Toxicity of a Binary Mixture of TiO2 and Imidacloprid Applied to Chlorella vulgaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Culture
2.2. Toxicity of TiO2 and IMD as Individual and Binary Mixtures
2.3. Algal Growth Analysis
3. Results
3.1. Growth of Chlorella vulgaris in Batch Culture
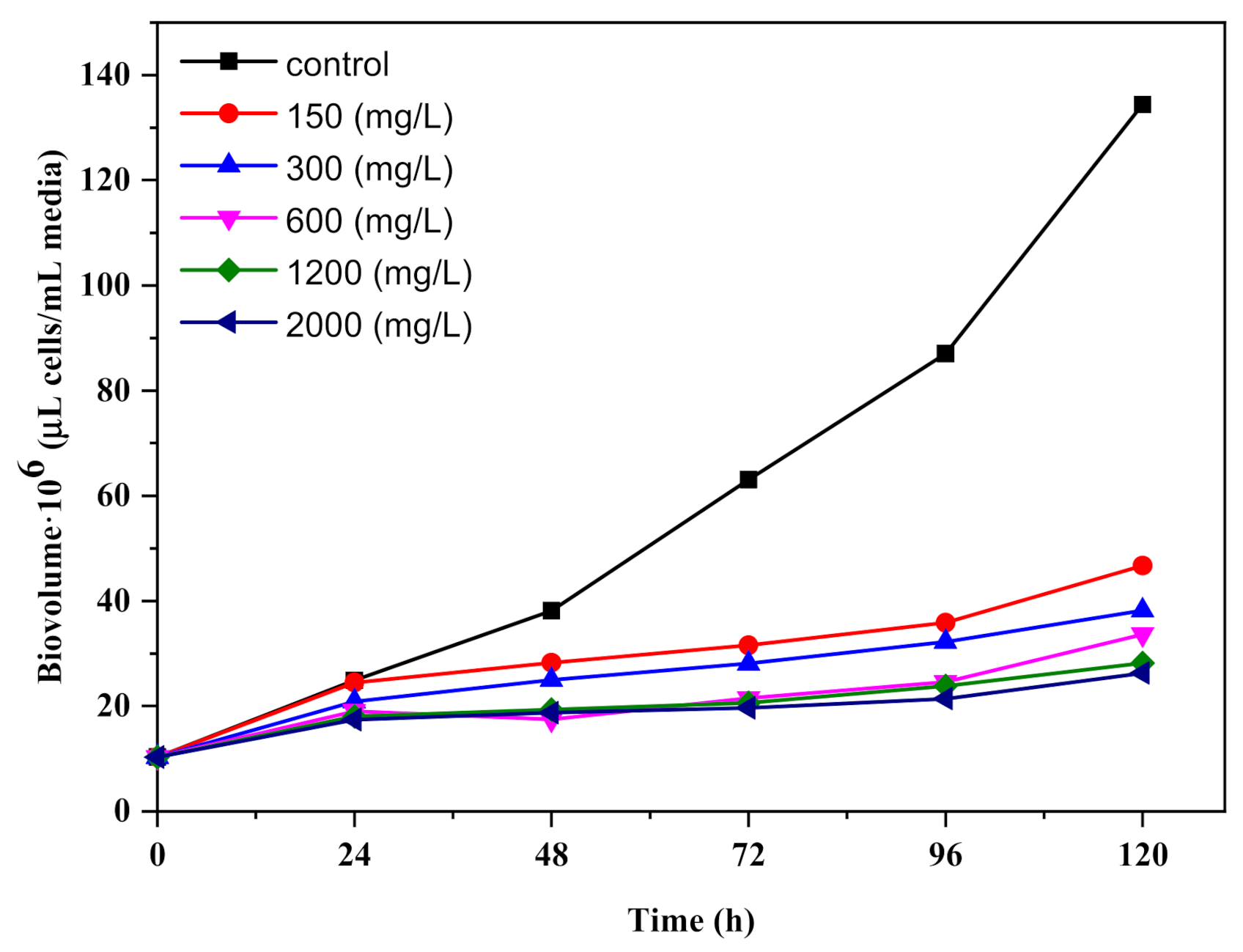
3.2. Growth of Chlorella vulgaris in Batch Culture under TiO2 Nanoparticle Stress
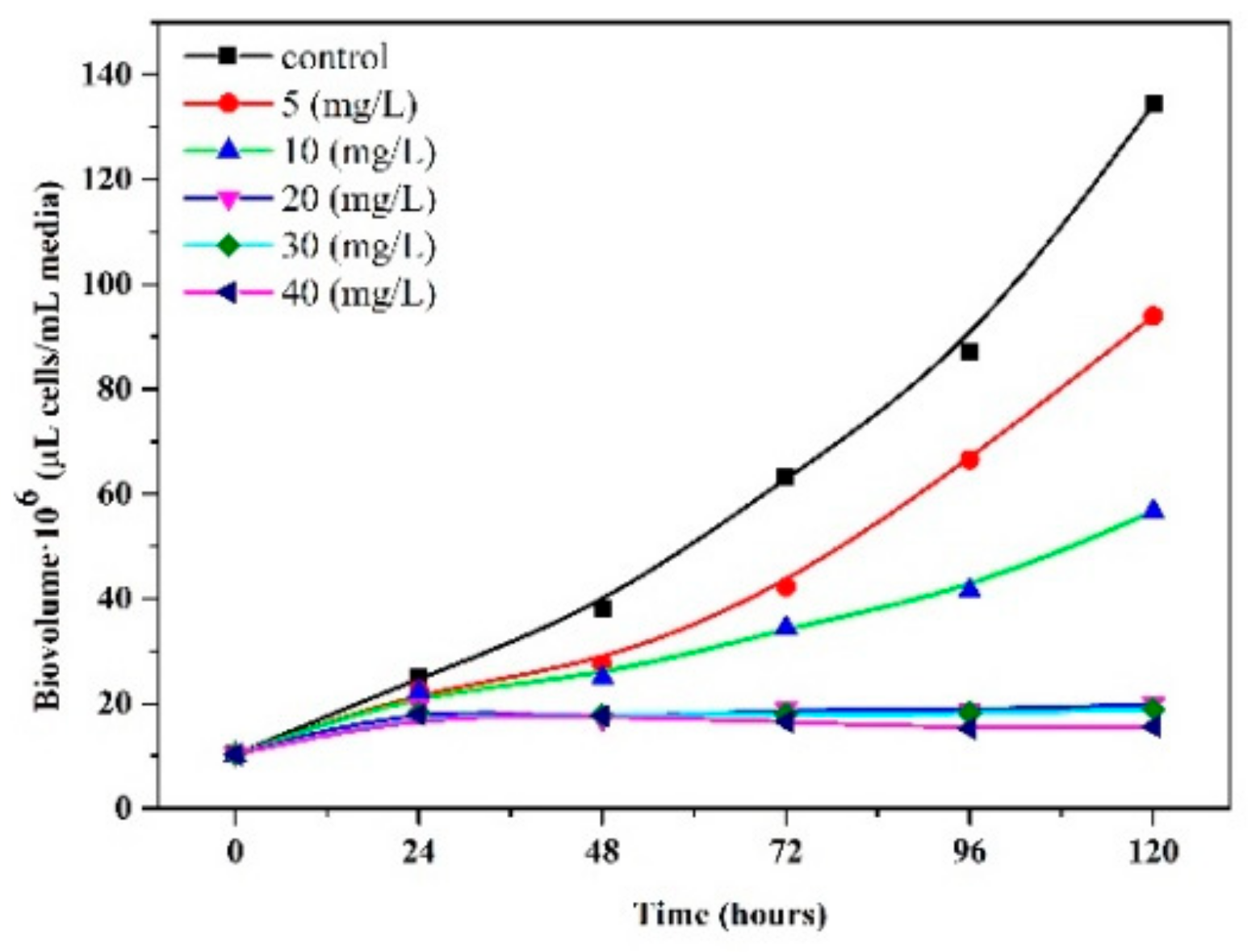
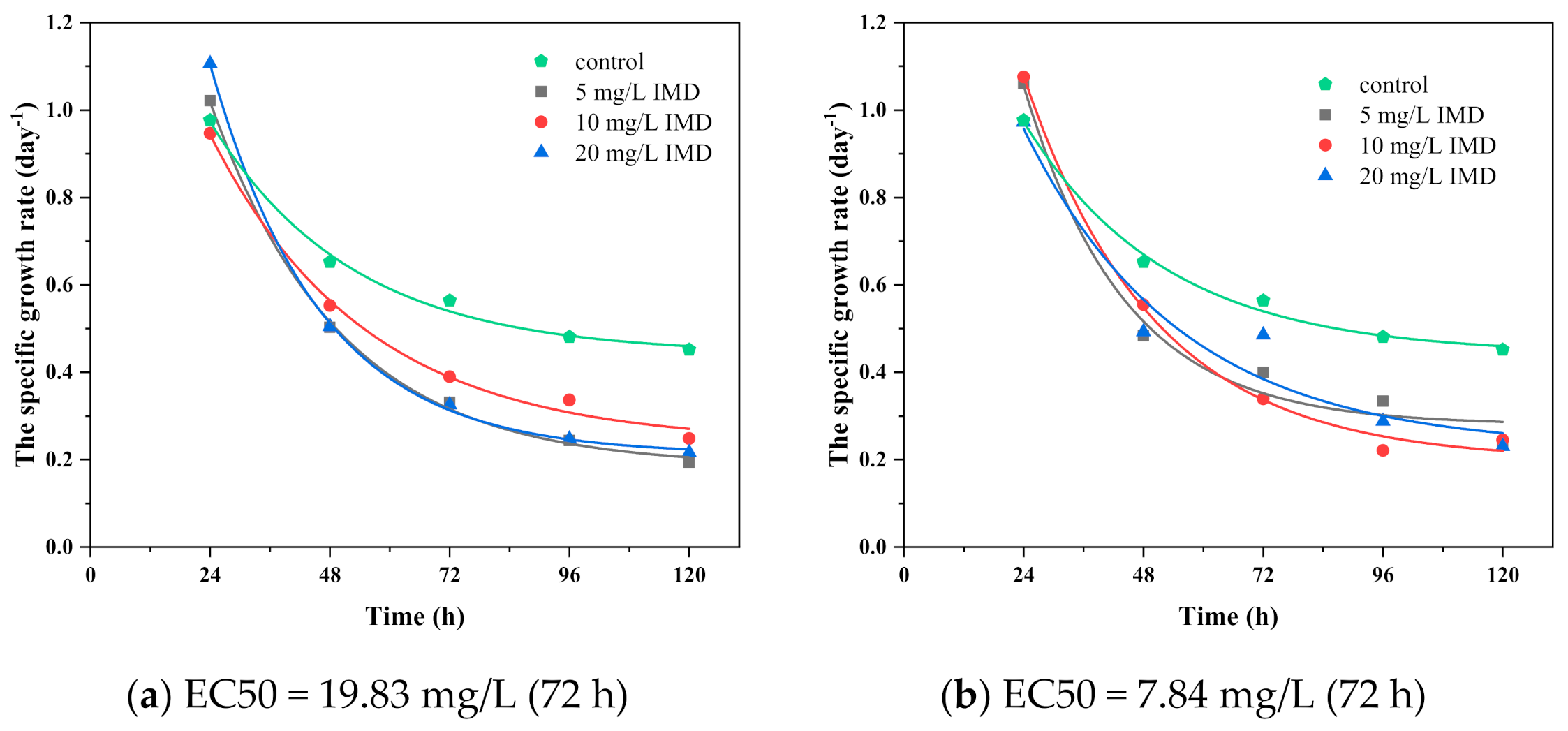
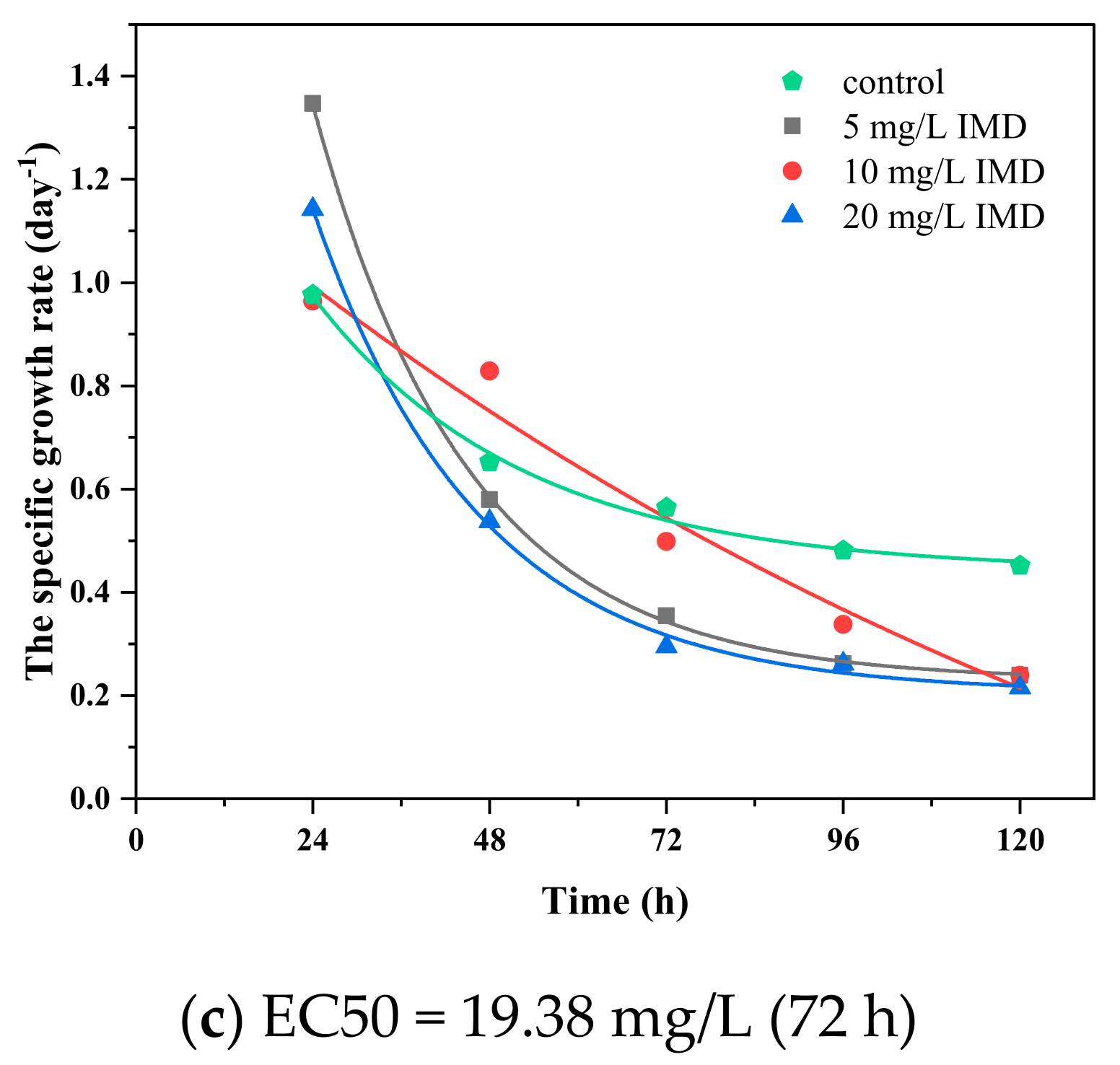
3.3. Effect of the Imidacloprid on Algal Growth and Cellular Diameter
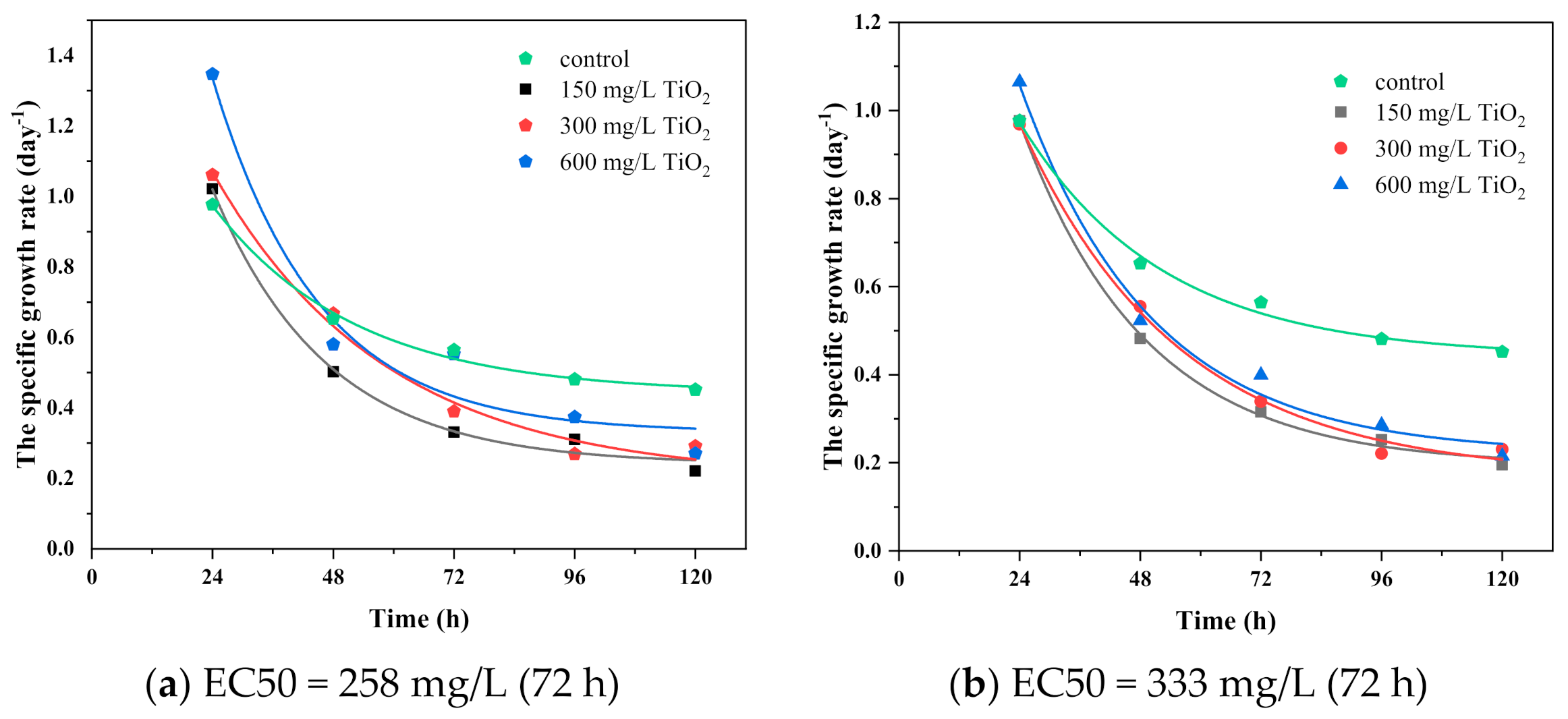
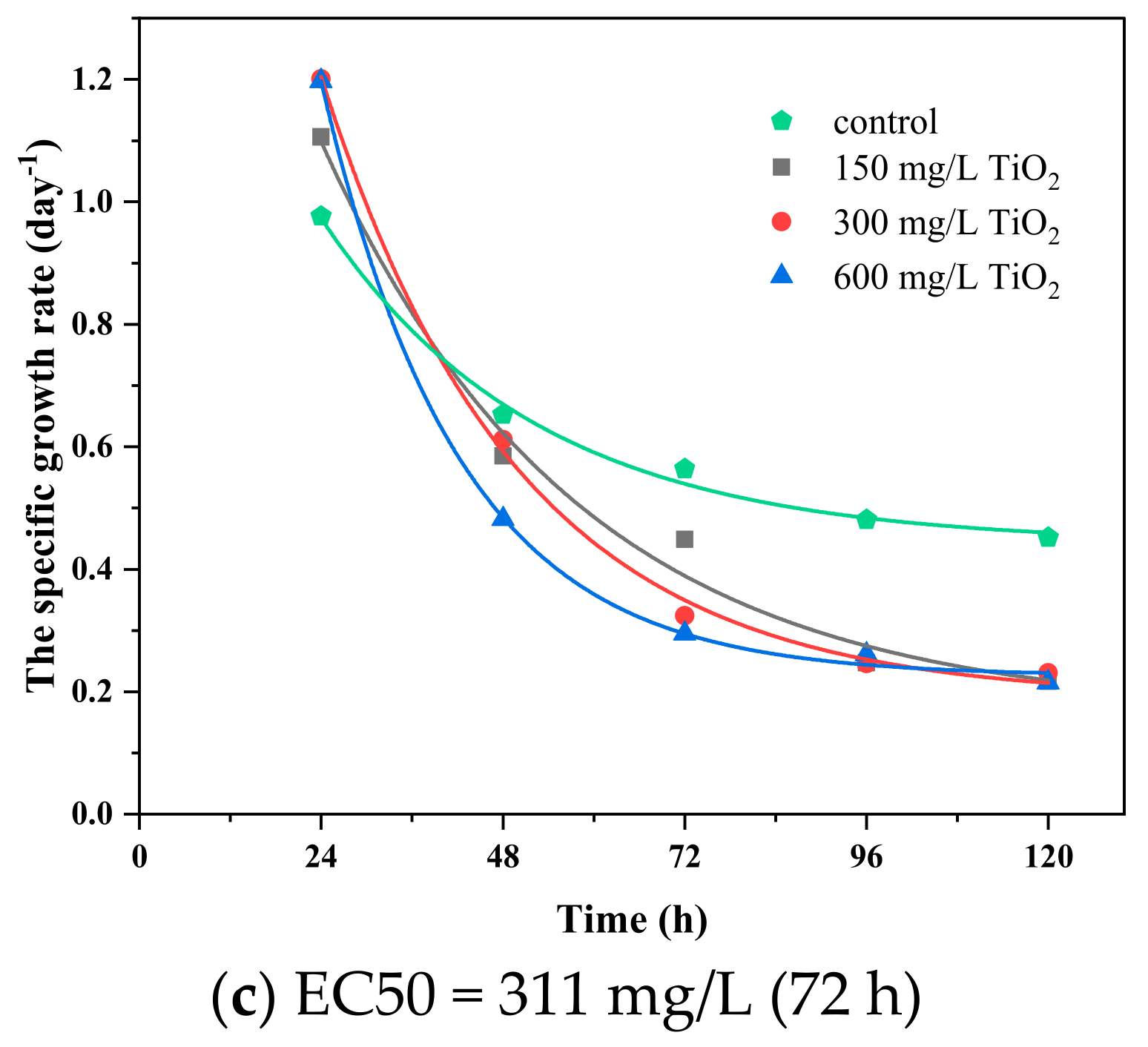
3.4. Synergy between TiO2 Nanoparticles and Imidacloprid on Algae Growth
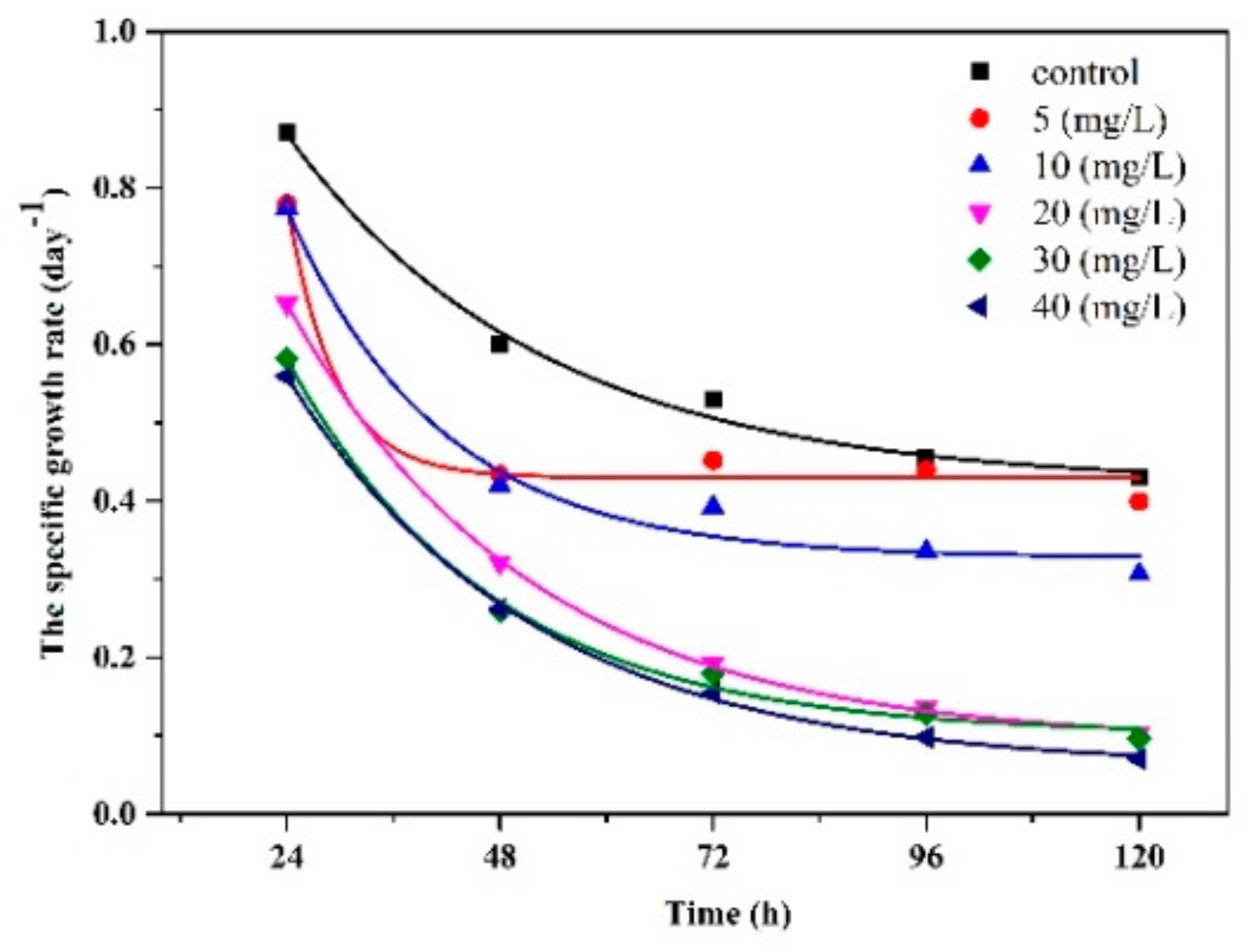
3.5. The Dose–Response and Growth Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdennouri, M.; Baâlala, M.; Galadi, A.; El Makhfouk, M.; Bensitel, M.; Nohair, K.; Sadiq, M.; Boussaoud, A.; Barka, N. Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem. 2016, 9, S313–S318. [Google Scholar] [CrossRef] [Green Version]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Andronic, L.; Andrasi, D.; Enesca, A.; Visa, M.; Duta, A. The influence of titanium dioxide phase composition on dyes photocatalysis. J. Sol-Gel Sci. Technol. 2011, 58, 201–208. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198, 301–309. [Google Scholar] [CrossRef]
