Guided Tissue Regeneration Treatment Yields Better Results in Class II Furcations in the Mandible Than in the Maxilla: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Method
2.1. Pre-Study Preparation
2.2. Study Design and Patients
- Diagnosis of chronic periodontitis;
- Clinical appearance of lingual or vestibular class II furcation defects with >3 mm horizontal width;
- Completion of an oral hygiene therapy comprising oral hygiene guidance, scaling, and root planning;
- O’ Leary plaque control level ≤ 20%;
- At least 2 mm of keratinized gingiva on the furcated teeth.
- Smoking habit;
- Severe systemic disease;
- Betel nut chewing habits;
- Alcohol consumption;
- Anticoagulant and antibiotic medication for 6 months prior to treatment;
- Poor oral hygiene;
- Pregnant or breastfeeding women;
- Allergy to collagen membrane;
- Lost to follow-up after 3, 6, and 9 months postoperatively.
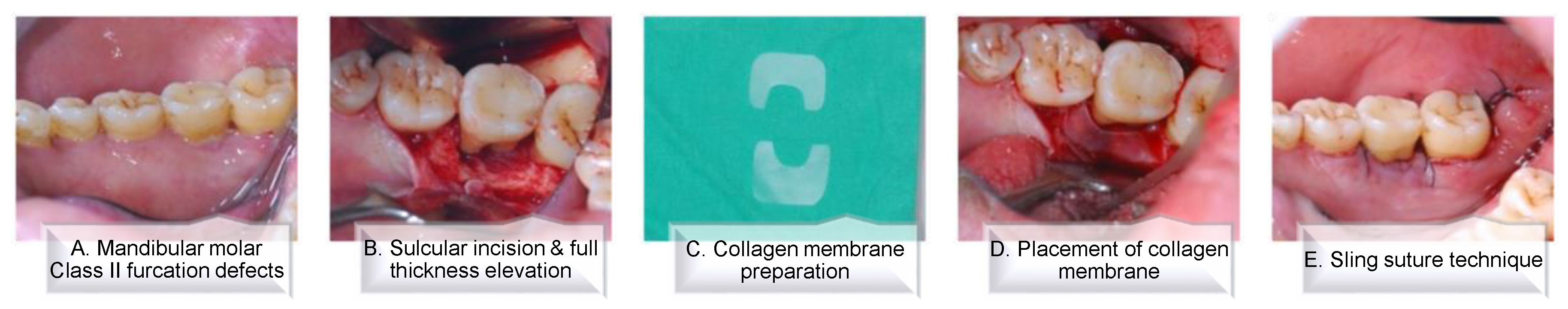
2.3. Surgical Procedure
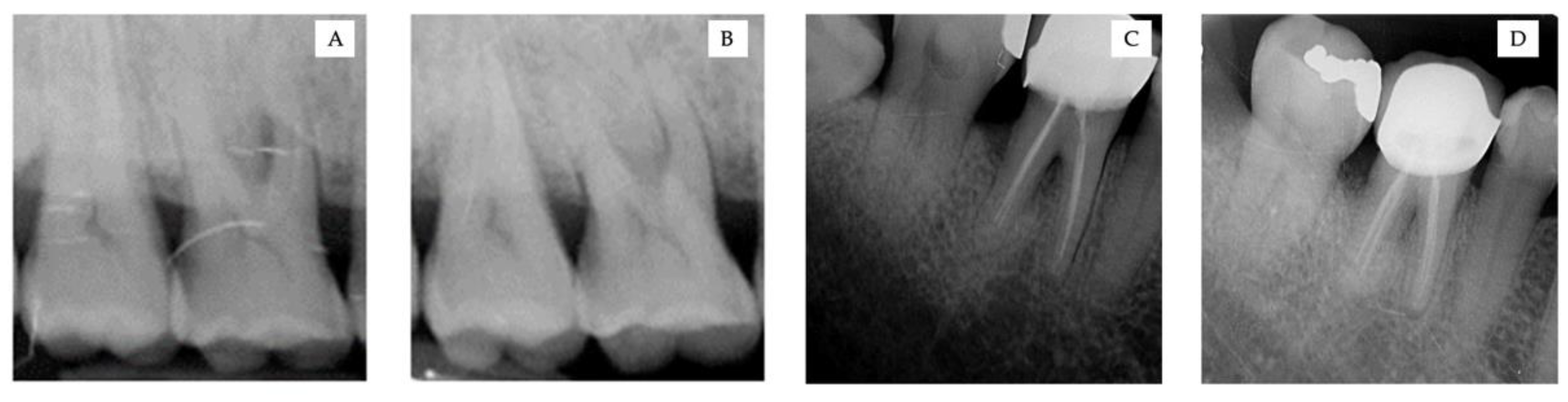
2.4. Data Collection and Measurements
2.5. Study Outcomes
2.6. Statistical Analyses
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Clinical and Radiographic Measurements
