The WOMEN-UP Solution, a Patient-Centered Innovative e-Health Tool for Pelvic Floor Muscle Training: Qualitative and Usability Study during Early-Stage Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Stage 1. Qualitative Assessment: Identify Key Stakeholders’ Needs
2.1.1. Participants
2.1.2. Data Analysis
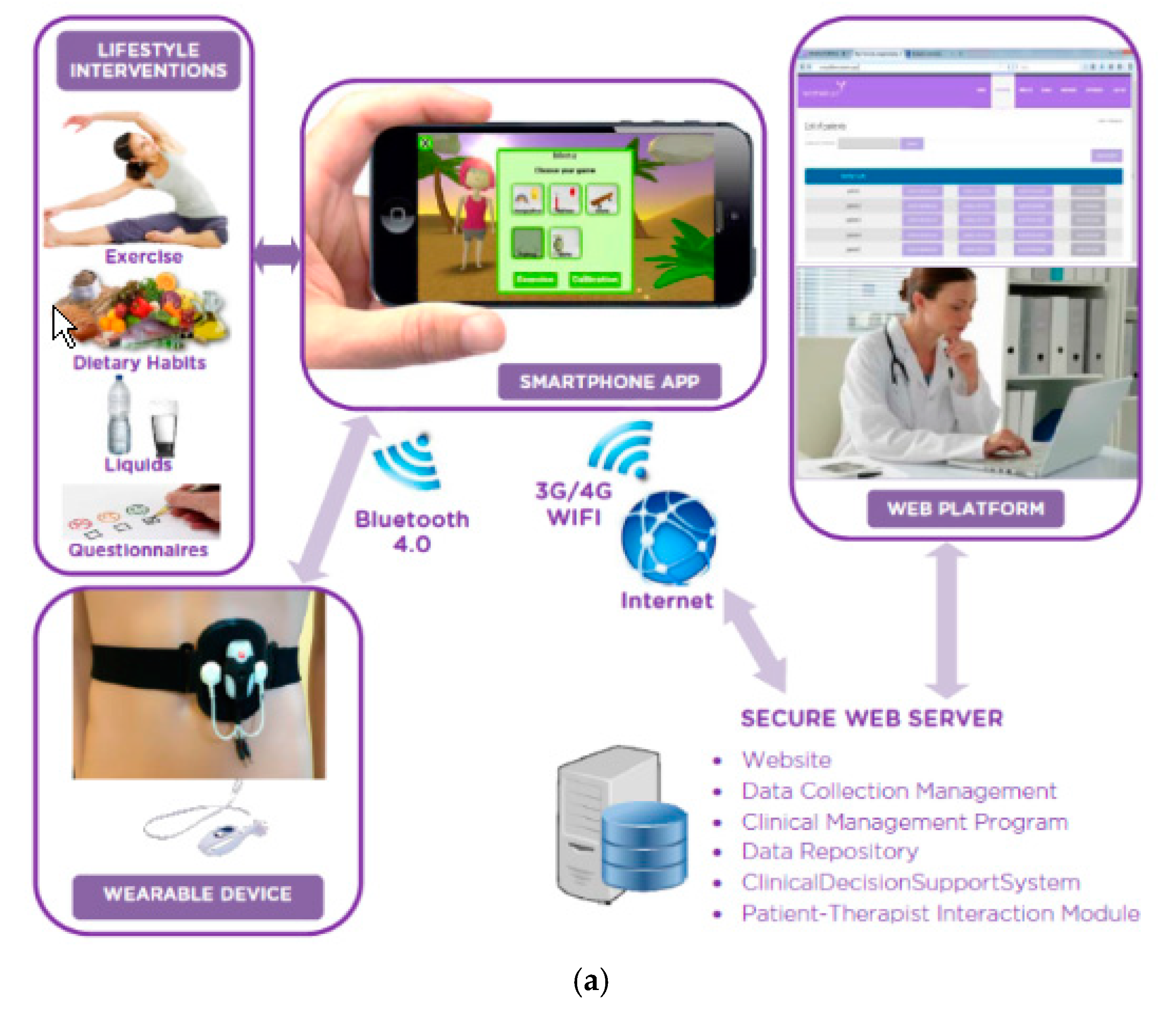
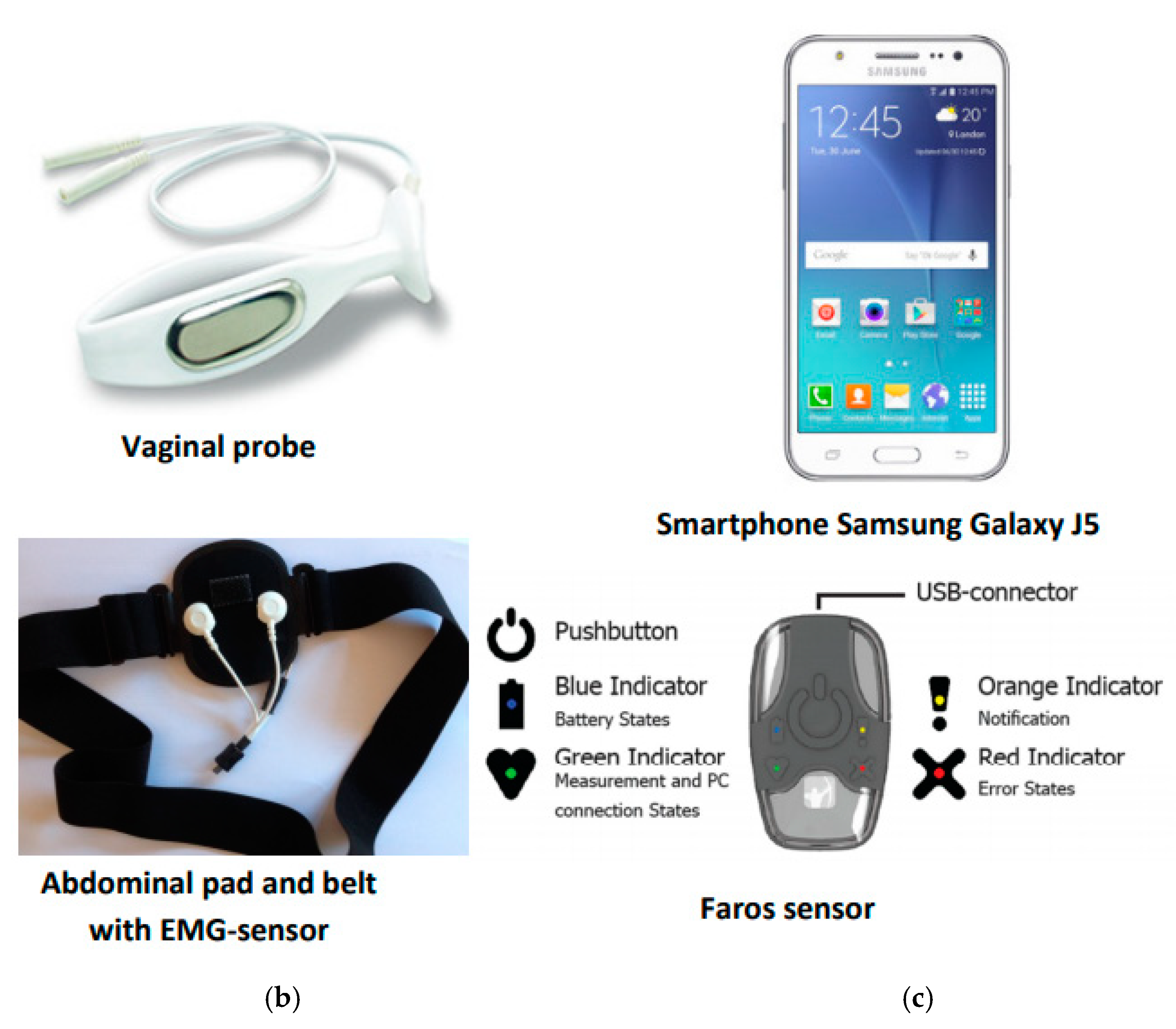
2.2. Stage 2. WOMEN-UP Solution: First Prototype
2.3. Stage 3. Usability Testing
2.3.1. Participants
2.3.2. Qualitative Analysis
2.3.3. Multidisciplinary Solution Optimization
3. Results
3.1. Stage 1. Qualitative Assessment: Identify Key Stakeholders’ Needs
- Adherence improvement:“Having the home device allows greater availability and gives them autonomy, which facilitates the adherence to the PFMT.”“The verbal and visual instructions facilitate the successful accomplishment of the PFMT, and consequently, improve concentration and adherence to treatment.”“Having a biofeedback device at home helps them to motivate themselves to continue doing the exercises, thus creating more adherence to PFMT.”“The possibility to train home increased the amount of exercises done during the study period.”
