Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
| Filters: [Title/Abstract] |
| #1 Ultrasonography [Mesh]: #2 Ultrasound; #3 Echography; #4 Sonography |
| #5 #1 OR #2 OR #3 OR #4 |
| #6 Exercise Therapy [Mesh]: #7 Motor control; #8 Stabilization exercise; #9 Rehabilitation Exercise |
| #10 #6 OR #7 OR #8 OR #9 |
| #11 Feedback, Sensory [Mesh]: #12 Biofeedback; #13 Visual Feedback; # 14 Audio Feedback; #15 Proprioceptive Feedback; #16 Sensorimotor Feedback |
| #17 #11 OR #12 OR #13 OR #14 OR #15 OR #16 |
| # 18 Muscle, Skeletal [Mesh] |
| #19 #5 AND #10 AND #17 AND #18 |
2.3. Study Eligibility Criteria
2.4. Study Appraisal and Synthesis Methods
3. Results
3.1. Study Selection
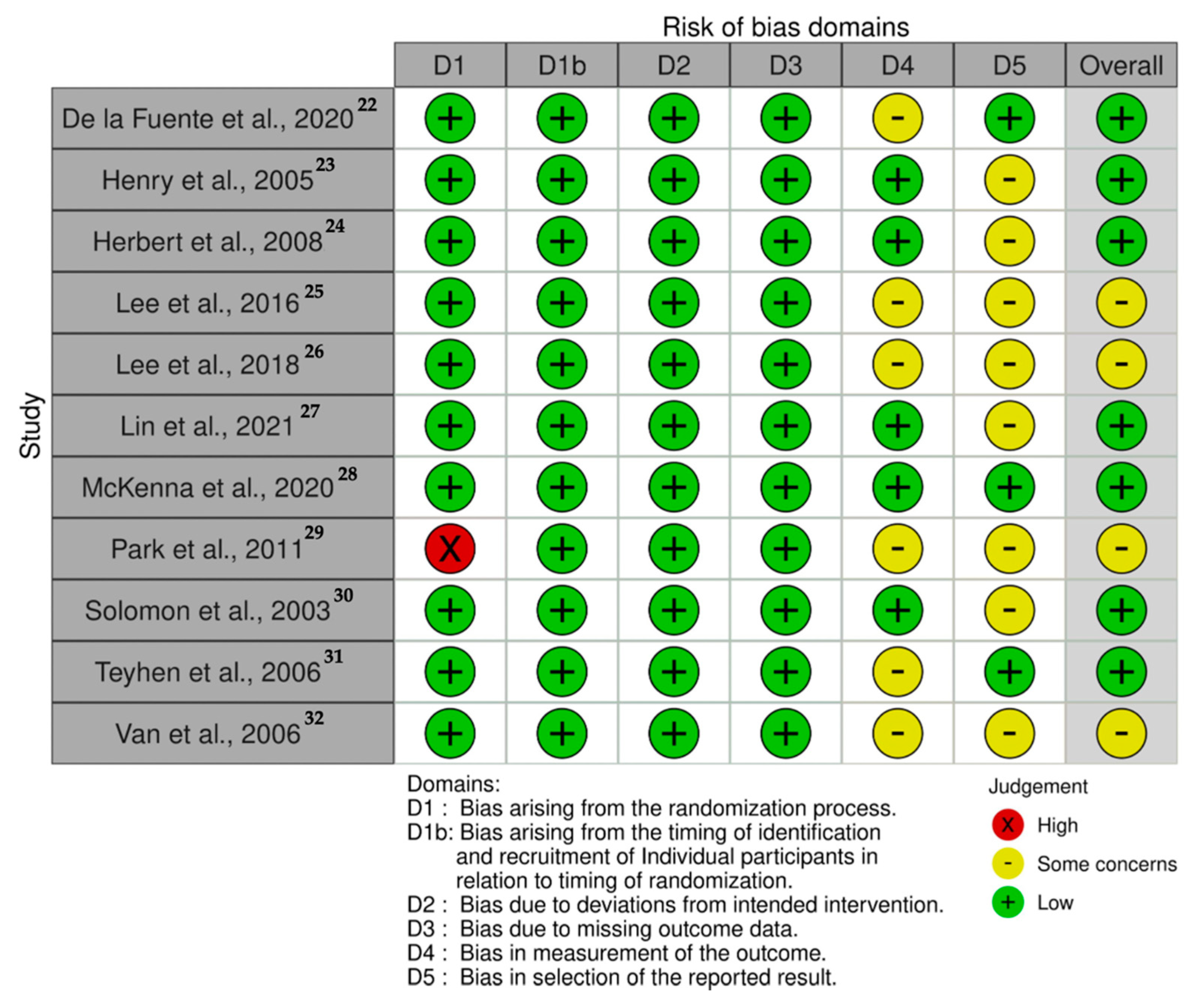
3.2. Methodological Quality and Risk of Bias
3.3. Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saragiotto, B.T.; Maher, C.; Yamato, T.; Costa, L.; Costa, L.D.C.M.; Ostelo, R.; Macedo, L. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst. Rev. 2016, 8, CD012004. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Hänsel, F. Chronic Non-specific Low Back Pain and Motor Control during Gait. Front. Psychol. 2018, 9, 2236. [Google Scholar] [CrossRef] [PubMed]
- Niederer, D.; Mueller, J. Sustainability effects of motor control stabilisation exercises on pain and function in chronic nonspecific low back pain patients: A systematic review with meta-analysis and meta-regression. PLoS ONE 2020, 15, e0227423. [Google Scholar] [CrossRef]
- Vera, D.M.; Calero, J.A.V.; Manzano, G.P.; Casas, P.M.; Martín, D.P.; Izquierdo, T.G. C0065 Different feedback methods to improve lumbar multifidus contraction. Br. J. Sprots Med. 2018, 52, A15–A16. [Google Scholar] [CrossRef]
- Blumenstein, B.; Bar-Eli, M.; Tenenbaum, G. Biofeedback Applications in Performance Enhancement: Brain and Body in Sport and Exercise; John Wiley & Sons: Sussex, UK, 2002. [Google Scholar]
- Valera-Calero, J.A.; Ojedo-Martín, C.; Fernández-De-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Hervás-Pérez, J.P. Reliability and Validity of Panoramic Ultrasound Imaging for Evaluating Muscular Quality and Morphology: A Systematic Review. Ultrasound Med. Biol. 2021, 47, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Arias-Buría, J.L.; Fernández-De-Las-Peñas, C.; Cleland, J.A.; Gallego-Sendarrubias, G.M.; Cimadevilla-Fernández-Pola, E. Echo-intensity and fatty infiltration ultrasound imaging measurement of cervical multifidus and short rotators in healthy people: A reliability study. Musculoskelet. Sci. Pract. 2021, 53, 102335. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Ellis, R.; Hodges, P.W.; Osullivan, C.; Hides, J.; Carnero, S.F.; Arias-Buria, J.L.; Teyhen, D.S.; Stokes, M.J. Imaging with ultrasound in physical therapy: What is the PT’s scope of practice? A competency-based educational model and training recommendations. Br. J. Sports Med. 2019, 53, 1447–1453. [Google Scholar] [CrossRef]
- Koppenhaver, S.L.; Hebert, J.; Parent, E.C.; Fritz, J.M. Rehabilitative ultrasound imaging is a valid measure of trunk muscle size and activation during most isometric sub-maximal contractions: A systematic review. Aust. J. Physiother. 2009, 55, 153–169. [Google Scholar] [CrossRef]
- Russo, M.; Deckers, K.; Eldabe, S.; Kiesel, K.; Gilligan, C.; Vieceli, J.; Crosby, P. Muscle Control and Non-specific Chronic Low Back Pain. Neuromodul. Technol. Neural Interface 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Neblett, R. Surface Electromyographic (SEMG) Biofeedback for Chronic Low Back Pain. Healthcare 2016, 4, 27. [Google Scholar] [CrossRef]
- Stokes, I.A.; Henry, S.M.; Single, R.M. Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clin. Biomech. 2003, 18, 9–13. [Google Scholar] [CrossRef]