- Malato, S.; Caceres, J.; Agüera, A.; Mezcua, M.; Hernando, D.; Vial, J.; Fernández-Alba, A.R. Degradation of imidacloprid in water by photo-fenton and TiO2 photocatalysis at a solar pilot plant: A comparative study. Environ. Sci. Technol. 2001, 35, 4359–4366. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.-Z.; Shi, X.-H.; Cao, Y.-C.; Zhou, C.-R. Solubility of imidacloprid in different solvents. J. Chem. Eng. Data 2008, 53, 615–618. [Google Scholar] [CrossRef]
- Fulton, M.H.; Key, P.B.; DeLorenzo, M.E. Organic chemical toxicology of fishes. In Fish Physiology; Keith, B., Tierney Anthony, P., Farrell, C.J.B., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 33, pp. 309–368. ISBN 9780123982544. [Google Scholar]
- Ahmari, H.; Heris, S.Z.; Khayyat, M.H. The effect of titanium dioxide nanoparticles and UV irradiation on photocatalytic degradation of Imidaclopride. Environ. Technol. 2018, 39, 536–547. [Google Scholar] [CrossRef]
- Loureiro, A.G.; Azoia, N.C.; Gomes, A.; Cavaco-Paulo, A. Albumin-based nanodevices as drug carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Flores, P.; Hellín, P.; Vela, N.; Navarro, G.; García-García, J.; Navarro, S. Implementation of a new modular facility to detoxify agro-wastewater polluted with neonicotinoid insecticides in farms by solar photocatalysis. Energy 2019, 175, 722–729. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Cáceres, J.; Fernández-Alba, A.R.; Agüera, A.; Rodríguez, A. Photocatalytic treatment of water-soluble pesticides by photo-Fenton and TiO2 using solar energy. Catal. Today 2002, 76, 209–220. [Google Scholar] [CrossRef]