- Performance improvement:“A key issue was also the easy-to-use technique with easily found start-buttons and no wires and very clear instructions.”“Devices help to improve the self-awareness of pelvic floor muscles by vaginal probe and by control over the use of the abdominal muscles, and allow better integration about the instructions given by the therapist.”“Self-assessment in each session may increase the motivation to overcome the results of the training itself.”
3.2. Stage 3. Usability Testing
3.2.1. Usability Aids
3.2.2. Usability Issues
3.2.3. Multidisciplinary Solution Optimization
4. Discussion
4.1. Stage 1. Qualitative Assessment: Identify Key Stakeholders’ Needs
4.2. Stage 3. Usability Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milsom, I.; Altman, D.; Cartwrigth, R.; Lapitan, M.C.; Nelson, R.; Sjöström, S.; Tikkinen, K.a.O. Epidemiology of urinary incontinence (UI) and other lower urinary tract symptoms (LUTS), pelvic organ prolapse (POP) and anal (AI)incontinence. In International Consultation on Incontinence, 6th ed.; Abrams, P., Cardozo, L., Wagg, A., Wein, A., Eds.; Health Publications Ltd.: London, UK, 2017; pp. 1–142. ISBN 978–0–9569607-3-3. [Google Scholar]
- Dumoulin, C. Adult Conservative Management. Committee 12. In International Consultation on Incontinence, 6th ed.; Abrams, P., Cardozo, L., Wagg, A., Wein, A., Eds.; Health Publications Ltd.: London, UK, 2017; pp. 1443–1628. ISBN 978–0–9569607-3-3. [Google Scholar]
- Novara, G.; Checcucci, E.; Crestani, A.; Abrate, A.; Esperto, F.; Pavan, N.; De Nunzio, C.; Galfano, A.; Giannarini, G.; Gregori, A.; et al. Telehealth in Urology: A Systematic Review of the Literature. How Much Can Telemedicine Be Useful during and after the COVID-19 Pandemic? Eur. Urol. 2020, 78, 786–811. [Google Scholar] [CrossRef]
- Grimes, C.L.; Balk, E.M.; Crisp, C.C.; Antosh, D.D.; Murphy, M.; Halder, G.E.; Jeppson, P.C.; Lebrun, E.E.W.; Raman, S.; Kim-Fine, S.; et al. A guide for urogynecologic patient care utilizing telemedicine during the COVID-19 pandemic: Review of existing evidence. Int. Urogynecol. J. 2020, 31, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Woods, M.; Amalberti, R.; Goodman, S.; Bergman, B.; Glasziou, P. Problems and promises of innovation: Why healthcare needs to rethink its love/hate relationship with the new. BMJ Qual. Saf. 2011, 20, i47–i51. [Google Scholar] [CrossRef] [PubMed]
- Borello-France, D.; Burgio, K.L.; Goode, P.S.; Ye, W.; Weidner, A.C.; Lukacz, E.S.; Jelovsek, J.-E.; Bradley, C.; Schaffer, J.; Hsu, Y.; et al. Adherence to Behavioral Interventions for Stress Incontinence: Rates, Barriers, and Predictors. Phys. Ther. 2013, 93, 757–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Or, C.K.L.; Karsh, B.-T. A Systematic Review of Patient Acceptance of Consumer Health Information Technology. J. Am. Med. Inform. Assoc. 2009, 16, 550–560. [Google Scholar] [CrossRef]
- Zapata, B.C.; Fernández-Alemán, J.L.; Idri, A.; Toval, A. Empirical Studies on Usability of mHealth Apps: A Systematic Literature Review. J. Med. Syst. 2015, 39, 1. [Google Scholar] [CrossRef]
- Gurupur, V.P.; Wan, T.T.H. Challenges in implementing eHealth interventions: A technical perspective. eHealth 2017, 3, 32. [Google Scholar] [CrossRef]
- Schnall, R.; Rojas, M.; Bakken, S.; Brown, W.; Carballo-Dieguez, A.; Carry, M.; Gelaude, D.; Mosley, J.P.; Travers, J. A user-centered model for designing consumer mobile health (mHealth) applications (apps). J. Biomed. Inform. 2016, 60, 243–251. [Google Scholar] [CrossRef]
- Brown, W.; Yen, P.-Y.; Rojas, M.; Schnall, R. Assessment of the Health IT Usability Evaluation Model (Health-ITUEM) for evaluating mobile health (mHealth) technology. J. Biomed. Inform. 2013, 46, 1080–1087. [Google Scholar] [CrossRef]