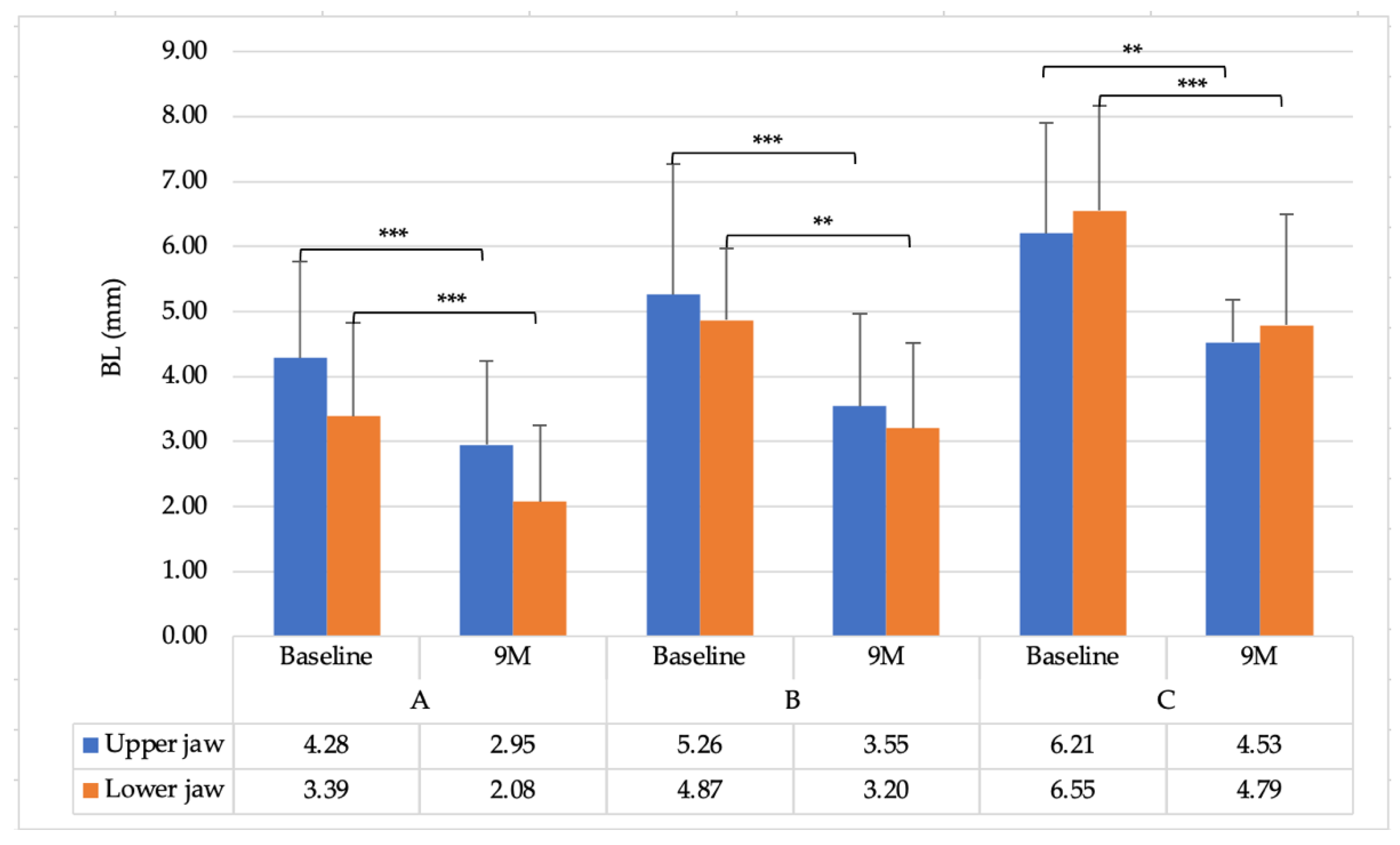
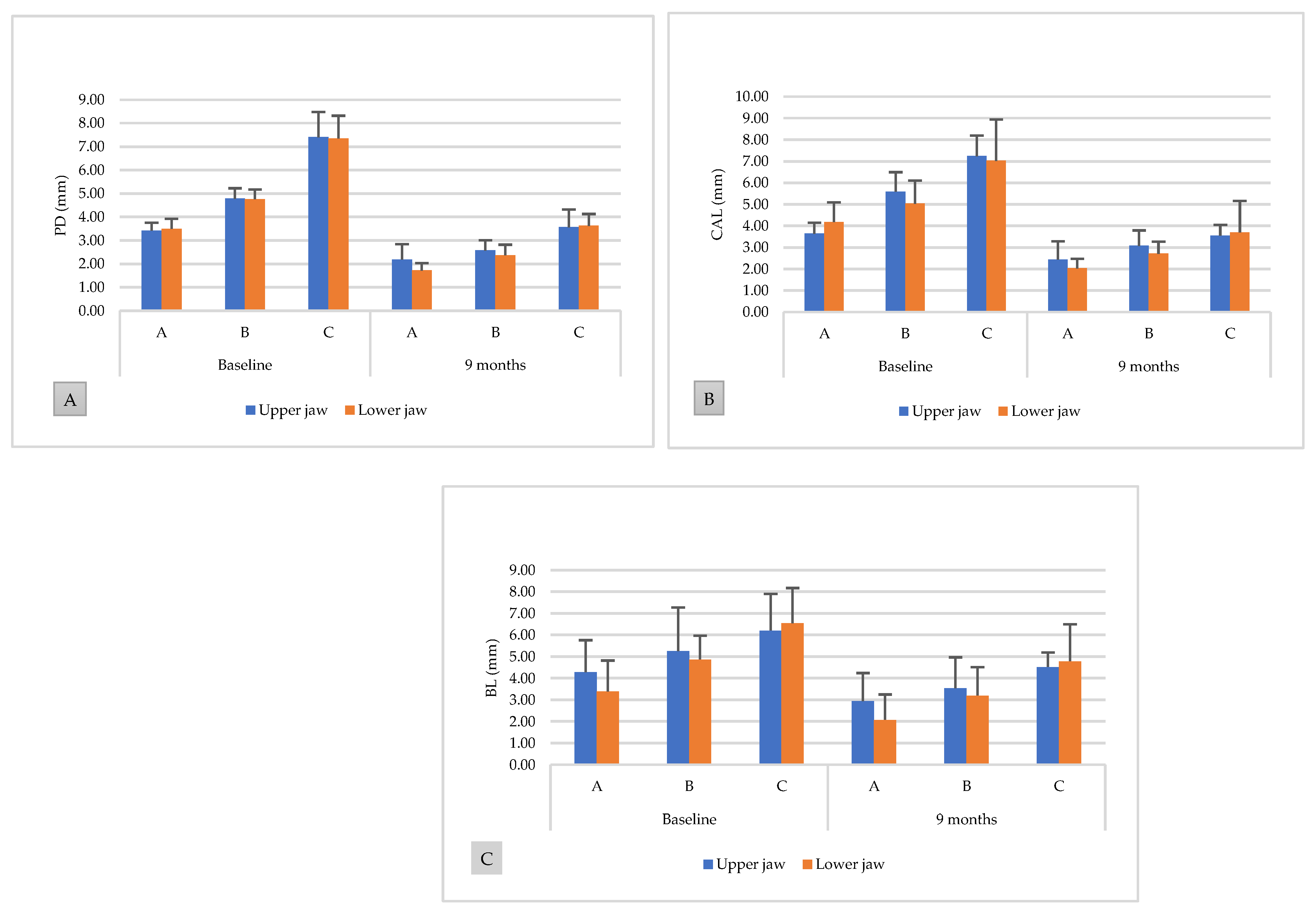
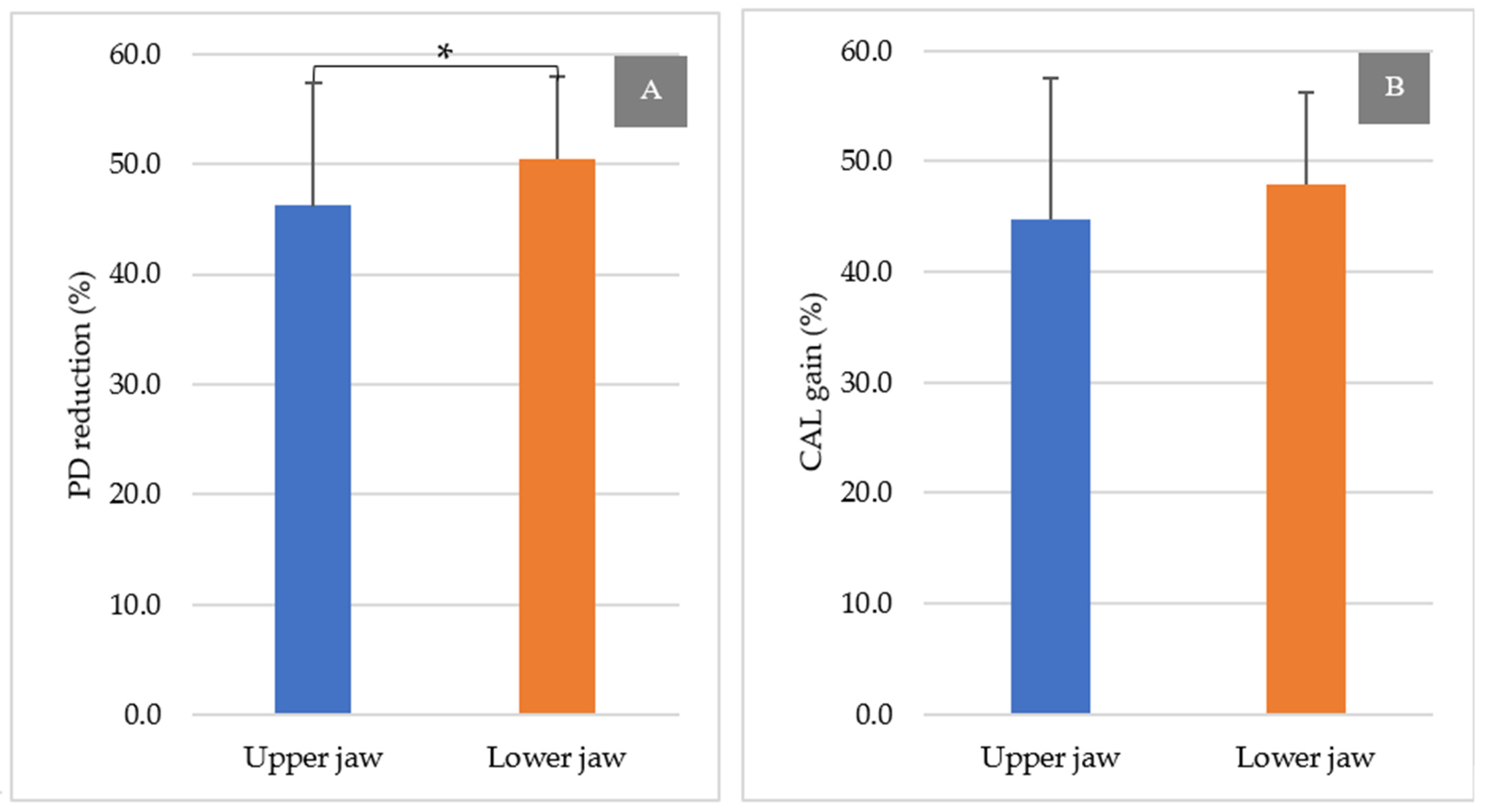
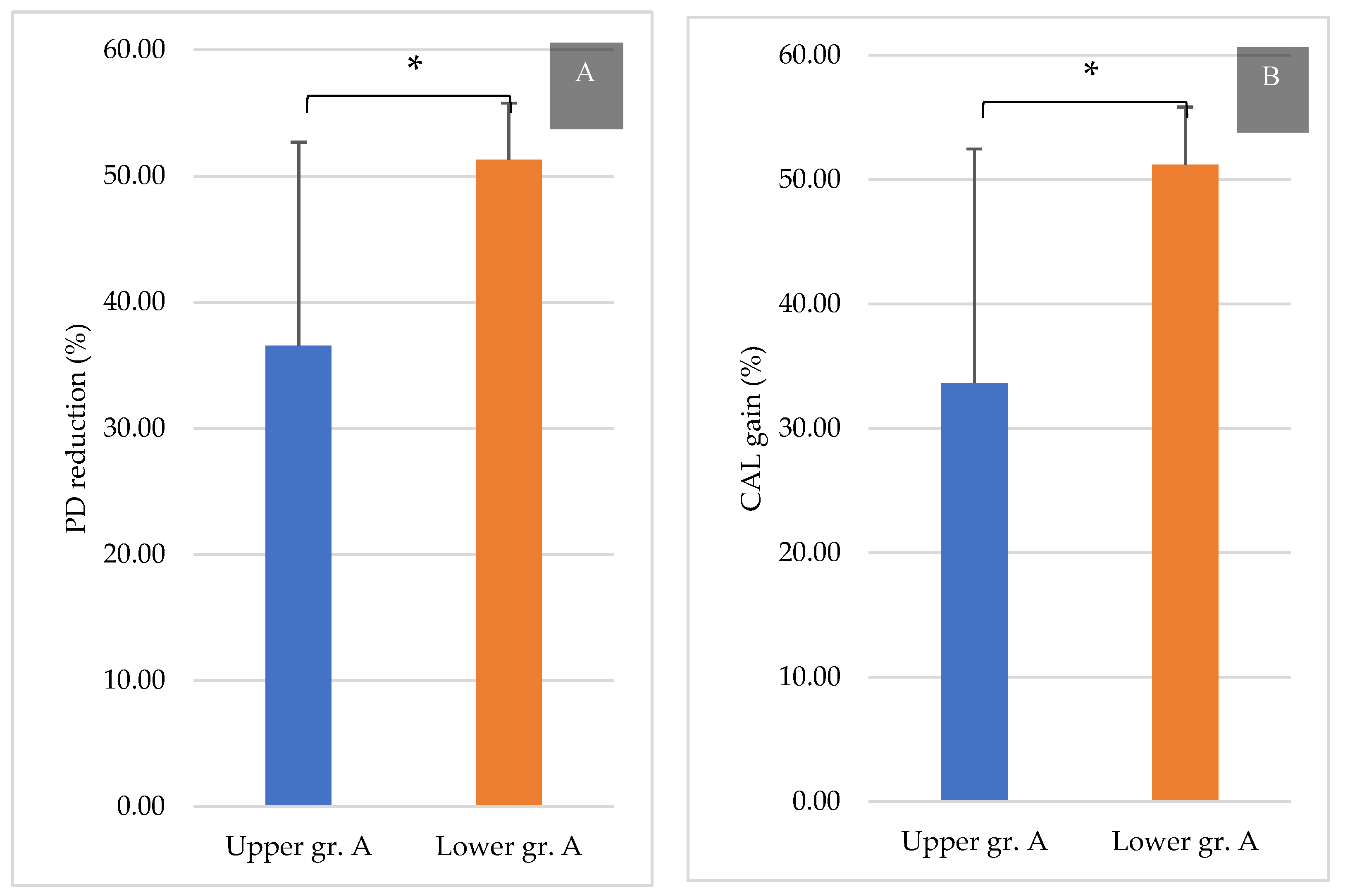
3.3. Comparison between the Upper and Lower Jaws
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Lang, N.P.; Lindhe, J. (Eds.) Clinical Periodontology and Implant Dentistry; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Nibali, L.; Zavattini, A.; Nagata, K.; Di Iorio, A.; Lin, G.-H.; Needleman, I.; Donos, N. Tooth loss in molars with and without furcation involvement—A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 156–166. [Google Scholar] [CrossRef]
- Hamp, S.E.; Nyman, S.; Lindhe, J. Periodontal treatment of multirooted teeth. Results after 5 years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar] [CrossRef]
- Sanz, M.; Jepsen, K.; Eickholz, P.; Jepsen, S. Clinical concepts for regenerative therapy in furcations. Periodontol. 2000 2015, 68, 308–332. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Christiansen, A.L.; Cortellini, P. Vertical subclassification predicts survival of molars with class II furcation involvement during supportive periodontal care. J. Clin. Periodontol. 2017, 44, 1140–1144. [Google Scholar] [CrossRef]