- Rózsa, G.; Náfrádi, M.; Alapi, T.; Schrantz, K.; Szabó, L.; Wojnárovits, L.; Takács, E.; Tungler, A. Photocatalytic, photolytic and radiolytic elimination of imidacloprid from aqueous solution: Reaction mechanism, efficiency and economic considerations. Appl. Catal. B Environ. 2019, 250, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Su, L.; Yin, X.; Pei, Y. Responses of Chlorella vulgaris exposed to boron: Mechanisms of toxicity assessed by multiple endpoints. Environ. Toxicol. Pharmacol. 2019, 70, 103208. [Google Scholar] [CrossRef]
- Van Der Heever, J.A.; Grobbelaar, J.U. The Use of Algae in Bioassays to Detect the Presence of Toxic Compounds in Natural Waters; Department of Botany and Genetics University of the Orange Free State: Bloemfontein, South Africa, 1995. [Google Scholar]
- Meseck, S.L.; Alix, J.H.; Wikfors, G.H. Photoperiod and light intensity effects on growth and utilization of nutrients by the aquaculture feed microalga, Tetraselmis chui (PLY429). Aquaculture 2005, 246, 393–404. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L.; Thinh, L.-V.; Kuo, C.; Padovan, A.; Sammy, N. Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J. Appl. Phycol. 1991, 3, 43–53. [Google Scholar] [CrossRef]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, L.; Dewez, D. Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; Zaidi, W.; Samadani, M.; Dewez, D. Toxicity of nickel oxide nanoparticles on a freshwater green algal strain of Chlorella vulgaris. Biomed Res. Int. 2017, 2017, 9528180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shariati, F.; Shirazi, M.A. Effect of SiO2 Nanoparticles on chlorophyll, carotenoid and growth of green micro-algae Dunaliella salina. Nanomed. Res. J. 2019, 4, 164–175. [Google Scholar] [CrossRef]
