia, Andrea Santarelli, Roberto Cortelazzi, and Lorenzo Lo Muzio. 2021. "Cocaine-Induced Midline Destructive Lesions (CIMDL): A Real Challenge in Diagnosis" International Journal of Environmental Research and Public Health 18, no. 15: 7831. https://doi.org/10.3390/ijerph18157831
APA StyleDi Cosola, M., Ambrosino, M., Limongelli, L., Favia, G., Santarelli, A., Cortelazzi, R., & Lo Muzio, L. (2021). Cocaine-Induced Midline Destructive Lesions (CIMDL): A Real Challenge in Diagnosis. International Journal of Environmental Research and Public Health, 18(15), 7831. https://doi.org/10.3390/ijerph18157831