- Dommisch, H.; Walter, C.; Dannewitz, B.; Eickholz, P. Resective surgery for the treatment of furcation involvement: A systematic review. J. Clin. Periodontol. 2020, 47, 375–391. [Google Scholar] [CrossRef] [Green Version]
- Hugoson, A.; Ravald, N.; Fornell, J.; Johard, G.; Teiwik, A.; Gottlow, J. Treatment of Class II Furcation Involvements in Humans with Bioresorbable and Nonresorbable Guided Tissue Regeneration Barriers. A Randomized Multi-Center Study. J. Periodontol. 1995, 66, 624–634. [Google Scholar] [CrossRef]
- Eickholz, P.; Kim, T.S.; Holle, R. Guided tissue regeneration with non-resorbable and biodegradable barriers: 6 months results. J. Clin. Periodontol. 1997, 24, 92–101. [Google Scholar] [CrossRef]
- Caffesse, R.; Smith, B.A.; Duff, B.; Morrison, E.; Merrill, D.; Becker, W. Class II Furcations Treated by Guided Tissue Regeneration in Humans: Case Reports. J. Periodontol. 1990, 61, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Yukna, C.; Yukna, R. Multi-Center Evaluation of Bioabsorbable Collagen Membrane for Guided Tissue Regeneration in Human Class II Furcations. J. Periodontol. 1996, 67, 650–657. [Google Scholar] [CrossRef]