- Crasto, C.F.B.; Montes, A.M.; Carvalho, P.; Carral, J.M.C. Pressure biofeedback unit to assess and train lumbopelvic stability in supine individuals with chronic low back pain. J. Phys. Ther. Sci. 2019, 31, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.M.; Teyhen, D.S. Ultrasound Imaging as a Feedback Tool in the Rehabilitation of Trunk Muscle Dysfunction for People with Low Back Pain. J. Orthop. Sports Phys. Ther. 2007, 37, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Persson, U.M.; Caulfield, B. Biofeedback in rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Dhammi, I.K.; Haq, R.U. How to write systematic review or meta-analysis. Indian J. Orthop. 2018, 52, 575–577. [Google Scholar] [CrossRef]
- Greenhalgh, T. How to read a paper: The Medline database. BMJ 1997, 315, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Booths, A. Brimful of STARLITE: Toward standards for reporting literature searches. J. Med. Libr. Assoc. 2006, 94, 421–430. [Google Scholar]
- Moseley, A.M.; Elkins, M.R.; Van der Wees, P.J.; Pinheiro, M.B. Using research to guide practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- De La Fuente, C.; Silvestre, R.; Baechler, P.; Gemigniani, A.; Grunewaldt, K.; Vassiliu, M.; Wodehouse, V.; Delgado, M.; Carpes, F.P. Intrasession Real-time Ultrasonography Feedback Improves the Quality of Transverse Abdominis Contraction. J. Manip. Physiol. Ther. 2020, 43, 816–823. [Google Scholar] [CrossRef]
- Henry, S.M.; Westervelt, K.C. The Use of Real-Time Ultrasound Feedback in Teaching Abdominal Hollowing Exercises to Healthy Subjects. J. Orthop. Sports Phys. Ther. 2005, 35, 338–345. [Google Scholar] [CrossRef]
- Herbert, W.J.; Heiss, D.G.; Basso, D.M. Influence of Feedback Schedule in Motor Performance and Learning of a Lumbar Multifidus Muscle Task Using Rehabilitative Ultrasound Imaging: A Randomized Clinical Trial. Phys. Ther. 2008, 88, 261–269. [Google Scholar] [CrossRef]
- Lee, S.; Han, S.; Lee, D. Comparison of abdominal muscle thickness according to feedback method used during abdominal hollowing exercise. J. Phys. Ther. Sci. 2016, 28, 2519–2521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, D.H.; Hong, S.K.; Lee, Y.-S.; Kim, C.-H.; Hwang, J.-M.; Lee, Z.; Kim, J.M.; Park, D. Is abdominal hollowing exercise using real-time ultrasound imaging feedback helpful for selective strengthening of the transversus abdominis muscle? Medicine 2018, 97, e11369. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, B.; Zheng, Y.; Huang, G.; Zeng, Q.; Wang, C. Effect of real-time ultrasound imaging for biofeedback on trunk muscle contraction in healthy subjects: A preliminary study. BMC Musculoskelet. Disord. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- McKenna, L.J.; Bonnett, L.; Panzich, K.; Lim, J.; Hansen, S.K.; Graves, A.; Jacques, A.; Williams, A.S. The Addition of Real-time Ultrasound Visual Feedback to Manual Facilitation Increases Serratus Anterior Activation in Adults with Painful Shoulders: A Randomized Crossover Trial. Phys. Ther. 2021, 17, pzaa208. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, H. The Use of Rehabilitative Ultrasound Imaging for Feedback from the Abdominal Muscles during Abdominal Hollowing in Different Positions. J. Phys. Ther. Sci. 2011, 23, 895–898. [Google Scholar] [CrossRef][Green Version]
- Solomon, M.; Pager, C.K.; Rex, J.; Roberts, R.; Manning, J. Randomized, Controlled Trial of Biofeedback with Anal Manometry, Transanal Ultrasound, or Pelvic Floor Retraining with Digital Guidance Alone in the Treatment of Mild to Moderate Fecal Incontinence. Dis. Colon Rectum 2003, 46, 703–710. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Miltenberger, C.E.; Deiters, H.M.; Del Toro, Y.M.; Pulliam, J.N.; Childs, J.D.; Boyles, R.E.; Flynn, T.W. The Use of Ultrasound Imaging of the Abdominal Drawing-in Maneuver in Subjects with Low Back Pain. J. Orthop. Sports Phys. Ther. 2005, 35, 346–355. [Google Scholar] [CrossRef]