- Ayatallahzadeh Shirazi, M.; Shariati, F.; Keshavarz, A.K.; Ramezanpour, Z. Toxic effect of aluminum oxide nanoparticles on green micro-algae dunaliella salina. Int. J. Environ. Res. 2015, 9, 585–594. [Google Scholar]
- Li, F.; Liang, Z.; Zheng, X.; Zhao, W.; Wu, M.; Wang, Z. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat. Toxicol. 2015, 158, 1–13. [Google Scholar] [CrossRef]
- Manzo, S.; Miglietta, M.L.; Rametta, G.; Buono, S.; Di Francia, G. Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta. Sci. Total Environ. 2013, 445, 371–376. [Google Scholar] [CrossRef]
- Romero, N.; Visentini, F.F.; Márquez, V.E.; Santiago, L.G.; Castro, G.R.; Gagneten, A.M. Physiological and morphological responses of green microalgae Chlorella vulgaris to silver nanoparticles. Environ. Res. 2020, 189, 109857. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Hajnal-Jafari, T.; Seman, V.; Stamenov, D.; Uric, S. Effect of Chlorella vulgaris on growth and photosynthetic pigment content in swiss chard (Beta vulgaris L. Subsp. Cicla). Polish J. Microbiol. 2020, 69, 235–238. [Google Scholar] [CrossRef]
- Moazami, N.; Ashori, A.; Ranjbar, R.; Tangestani, M.; Eghtesadi, R.; Nejad, A.S. Large-scale biodiesel production using microalgae biomass of Nannochloropsis. Biomass Bioenergy 2012, 39, 449–453. [Google Scholar] [CrossRef]
- Cunha, C.; Lopes, J.; Paulo, J.; Faria, M.; Kaufmann, M.; Nogueira, N.; Ferreira, A.; Cordeiro, N. The effect of microplastics pollution in microalgal biomass production: A biochemical study. Water Res. 2020, 186, 116370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Lao, Y.; Lv, X.; Tao, Y.; Huang, B.; Wang, J.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci. Total Environ. 2016, 565, 818–826. [Google Scholar] [CrossRef]