- Agarwal, S.; LeFevre, A.; Lee, J.; L’Engle, K.; Mehl, G.; Sinha, C.; Labrique, A. Guidelines for reporting of health interventions using mobile phones: Mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ 2016, 352, i1174. [Google Scholar] [CrossRef]
- Jaspers, M.W. A comparison of usability methods for testing interactive health technologies: Methodological aspects and empirical evidence. Int. J. Med. Inform. 2009, 78, 340–353. [Google Scholar] [CrossRef]
- Giunti, G.; Mylonopoulou, V.; Romero, O.R. More stamina, a gamified eHealth solution for persons with multiple sclerosis: Research through design. J. Med. Internet Res. 2018, 6, e51. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Slevin, P.; Ward, T.; Caulfield, B. A Wearable Sensor-Based Exercise Biofeedback System: Mixed Methods Evaluation of Formulift. JMIR eHealth uHealth 2018, 6, e33. [Google Scholar] [CrossRef]
- Thakar, R.; Cundiff, G.W. In pursuit of patient-centered innovation: The role of professional organizations. Int. Urogynecol. J. 2020, 31, 423–428. [Google Scholar] [CrossRef]
- Herderschee, R.; Hay-Smith, E.J.C.; Herbison, G.P.; Roovers, J.P.; Heineman, M.J. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst. Rev. 2011, CD009252. [Google Scholar] [CrossRef] [PubMed]
- Tăut, D.; Pintea, S.; Roovers, J.-P.W.R.; Mañanas, M.A.; Băban, A. Play seriously: Effectiveness of serious games and their features in motor rehabilitation. A meta-analysis. NeuroRehabilitation 2017, 41, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Hertzum, M.; Holmegaard, K.D. Thinking Aloud Influences Perceived Time. Hum. Factors J. Hum. Factors Ergon. Soc. 2014, 57, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Clemmensen, T.; Yssing, C. Getting access to what goes on in people’s heads? In Proceedings of the Second Nordic Conference on Human-Computer Interaction-NordiCHI ’02, New York, NY, USA, 19–23 October 2002; Volume 101. [Google Scholar] [CrossRef]
- Zhang, J.; Johnson, T.; Patel, V.L.; Paige, D.L.; Kubose, T. Using usability heuristics to evaluate patient safety of medical devices. J. Biomed. Inform. 2003, 36, 23–30. [Google Scholar] [CrossRef]
- Venkatesh, V.; Thong, J.Y.; Xu, X. Consumer Acceptance and Use of Information Technology: Extending the Unified Theory of Acceptance and Use of Technology. MIS Q. 2012, 36, 157. [Google Scholar] [CrossRef]
- Nielsen, J. Usability Engineering; Morgan Kaufmann Publishers Inc.: San Francisco, CA, USA, 1994; ISBN 9780080520292. [Google Scholar]
- Bhattacharyya, O.; Blumenthal, D.; Stoddard, R.; Mansell, L.; Mossman, K.; Schneider, E.C. Redesigning care: Adapting new improvement methods to achieve person-centred care. BMJ Qual. Saf. 2018, 28, 242–248. [Google Scholar] [CrossRef]
- Vermeir, J.F.; White, M.J.; Johnson, D.; Crombez, G.; Van Ryckeghem, D.M.L. The Effects of Gamification on Computerized Cognitive Training: Systematic Review and Meta-Analysis. JMIR Serious Games 2020, 8, e18644. [Google Scholar] [CrossRef] [PubMed]
- Tarver, M.E.; Neuland, C. Integrating Patient Perspectives into Medical Device Regulatory Decision Making to Advance Innovation in Kidney Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 636–638. [Google Scholar] [CrossRef] [PubMed]
| Feature | Option | Responses | % Participants |
|---|---|---|---|
| Vaginal probe shape |
| 11 | 50.0% |
| 5 | 22.7% | |
| 4 | 18.2% | |
| 2 | 9.1% | |
| Valid responses | 22 | 100% | |
| Vaginal probe material |
| 19 | 86.4% |
| 18 | 81.8% | |
| 12 | 54.5% | |
| 10 | 45.5% | |
| 9 | 40.9% | |
| 9 | 40.9% | |
| 7 | 31.8% | |
| 1 | 4.5% | |
| 1 | 4.5% | |
| Total responses | 87 | ||
| Device perception |
| 18 | 81.8% |
| 17 | 77.3% | |
| 17 | 77.3% | |
| 16 | 72.7% | |
| 15 | 68.2% | |
| 14 | 63.6% | |
| 12 | 54.5% | |
| Total responses | 110 | ||
| Motivation to exercise |
| 18 | 81.8% |
| 2 | 9.1% | |
| Valid responses | 20 | 90.9% | |
| Empty responses | 2 | 9.1% | |
| Feedback preferences |
| 6 | 27.3% |
| 5 | 22.7% | |
| 2 | 9.1% | |
| 2 | 9.1% | |
| Valid responses | 15 | 68.2% | |