- Van, K.; Hides, J.; Richardson, C.A. The Use of Real-Time Ultrasound Imaging for Biofeedback of Lumbar Multifidus Muscle Contraction in Healthy Subjects. J. Orthop. Sports Phys. Ther. 2006, 36, 920–925. [Google Scholar] [CrossRef]
- Meier, M.L.; Vrana, A.; Schweinhardt, P. Low Back Pain: The Potential Contribution of Supraspinal Motor Control and Proprioception. Neuroscientist 2019, 25, 583–596. [Google Scholar] [CrossRef]
- Tsao, H.; Danneels, L.A.; Hodges, P.W. ISSLS prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine 2011, 36, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Niederer, D.; Engel, T.; Vogt, L.; Arampatzis, A.; Banzer, W.; Beck, H.; Catalá, M.M.; Brenner-Fliesser, M.; Güthoff, C.; Haag, T.; et al. Motor Control Stabilisation Exercise for Patients with Non-Specific Low Back Pain: A Prospective Meta-Analysis with Multilevel Meta-Regressions on Intervention Effects. J. Clin. Med. 2020, 9, 3058. [Google Scholar] [CrossRef] [PubMed]
- Owen, P.J.; Miller, C.T.; Mundell, N.L.; Verswijveren, S.J.J.M.; Tagliaferri, S.D.; Brisby, H.; Bowe, S.J.; Belavy, D.L. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sports Med. 2020, 54, 1279–1287. [Google Scholar] [CrossRef]
- Saragiotto, B.; Maher, C.; Yamato, T.; Costa, L.; Costa, L.D.C.M.; Ostelo, R.; Macedo, L. Motor Control Exercise for Nonspecific Low Back Pain. Spine 2016, 41, 1284–1295. [Google Scholar] [CrossRef]
- Macedo, L.G.; Saragiotto, B.T.; Yamato, T.P.; Costa, L.O.; Menezes Costa, L.C.; Ostelo, R.W.; Maher, C.G. Motor control exercise for acute non-specific low back pain. Cochrane Database Syst. Rev. 2016, 2, CD012085. [Google Scholar] [CrossRef] [PubMed]

| Reference. | Study Type | PEDro Scale Items | Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| De la Fuente et al., 2020 [22] | RCT | + | + | − | + | − | − | + | + | + | + | + | 7 |
| Henry et al., 2005 [23] | RCT | + | + | − | + | − | − | + | + | + | + | − | 6 |
| Herbert et al., 2008 [24] | RCT | + | + | + | + | − | − | + | + | + | + | − | 7 |
| Lee et al., 2016 [25] | RCT | + | + | − | + | − | − | − | + | + | + | − | 5 |
| Lee et al., 2018 [26] | RCT | + | + | − | + | − | − | + | + | + | + | − | 6 |
| Lin et al., 2021 [27] | RCT | + | + | − | + | − | − | − | + | + | + | − | 5 |
| McKenna et al., 2020 [28] | RCT | + | + | + | + | − | − | + | + | + | + | + | 8 |
| Park et al., 2011 [29] | CT | + | − | − | + | − | − | − | + | + | + | − | 4 |
| Solomon et al., 2003 [30] | RCT | + | + | + | + | − | − | + | + | + | + | − | 7 |
| Teyhen et al., 2006 [31] | RCT | + | + | + | + | − | + | + | + | + | + | + | 9 |
| Van et al., 2006 [32] | RCT | + | + | + | + | − | − | + | + | + | + | − | 7 |
| Study | Population | Comparator | Interventions | Tasks | Muscles Assessed | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| De la Fuente et al., 2020 [22] | n = 20 healthy participants (7M/13F) Age: 25 ± 5 years. Height: 166 ± 10 cm. Weight: 64 ± 6 kg. BMI: 22.2 ± 5.8 kg/m2 | Visual biofeedback (RUSI) vs. Verbal biofeedback | Participants were placed in a supine position (45° of hip flexion, 90° of knee flexion, the arms close to the trunk in a comfortable position, and the forearms in pronation). Both groups were instructed about the protocols during 5 min before the experiment, using a video. RUSI group watched echography images and were advised to pay attention to the changes in thickness of the TrA. Verbal biofeedback group paid attention to the perception of contraction in the muscles | Four repetitions of the AHE (sustaining an abdominal contraction lasting 7 s after 1 cycle of full inspiration and expiration), with 2 min of rest between repetitions. One basal measure + 3 measures with biofeedback. | Transversus Abdominis | Normalized Thickness: Difference between the measurement from each repetition and the basal measure, divided by the basal condition, and expressed in arbitrary units. Normalized Pressure: Difference of pressure