- Du, Z.Y.; Lucker, B.F.; Zienkiewicz, K.; Miller, T.E.; Zienkiewicz, A.; Sears, B.B.; Kramer, D.M.; Benning, C. Galactoglycerolipid lipase PGD1 is involved in thylakoid membrane remodeling in response to adverse environmental conditions in chlamydomonas. Plant Cell 2018, 30, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ke, S.; Sun, C.; Xu, X.; Chen, J.; Yao, L. Fate and toxicity of silver nanoparticles in freshwater from laboratory to realistic environments: A review. Environ. Sci. Pollut. Res. 2019, 26, 7390–7404. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Sinram, T.; Schlich, K.; Nickel, C.; Dickehut, H.P.; Schmidt, M.; Kühnel, D. Attachment efficiency of nanomaterials to algae as an important criterion for ecotoxicity and grouping. Nanomaterials 2020, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Parashar, A.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. Differential effects of P25 TiO2 nanoparticles on freshwater green microalgae: Chlorella and Scenedesmus species. Aquat. Toxicol. 2016, 176, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Metzler, D.M.; Li, M.; Erdem, A.; Huang, C.P. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J. 2011, 170, 538–546. [Google Scholar] [CrossRef]
- Gunawan, C.; Sirimanoonphan, A.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Submicron and nano formulations of titanium dioxide and zinc oxide stimulate unique cellular toxicological responses in the green microalga Chlamydomonas reinhardtii. J. Hazard. Mater. 2013, 260, 984–992. [Google Scholar] [CrossRef]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef]