- Djurkin, A.; Toma, S.; Brecx, M.C.; Lasserre, J.F. Treatment of mandibular Class II furcations using bovine-derived bone xenograft with or without a collagen membrane: A randomized controlled trial. Quintessence Int. 2019, 50, 652–660. [Google Scholar]
- Salamanca, E.; Tsai, C.-Y.; Pan, Y.-H.; Lin, Y.-T.; Huang, H.-M.; Teng, N.-C.; Lin, C.-T.; Feng, S.-W.; Chang, W.-J. In Vitro and In Vivo Study of a Novel Porcine Collagen Membrane for Guided Bone Regeneration. Materials 2016, 9, 949. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-J.; Lin, S.-L.; Chang, W.-J.; Salamanca, E.; Feng, S.-W.; Teng, N.-C.; Huang, H.-M. A novel porcine collagen GTR membrane for treatment of Class II molar furcation involvement. J. Polym. Eng. 2014, 34, 237–240. [Google Scholar] [CrossRef]
- Huynh-Ba, G.; Kuonen, P.; Hofer, D.; Schmid, J.; Lang, N.P.; Salvi, G.E. The effect of periodontal therapy on the survival rate and incidence of complications of multirooted teeth with furcation involvement after an observation period of at least 5 years: A systematic review. J. Clin. Periodontol. 2009, 36, 164–176. [Google Scholar] [CrossRef]