between each repetition and the basal measure, divided by the basal condition, and in arbitrary units. | Post hoc power = 0.804. Group differences were found (p = 0.006) without interactions (p = 0.994) or repetition effects (p = 0.468). RUSI feedback resulted in larger changes in thickness than the verbal feedback alone (p < 0.05). The bias between thickness and pressure for feedback with and without ultrasonography was 0.0490 and −0.0080 respectively. Significant correlation was not found between pressure measurement and thickness. The lowest minimal detectable changes were achieved by using the ultrasonography feedback. |
| Henry et al., 2005 [23] | n = 48 healthy participants (6M/42F) Age: 21.3–23.1 years. Height: 1.7 ± 0.1 m. Weight: 62.5–64.0 kg. BMI: 22.2 ± 5.8 kg/m2 | Visual Feedback (RUSI) vs. Minimal verbal Feedback vs. Common clinical feedback (verbal descriptive feedback of any observed substitution patterns, verbal corrective feedback, and cutaneous feedback from palpation) | Participants were placed in a supine position with hips flexed between 40° and 80° and knees flexed between 60° and 120°. All groups received instruction in how to perform an AHE. Feedback was given after the first trial and after every other trial thereafter. If the subject appeared to be having difficulty performing the AHE, then the verbal corrective feedback also included a rewording of the instructions to promote understanding. | Each subject was given 2 warm-up trials of the AHE, followed by 10 trials of the AHE, which were assessed as correct or incorrect. Subjects able to perform 3 consecutives correct AHEs on the retention test, as in the initial test, were considered to have retained the ability to perform the AHE correctly. | Transversus Abdominis Internal Oblique External Oblique | Number of trials needed for an individual to consistently perform an AHE. Subjects’ ability to retain the correct performance of the AHE up to 4 days later. | The ability to perform the AHE differed among groups (p < 0.001). During the initial session, 12.5% of subjects in verbal feedback group, 50.0% of subjects in common clinical feedback group, and 87.5% of subjects in RUSI group were able to perform 3 consecutive AHEs. There was a difference among groups in the mean number of trials until performance criterion was reached (p = 0.0006). No differences were noted among feedback groups with regard to the proportions of subjects able to reach the retention criterion. |
| Herbert et al., 2008 [24] | n = 28 healthy participants (9M/19F) Age: 28 ± 8 years. BMI: 24.0 ± 0.7 kg/m2 | Constant feedback vs. Variable feedback | Participants were positioned prone on the treatment table with the hips in the neutral position Real-time RUSI of the multifidus muscle at the level of S1 was recorded, transferred to the video recording system, and projected on the television monitor to provide visual feedback. Constant feedback group received visual feedback of the real-time RUSI of successful or unsuccessful multifidus muscle activation on the monitor, but were not given verbal feedback. Variable feedback group received delayed feedback after performing a number of repetitions of the exercise, based on a pre- determined schedule. | Subjects attended 15-min exercise training sessions in the laboratory, twice a week, for a total of 8 training sessions. Participants were asked to recruit the multifidus muscle without extraneous movements and to hold each contraction for 3 s. It also informed the subjects that the training session would consist of 12 repetitions of the exercise and that a successful performance outcome was visualization of muscle movement on the monitor. | Lumbar multifidus muscle | Performance success: Defined as isolated isometric recruitment of the first sacral level (S1) multifidus muscle without substitution of extraneous movements