- Al-Awady, M.J.; Greenway, G.M.; Paunov, V.N. Nanotoxicity of polyelectrolyte-functionalized titania nanoparticles towards microalgae and yeast: Role of the particle concentration, size and surface charge. RSC Adv. 2015, 5, 37044–37059. [Google Scholar] [CrossRef] [Green Version]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Johnson, J.D.; Pettis, J.S. A survey of imidacloprid levels in water sources potentially frequented by honeybees (Apis mellifera) in the eastern USA. Water. Air. Soil Pollut. 2014, 225. [Google Scholar] [CrossRef] [Green Version]
- Malev, O.; Klobučar, R.S.; Fabbretti, E.; Trebše, P. Comparative toxicity of imidacloprid and its transformation product 6-chloronicotinic acid to non-target aquatic organisms: Microalgae Desmodesmus subspicatus and amphipod Gammarus fossarum. Pestic. Biochem. Physiol. 2012, 104, 178–186. [Google Scholar] [CrossRef]
- Ye, N.; Wang, Z.; Wang, S.; Peijnenburg, W.J.G.M. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Isac, L.; Cazan, C.; Enesca, A.; Andronic, L. Copper sulfide based heterojunctions as photocatalysts for dyes photodegradation. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adochite, C.; Andronic, L. Aquatic toxicity of photocatalyst nanoparticles to green microalgae Chlorella vulgaris. Water 2020, 13, 77. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, K.; Zhang, L.; Yang, K.; Lin, D. Distinct effects of soluble and bound exopolymeric substances on algal bioaccumulation and toxicity of anatase and rutile TiO2 nanoparticles. Environ. Sci. Nano 2018, 5, 720–729. [Google Scholar] [CrossRef]