- Fleischer, H.C.; Mellonig, J.T.; Brayer, W.K.; Gray, J.L.; Barnett, J.D. Scaling and Root Planing Efficacy in Multirooted Teeth. J. Periodontol. 1989, 60, 402–409. [Google Scholar] [CrossRef]
- Metzler, D.G.; Seamons, B.C.; Mellonig, J.T.; Gher, M.E.; Gray, J.L. Clinical Evaluation of Guided Tissue Regeneration in the Treatment of Maxillary Class II Molar Furcation Invasions. J. Periodontol. 1991, 62, 353–360. [Google Scholar] [CrossRef]
- Mellonig, J.T.; Seamons, B.C.; Gray, J.L.; Towle, H.J. Clinical evaluation of guided tissue regeneration in the treatment of grade II molar furcation invasions. Int. J. Periodontics Restor. Dent. 1994, 14, 254–271. [Google Scholar]
- Gher, M.W.; Dunlap, R.W. Linear Variation of the Root Surface Area of the Maxillary First Molar. J. Periodontol. 1985, 56, 39–43. [Google Scholar] [CrossRef]
- Dunlap, R.M.; Gher, M.E. Root Surface Measurements of the Mandibular First Molar. J. Periodontol. 1985, 56, 234–238. [Google Scholar] [CrossRef]
- Al-Shammari, K.F.; Kazor, C.E.; Wang, H.-L. Molar root anatomy and management of furcation defects. J. Clin. Periodontol. 2001, 28, 730–740. [Google Scholar] [CrossRef]
- Lu, H.-K.J. Topographical Characteristics of Root Trunk Length Related to Guided Tissue Regeneration. J. Periodontol. 1992, 63, 215–219. [Google Scholar] [CrossRef]