such as Valsalva, pelvic tilt, arching the back, lifting the upper trunk, or lifting the lower extremity. Retention success: Each subject returned after 1 and 4 weeks. Same procedures were repeated, except that no augmented feedback was provided. | Both groups had similar performances of multifidus muscle recruitment (p = 0.26). Constant feedback group had good success (80%) that was maintained at session 8 (84%), with no difference between sessions 1 and 8 (p = 0.19). Variable feedback group gradually increased success between sessions 1 and 8 (p = 0.002). Both groups sustained their session 8 success when tested for short-term retention at 1 week (Both, p > 0.36). At the long-term retention test, the variable feedback group outperformed the constant feedback group (p = 0.04), indicating superior motor learning. |
| Lee et al., 2016 [25] | n = 30 healthy participants Age: 20.3–21.1 years Height: 1.66–1.67 m Weight: 55.3–57.0 kg | Visual biofeedback (RUSI) vs. Pressure biofeedback unit vs. Basic training | Participants were placed in a crooked lying position with their knees flexed to 90°. Basic training group received verbal and manual contact biofeedback. Pressure biofeedback group were told to maintain the manometer at 10 mm Hg, starting from 40 mm Hg. RUSI group received training with monitoring of possible contraction of their muscles in the screen. | All of the subjects received AHE training for 15 min. After training, the subjects were measured three times being at rest in a supine position and performing the AHE with which they were trained. | Transversus Abdominis Internal Oblique External Oblique | Thickness measured with ultrasound imaging. | All the groups showed greater TrA thickness (p < 0.01) but no changes in IO nor EO (p > 0.05). During AHE, the thickness of the musculus transversus abdominis differed significantly among the groups (p < 0.05). No significant differences were observed between the basic training and the pressure biofeedback groups, and between the pressure biofeedback and the RUSI groups (p > 0.05). However, significant differences between basic training and RUSI were found for TrA (p < 0.05). No significant difference was observed among the three groups regarding the thicknesses of the internal oblique abdominal and external oblique abdominal muscles during AHE (p > 0.05). |
| Lee et al., 2018 [26] | n = 20 healthy participants Age: 29.0 ± 3.0 years BMI: 22.1 ± 1.7 kg/m2 | Conventional feedback vs. Visual feedback (RUSI) | Subjects were placed in a supine hook-lying position. Subjects in conventional feedback group were trained AHE using verbal and tactile feedback. Subjects in RUSI group, in addition to the initial education about the conventional feedback, were educated about visual feedback provided with real-time ultrasound imaging. | All subjects received education session about AHE with conventional (verbal and tactile) feedback for 30 min. After the session, the baseline assessment of the muscle activity during AHE was recorded using the surface electromyogra- phy. | Transversus Abdominis Internal Oblique External Oblique | Ultrasonography Thickness measurement of the 3 muscles. Electromiography Percentages of maximal voluntary contraction were calculated by normalization with maximal voluntary contraction to evaluate how efficiently TrA-IO muscles were activated. Maximal voluntary contraction values of TrA-IO were obtained by maximally twisting upper-body to ipsilateral side against physiatrist’s manual resistance. | After 2 weeks of AHE training, the thicknesses of TrA, IO, and EO muscles in resting were not significantly changed in both groups. Thicknesses of contracted TrA and IO muscles during AHE were significantly increased than those of resting state in both of real-time ultrasound imaging and conventional feedback group (p < 0.05). The difference between resting and contraction of TrA muscle thickness in real-time ultrasound imaging feedback group was significantly higher than conventional feedback group (p < 0.05), but no for IO (p > 0.05). Root mean squares and maximal voluntary contraction values in TrA-IO increased without statistical significance in both groups (p > 0.05). The difference in maximal voluntary contraction value of TrA-IO was significantly higher in RUSI group than conventional feedback group (p < 0.05). The ratio of root mean squares values of TrA-IO/EO muscles was significantly higher in RUSI group. |