| Empty responses | 7 | 31.8% |
| Topic | Recommendations | Risks |
|---|---|---|
| global functionality |
|
|
| mechanical design |
|
|
| system features(in order ofimportance) |
|
|
| device instructions |
|
|
| feedback |
|
|
| patient–professionalinteractionpreferences |
|
|
| system interface |
|
|
| serious games |
|
|
| Category | Summarized Facilitator | Description | Number of Facilitators |
|---|---|---|---|
| Mobile Application general | Positive attitude toward the system | Positive attitude towards the system, application and the flexibility provided by the solution | 7 |
| Calibration | Easy-to-learn calibration | The calibration of the devices has a fast learning curve | 1 |
| Serious games | Good biofeedback | Users appreciate the usefulness of direct visual feedback on the muscular contractions and the final scores provided | 7 |
| Good control during games | Additional feedback given to the user (next actions to be performed, remaining time) helps the feeling of being in control of exercising | 3 | |
| Good game design | Games are well designed for these kind of rehabilitation exercises | 1 | |
| Games facilitate exercising | Exercises could not have been done without the games | 4 | |
| Game choice | Users can choose from different games, based on their preferences, to perform the same exercise | 2 | |
| Enjoyable gaming | Games make exercising fun and enjoyable | 4 | |
| Devices | Functioning and comfort | Users feel comfortable with the use of the wearable device | 2 |
| Category | Issues | Type | Description | Comments |
|---|---|---|---|---|
| Mobile Application general | 9 | 3 major, 2 minor 1 trivial and 3 nihil | Not all the functionalities were in the App, outdated design and sometimes used medical jargon abbreviations (PFMT) | As this is a pre-product test step, the online platform was chosen to summarize all functionalities since it was easier to increase the software functions without needing to install new App versions constantly. In addition, design was applied for functionality above cosmetics. |
| Mobile Application navigation | 7 | 6 trivial and 1 minor | User could not use back button and the design (visibility) of some buttons could be improved. | Some issues were due to an incomplete development at the time of testing. |
| Calibration | 9 | 4 trivial, 4 minor and 1 major. | The major issue was due to an absence of assistance/messages in case of error in the process. In addition, the display of the calibration process can be improved with more information and clear visibility along the whole process to guide the user. | Improvement implemented by a simpler calibration method. |
| Serious games | 13 | 5 major, 3 minor and 5 trivial. | Some games and menus were unclear and with lags. Absence of encouragement and levels in some games. The visualization of some elements could be improved. | Major and minor issues information was used to help improving the application for future versions. The absence of description was improved by means of implementing a demo screen before playing, |
| Gaming biofeedback | 6 | 2 minor and 4 trivial. | Most of the issues are referred to the score visualization, since it complicates the visualization of the game and it is uninformative. They also refer to the abdominal muscle activity: visualization and artifacts. | The score issues were improved by showing the information graphically and concentrated in the post-game screen. Respecting the abdominal muscle activity, instructions were added to explain to the user how to avoid artifacts. |
| Training evaluation | 5 | 1 major, 2 minor and 2 trivial | Major and Minor issues referred to lack of feedback information after performing the exercises and also on how to perform the exercises (relaxation and contraction). | The online platform contains the feedback information since it should also be accessible by the therapist, as mentioned in the Mobile Application General section. Other issues were used to help improving the application. |