- Babic, K.; Tomašic, V.; Gilja, V.; Le Cunff, J.; Gomzi, V.; Pintar, A.; Žerjav, G.; Kurajica, S.; Duplancic, M.; Zelic, I.E.; et al. Photocatalytic degradation of imidacloprid in the flat-plate photoreactor under UVA and simulated solar irradiance conditions—The influence of operating conditions, kinetics and degradation pathway. J. Environ. Chem. Eng. 2021, 9, 105611. [Google Scholar] [CrossRef]
- Nyholm, N. Response variable in algal growth inhibition tests—Biomass or growth rate? Water Res. 1985, 19, 273–279. [Google Scholar] [CrossRef]
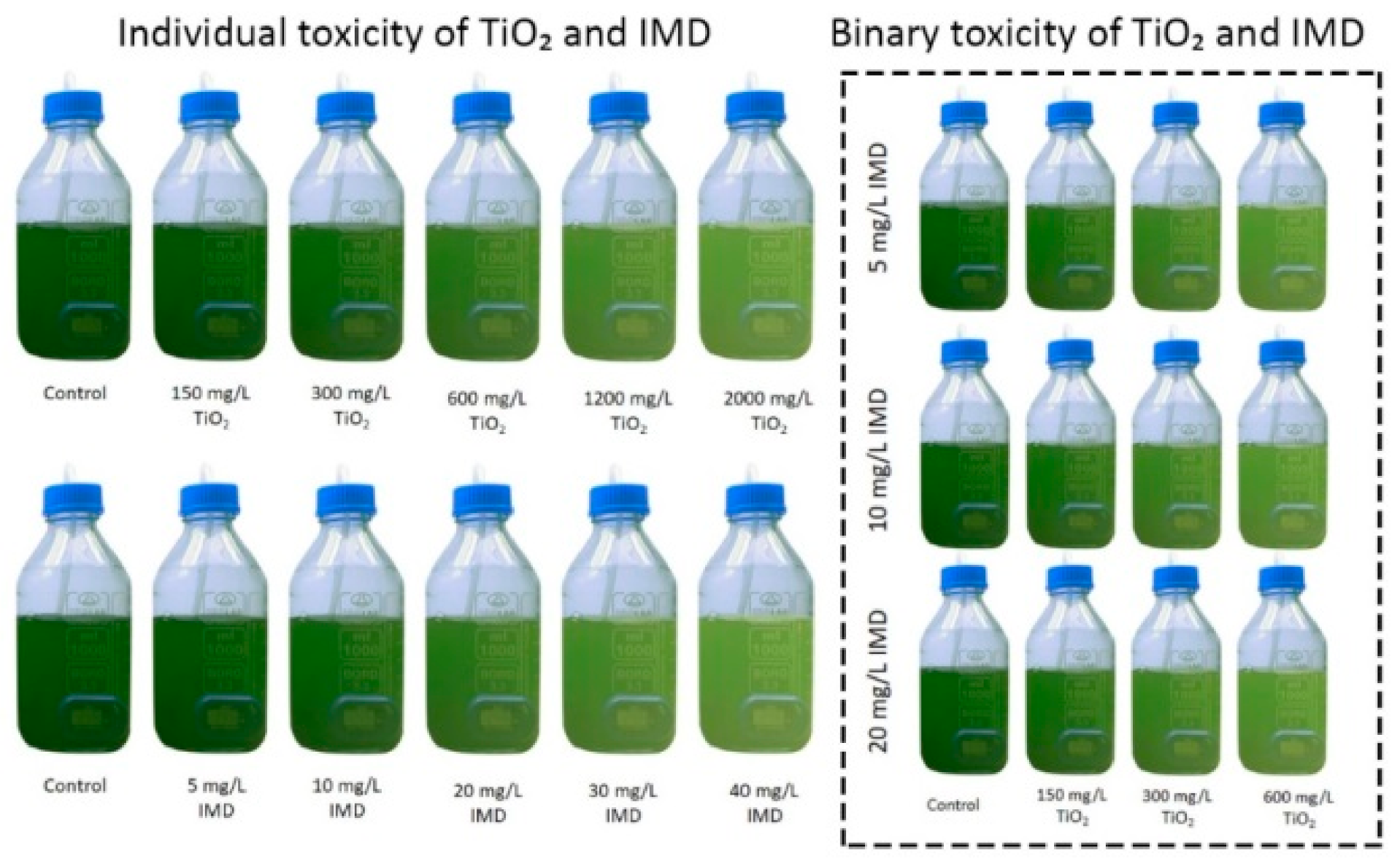
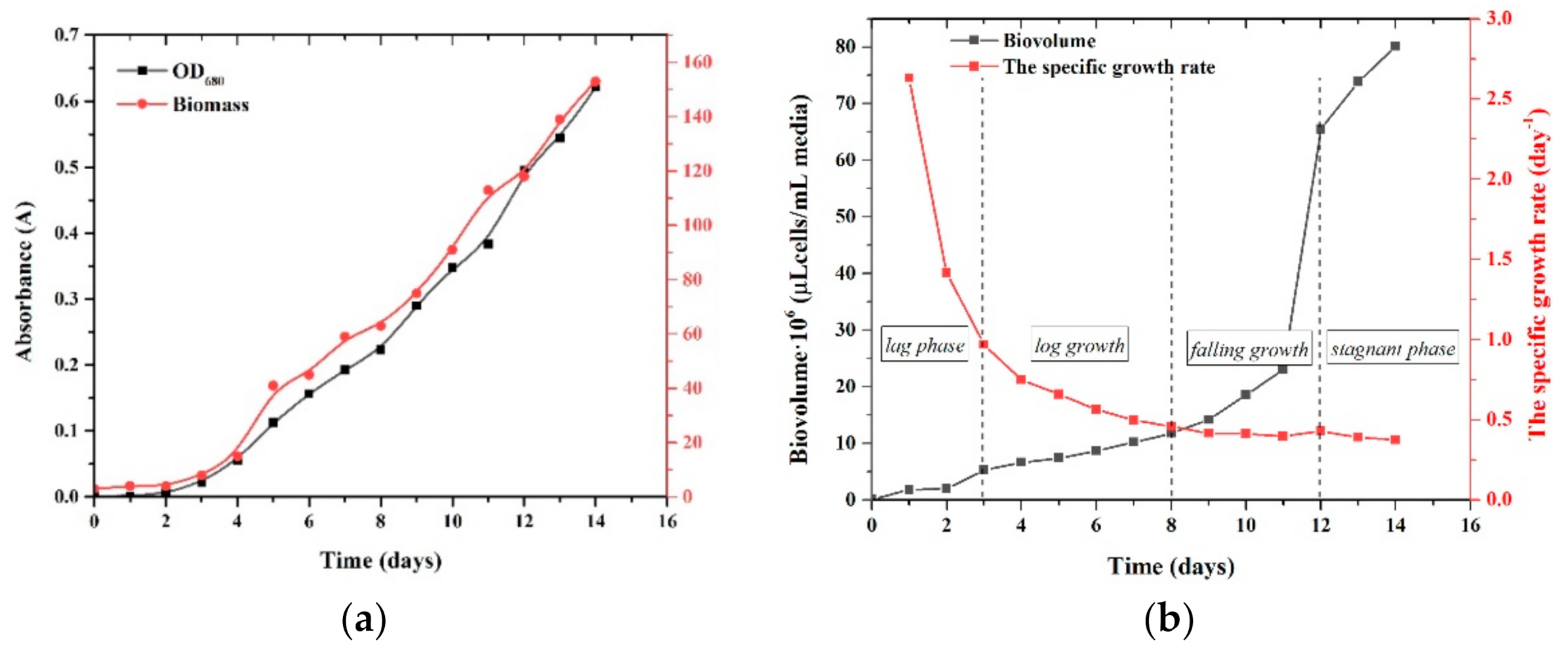
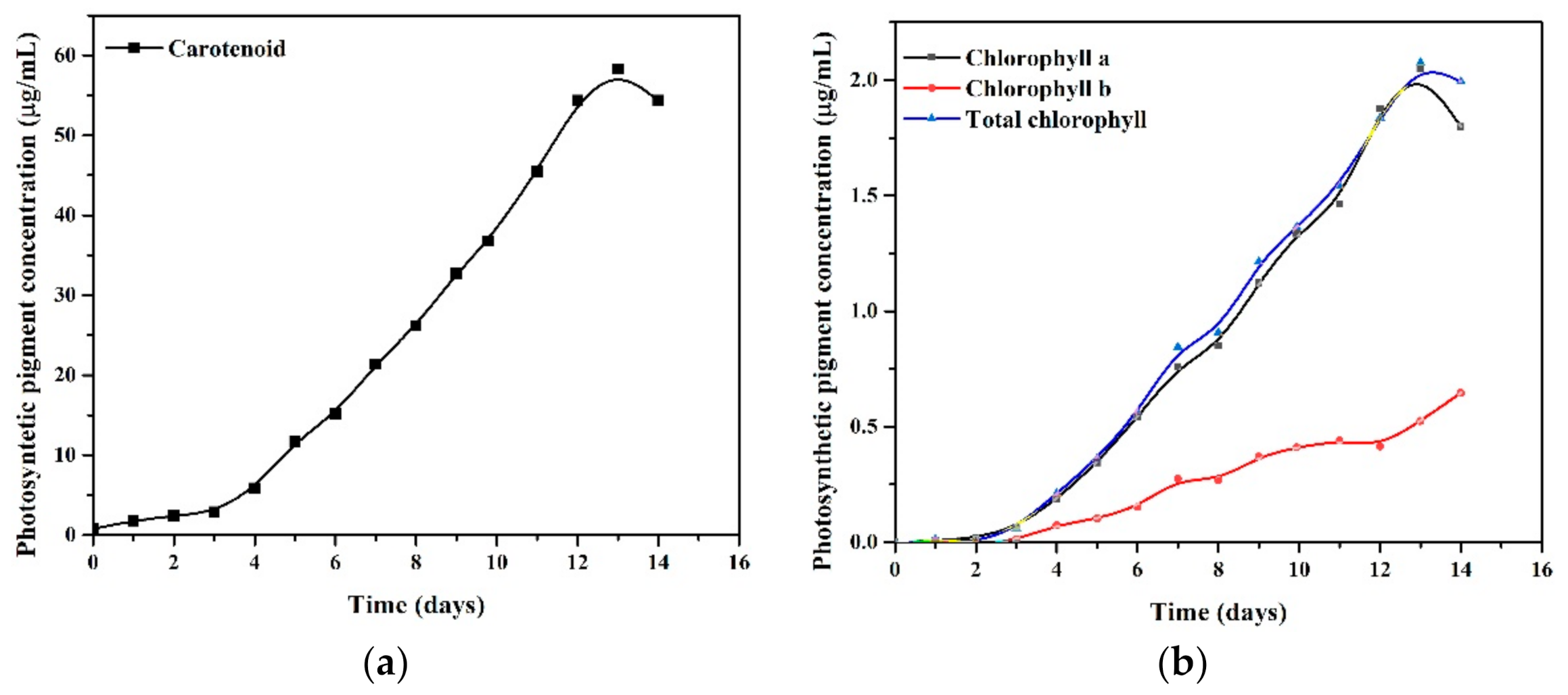
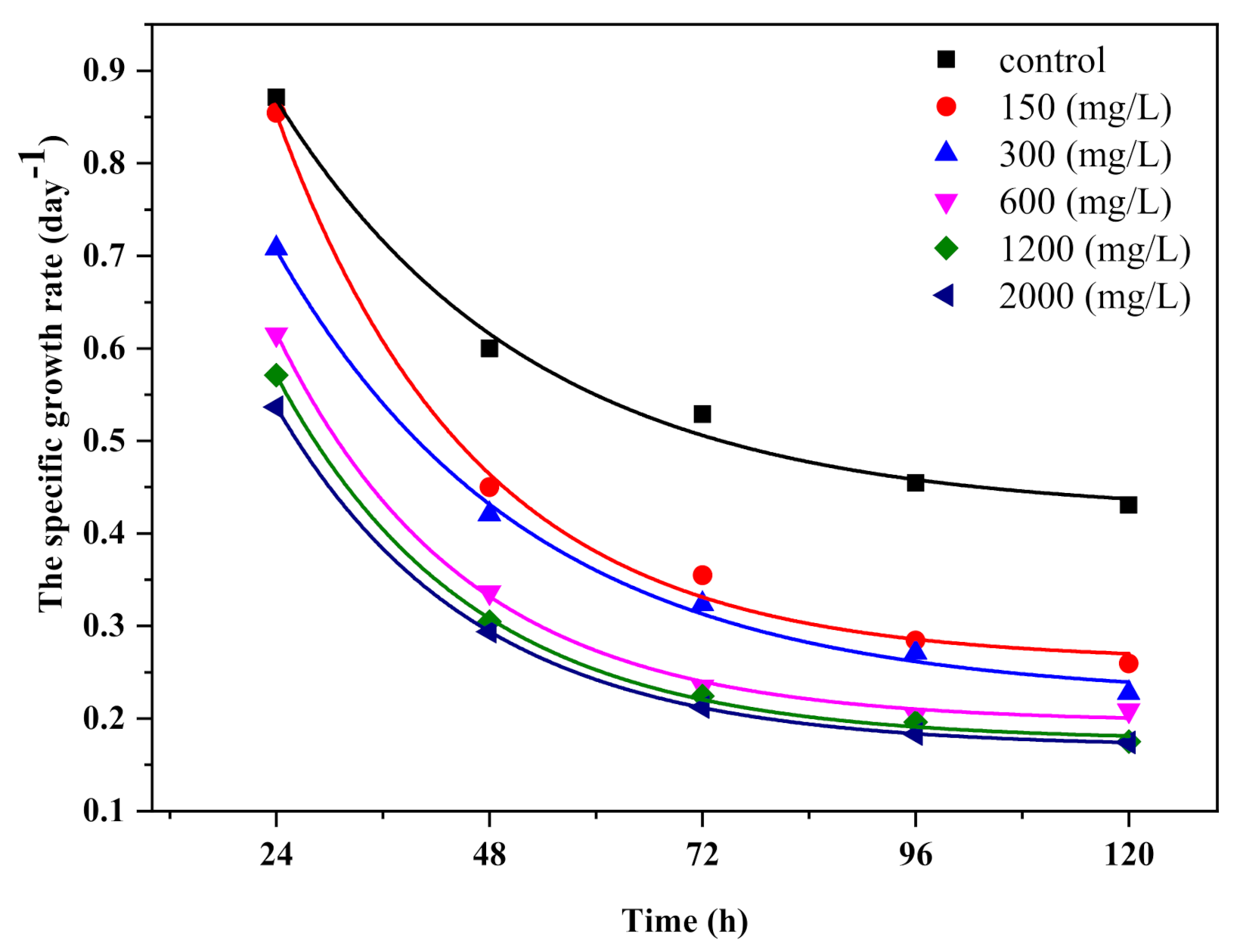
| Species | Toxicological Effect | References | |
|---|---|---|---|
| EC50 (mg/L) | Time (h) | ||
| Chlorella vulgaris | 388 | 72 | This study |
| 428 | 96 | ||
| 293 | 120 | ||
| Karenia brevis | 10.69 | 72 | [25] |
| Skeletonema costatum | 7.37 | 72 | [25] |
| Raphidocelis subcapitata | 126.9 | [38] | |
| Chlorella | 2.160 ± 0.06 | 72 | [39] |
| Scenedesmus | 4.139 ± 0.11 | 72 | [39] |
| Phaeodactylum tricornutum | 167.71 | 120 | [35] |
| Pseudokirchneriella subcapitata | 113 | 96 | [40] |
| Chlamydomonas reinhardtii | >100 | 192 | [41] |
| Nitzschia closterium | 88.78 | 96 | [42] |
| Toxicant | Concentration | 72 h | 96 h | 120 h | ||||
|---|---|---|---|---|---|---|---|---|
| TiO2 | IMD | EC20 | EC50 | EC20 | EC50 | EC20 | EC50 | |