| Lin et al., 2021 [27] | n = 40 healthy participants (9M/31F) Age: 25.9–26.6 years Height: 1.62–1.63 m Weight: 55.6–56.2 kg BMI: 21.0–21.0 kg/m2 | Verbal biofeedback vs. Visual feedback (RUSI) | During contraction, subjects in the experimental group were required to watch the real-time ultrasound imaging and maintain continuous contraction with maximum effort. Images of the right LM at rest and during maximum isometric contraction were acquired. Images of the right TrA muscle were acquired at rest and during the ADIM maneuver. | All participants were firstly given a verbal explanation regarding the purpose and operation procedure of the experiment and the anatomical structure and function of the muscles before the test. Image acquisition for each condition and each time point (Trest, Tc-max, Tc-15 s, Tc-30 s) was repeated three times. | Lumbar Multifidus Transversus Abdominis | Lumbar multifidus thickness Three separate resting ultrasound images were collected immediately after ex- halation TrA Thickness ADIM was used to assess the altered muscle thickness associated with a voluntary contraction of the TrA muscle. | No significant differences were found in the thickness of LM at rest (p > 0.999), Tc-max (p > 0.999), and T15 s (p = 0.414) between the two groups. The ability to recruit LM muscle contraction differed between groups at T30 s (p = 0.006), with subjects in the experimental group that received visual ultrasound biofeedback maintaining a relative maximum contraction. No significant differences were found in the TrA muscle thickness at rest (p > 0.999) and Tc-max (p > 0.999) between the two groups. Significant differences of contraction thickness were found at T15 s (p = 0.031) and T30 s (p = 0.010) between the two groups during the ADIM, with greater TrA muscle contraction thickness in the experimental group. |
| McKenna et al., 2020 [28] | n = 27 patients with unilateral subacromial pain (15M/12F) Age: 54.4–56.8 years BMI: 24.6–29.5 kg/m2 NPRS score: 1.0–2.0 | Manual facilitation vs. Manual facilitation + RUSI | Participants performed all interventions in the supine position. Participants received individual training in either activating the SA using RUSI feedback with manual facilitation or training with manual facilitation only at the first session. At the second session, the participant received the intervention they did not receive on the first session. | Five practice serratus punches were performed continuously at an approximate speed of 3 s per punch with the participant cued to “reach up”. One minute of rest was then allowed, followed by a further 10 intervention repetitions with ongoing verbal cueing and encouragement, for a total of 15 repetitions during intervention. | Serratus anterior | Electromiography Levels of SA activation (normalized to a maximal voluntary isometric contraction). | The predicted marginal mean difference between interventions was 55.5% (95% CI = 13.9% to 97.1%) (p = 0.009), favoring the addition of RUSI feedback. |