| Web platform | 4 | 1 major and 4 trivial. | The major issue is referring to the poor visualization of the results. Trivial issues refer to some issues on the information display. | In the major issue, the visualization was more intended to be useful for the study outcomes. The other issues were used to help improve the web platform. |
| Devices | 12 | 1 minor, 9 trivial and 2 nihil. | Most of the issues referred to the feeling of the devices. Vaginal: unpleasant, rigid, lack of low-battery warning, only 1 size; abdominal: narrow belt, hook-in system. Also lack of available instructions. | In most cases, the issues referred to the design, which maintained unaltered for measurability reasons. |
| Device connectivity | 4 | 1 major, 2 minor and 1 trivial | Lack of assistance and messages when connecting or having troubles connecting. Also, names of the devices were unclear. | Improvements implemented through the manual, increasing the visual pop-up, when possible. Issues concerning Android were unaltered. |
| Training schedule | 4 | 1 minor, 2 trivial and 1 nihil. | In general, the user was confused with the names given to the training sessions when having to select to recover not-performed sessions. They were marked as uncompleted and the user was disappointed with such labels. | In general, they were expected issues due to an incomplete development at time of testing. |
| Communications with the user | 7 | 1 severe, 1 minor, 2 trivial and 3 nihil. | User reported some errors concerning not being aware of receiving messages since they were shown at the moment the user uses the App, such as communications of the therapist, reminders (questionnaires, exercises…), etc. Other reported issues were the web accessibility of the instructions (only on paper). | Some of them were expected since uncomplete development at time of testing and were improved later on. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anglès-Acedo, S.; López-Frías, L.; Soler, V.; Alonso, J.F.; Kastelein, A.W.; Graaf, B.C.d.; Vodegel, E.V.; Tervo, J.; Baban, A.; Espuña-Pons, M.; et al. The WOMEN-UP Solution, a Patient-Centered Innovative e-Health Tool for Pelvic Floor Muscle Training: Qualitative and Usability Study during Early-Stage Development. Int. J. Environ. Res. Public Health 2021, 18, 7800. https://doi.org/10.3390/ijerph18157800
Anglès-Acedo S, López-Frías L, Soler V, Alonso JF, Kastelein AW, Graaf BCd, Vodegel EV, Tervo J, Baban A, Espuña-Pons M, et al. The WOMEN-UP Solution, a Patient-Centered Innovative e-Health Tool for Pelvic Floor Muscle Training: Qualitative and Usability Study during Early-Stage Development. International Journal of Environmental Research and Public Health. 2021; 18(15):7800. https://doi.org/10.3390/ijerph18157800
Chicago/Turabian StyleAnglès-Acedo, Sònia, Lorena López-Frías, Vicenç Soler, Joan Francesc Alonso, Arnoud W. Kastelein, Boris C. de Graaf, Eva. V. Vodegel, Jaana Tervo, Adriana Baban, Montserrat Espuña-Pons, and et al. 2021. "The WOMEN-UP Solution, a Patient-Centered Innovative e-Health Tool for Pelvic Floor Muscle Training: Qualitative and Usability Study during Early-Stage Development" International Journal of Environmental Research and Public Health 18, no. 15: 7800. https://doi.org/10.3390/ijerph18157800
APA StyleAnglès-Acedo, S., López-Frías, L., Soler, V., Alonso, J. F., Kastelein, A. W., Graaf, B. C. d., Vodegel, E. V., Tervo, J., Baban, A., Espuña-Pons, M., & on behalf of the WOMEN-UP Consortium. (2021). The WOMEN-UP Solution, a Patient-Centered Innovative e-Health Tool for Pelvic Floor Muscle Training: Qualitative and Usability Study during Early-Stage Development. International Journal of Environmental Research and Public Health, 18(15), 7800. https://doi.org/10.3390/ijerph18157800