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| TiO2 | 150…2000 | - | 529.67 | 388.14 | 567.72 | 428.39 | 1041.66 | 293.48 |
| IMD | - | 5…40 | 17.27 | 13.00 | 16.21 | 11.76 | 16.09 | 11.95 |
| Binary system of TiO2 and IMD | 150 | 5…20 | 23.43 | 19.83 | 11.82 | 10 | 10.58 | 8.95 |
| 300 | 5…20 | 9.26 | 7.84 | 21.86 | 18.50 | 9.18 | 7.77 | |
| 600 | 5…20 | 22.90 | 19.38 | 10.69 | 9.05 | 11.82 | 10.00 | |
| 150…600 | 5 | 305.37 | 258.42 | 409.31 | 346.39 | 342.26 | 289.64 | |
| 150…600 | 10 | 394.42 | 333.78 | 270.52 | 228.93 | 375.14 | 317.47 | |
| 150…600 | 20 | 367.63 | 311.11 | 300.92 | 254.65 | 191.84 | 226.69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adochite, C.; Andronic, L. Toxicity of a Binary Mixture of TiO2 and Imidacloprid Applied to Chlorella vulgaris. Int. J. Environ. Res. Public Health 2021, 18, 7785. https://doi.org/10.3390/ijerph18157785
Adochite C, Andronic L. Toxicity of a Binary Mixture of TiO2 and Imidacloprid Applied to Chlorella vulgaris. International Journal of Environmental Research and Public Health. 2021; 18(15):7785. https://doi.org/10.3390/ijerph18157785
Chicago/Turabian StyleAdochite, Cristina, and Luminita Andronic. 2021. "Toxicity of a Binary Mixture of TiO2 and Imidacloprid Applied to Chlorella vulgaris" International Journal of Environmental Research and Public Health 18, no. 15: 7785. https://doi.org/10.3390/ijerph18157785
APA StyleAdochite, C., & Andronic, L. (2021). Toxicity of a Binary Mixture of TiO2 and Imidacloprid Applied to Chlorella vulgaris. International Journal of Environmental Research and Public Health, 18(15), 7785. https://doi.org/10.3390/ijerph18157785