| Park et al., 2011 [29] | n = 42 healthy males Age: 22.6–23.2 years Height: 1.75–1.76 m Weight: 67.8–67.9 kg BMI: 21.8–22.2 kg/m2 | RUSI feedback vs. No feedback | Participants were placed in 4 different positions. The experimental group performed AHE with RUSI feedback. The control group performed AHE with no RUSI feedback. | All the subjects were familiarized with AHE with a 30-min training. Measurements were conducted 3 times in each position with 2-min resting between measurements. | Transversus Abdominis Internal Oblique External Oblique | Ultrasound imaging Thickness differences between rest and AHE were compared between the two groups. | The difference in internal IO thickness changes between the groups were significant. The differences in EO thickness changes were only significant among the positions. A post hoc analysis of the differences in EO thickness changes among the positions found significant differences between the crook lying and four-point kneeling positions. The TrA thickness changes showed significant interaction between group and position. |
| Solomon et al., 2003 [30] | n = 120 patients with mild to moderate fecal incontinence with at least mild neuropathy (13M/107F) Age: 62.0 ± 12.8 years Exercise compliance: 83.0% | Digital examination feedback vs. Transanal RUSI vs. Anal manometry | All patients were lying in the left lateral position. In the digital examination group, patients performed a full set of supervised exercises guided by digital per anal examination of the external sphincter. In the RUSI group, patients were taught how to contract the anal sphincters while watching the real-time ultrasound display on the monitor screen, and a full set of exercises were performed during each treatment session. In the anal manometry group, Patients were taught how to contract and relax the anal sphincters while attending to the pressures generated in the anal canal, and a full set of exercises were performed during each treatment session. | All participants performed a full set of exercises, consisting of ten five-second sphincter contractions, each at one-second intervals, repeated ten times (a total of 100 contractions). All patients were urged to perform an identical set of exercises twice per day between outpatient visits and were asked to estimate the percentage of exercises they had actually completed. | Pelvic floor | St. Mark’s Hospital fecal incontinence score Pescatori fecal incontinence score Patient’s self-assessment of fecal incontinence severity using a visual analog scale Investigator’s assessment of fecal incontinence severity using a visual analog scale. Quality-of-life measure using Direct Questioning of Objectives Resting and maximal squeeze anal canal manometric pressures Isotonic fatigue time Isometric fatigue contractions | One hundred two patients (85 percent) completed the four-month treatment program. Across all treatment allocations, patients experienced modest but highly significant improvements in all nine outcome measures during treatment, with 70 percent of all patients perceiving improvement in symptom severity and 69 percent of patients reporting improved quality of life. With the possible exception of isotonic fatigue time, there were no significant differences between the three treatment groups in compliance, physiologic sphincter strength, and clinical or quality-of-life measures. Correlations between physiologic measures and clinical outcomes were much stronger with ultrasound-based measures than with manometry. |
| Teyhen et al., 2005 [31] | n = 30 patients with chronic low back pain (18M/12F) Age: 62.0 ± 12.8 years Exercise compliance: 83.0% | Tactile and verbal feedback vs. Tactile, verbal and RUSI feedback | All patients were placed on quadruped position. In both groups, tactile and verbal instructions were provided to all subjects in each position. After the training in quadruped, patients were then randomly assigned to receive further instruction using traditional training (visual + tactile feedback) or traditional training with biofeedback in the ADIM. | To determine the baseline performance of the patient’s ability to per- form the ADIM prior to training, subjects were instructed to contract their abdominals by bringing their belly button up and in towards their spine. No other instruction or tactile cues were provided. After baseline measurements were obtained, all subjects received an education session and training in the ADIM in 3 positions: quadruped, seated and supine. A total of 5 contraction attempts, each with a 10-s hold, were performed in each of the 3 positions. | Transversus Abdominis Internal Oblique External Oblique | Ultrasound imaging Thickness differences between rest and ADIM. In addition, a reliability analysis was performed. Performance retention At the end of the first session, all subjects received instruction on the home exercise program and were asked to return after 4 days. | Intrarater reliability measuring lateral abdominal muscle thickness exceeded 0.93. On average, patients in both groups demonstrated a 2-fold increase in the thickness of the TrA during the ADIM. Performance of the ADIM did not differ between the groups. |
| Van et al., 2006 [32] | n = 25 healthy participants (6M/19F) Age: 19.1–19.9 years | Verbal feedback vs. Verbal and RUSI feedback | Subjects were placed in a prone position. All subjects received feedback on the number of millimeters of increase in muscle thickness that occurred with contraction of the multifidus (KR), with the aim being to increase this value. In addition to the provision of KR, subjects in the other group received biofeedback in the form of visual observation of the ultrasound image of the muscle contraction as it occurred. | Prior to testing in the acquisition phase, all subjects received the same initial explanation relating to the multifidus muscle. Each subject performed a total of 10 contractions (acquisition phase) with 20 s of rest between measurements. After completing the 10 trials in the acquisition phase, all subjects were asked to return in 1 week for follow-up assessments (retention phase). | Lumbar multifidus | Ultrasound imaging To assess multifidus muscle contraction, the difference between the multifidus muscle thickness at rest and during contraction was calculated. | Subjects from both groups improved their voluntary contraction of the multifidus muscle in the acquisition phase (p < 0.001) and the ability to recruit the multifidus muscle differed between groups (p < 0.05), with subjects in the group that received visual ultrasound biofeedback achieving greater improvements. In addition, the group that received visual ultrasound biofeedback retained their improvement in performance from week 1 to week 2 (p > 0.90), whereas the performance of the other group decreased (p < 0.05). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valera-Calero, J.A.; Fernández-de-las-Peñas, C.; Varol, U.; Ortega-Santiago, R.; Gallego-Sendarrubias, G.M.; Arias-Buría, J.L. Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7554. https://doi.org/10.3390/ijerph18147554
Valera-Calero JA, Fernández-de-las-Peñas C, Varol U, Ortega-Santiago R, Gallego-Sendarrubias GM, Arias-Buría JL. Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(14):7554. https://doi.org/10.3390/ijerph18147554
Chicago/Turabian StyleValera-Calero, Juan Antonio, César Fernández-de-las-Peñas, Umut Varol, Ricardo Ortega-Santiago, Gracia María Gallego-Sendarrubias, and José Luis Arias-Buría. 2021. "Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review" International Journal of Environmental Research and Public Health 18, no. 14: 7554. https://doi.org/10.3390/ijerph18147554
APA StyleValera-Calero, J. A., Fernández-de-las-Peñas, C., Varol, U., Ortega-Santiago, R., Gallego-Sendarrubias, G. M., & Arias-Buría, J. L. (2021). Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. International Journal of Environmental Research and Public Health, 18(14), 7554. https://doi.org/10.3390/ijerph18147554