- Garrett, S.; Polson, A.M.; Stoller, N.H.; Drisko, C.L.; Caton, J.G.; Harrold, C.Q.; Bogle, G.; Greenwell, H.; Lowenguth, R.A.; Duke, S.P.; et al. Comparison of a bioabsorbable GTR barrier to a non-absorbable barrier in treating human class II furcation defects. A multi-center parallel design randomized single-blind trial. J. Periodontol. 1997, 68, 667–675. [Google Scholar] [CrossRef]
- Jepsen, S.; Gennai, S.; Hirschfeld, J.; Kalemaj, Z.; Buti, J.; Graziani, F. Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2019, 47, 352–374. [Google Scholar] [CrossRef]
- Report, T.F. American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J. Periodontol. 2015, 86, 835–838. [Google Scholar]
- Puri, S. The evaluation of effectiveness of combination of bone graft, bioresorbable membrane and lasers in the treatment of mandibular grade ii furcation defects in humans: A case series. Int. J. Appl. Dent. Sci. 2019, 5, 359–362. [Google Scholar]
- Taheri, M.; Molla, R.; Radvar, M.; Sohrabi, K.; Najafi, M. An evaluation of bovine derived xenograft with and without a bioabsorbable collagen membrane in the treatment of mandibular Class II furcation defects. Aust. Dent. J. 2009, 54, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, R.; Lindhe, J. Guided tissue regeneration in the treatment of degree II furcations in maxillary molars. J. Clin. Periodontol. 1995, 22, 756–763. [Google Scholar] [CrossRef]
- Houser, B.E.; Mellonig, J.T.; Brunsvold, M.A.; Cochran, D.L.; Meffert, R.M.; Alder, M.E. Clinical evaluation of anorganic bovine bone xenograft with a bioabsorbable collagen barrier in the treatment of molar furcation defects. Int. J. Periodontics Restor. Dent. 2001, 21, 161–169. [Google Scholar]
- Bowers, G.M.; Schallhorn, R.G.; McClain, P.K.; Morrison, G.M.; Morgan, R.; Reynolds, M.A. Factors Influencing the Outcome of Regenerative Therapy in Mandibular Class II Furcations: Part I. J. Periodontol. 2003, 74, 1255–1268. [Google Scholar] [CrossRef]
- Wang, H.-L.; O’Neal, R.B.; Thomas, C.L.; Shyr, Y.; MacNeil, R.L. Evaluation of an Absorbable Collagen Membrane in Treating Class II Furcation Defects. J. Periodontol. 1994, 65, 1029–1036. [Google Scholar] [CrossRef]
- Majzoub, J.; Barootchi, S.; Tavelli, L.; Wang, C.W.; Travan, S.; Wang, H.L. Treatment effect of guided tissue regeneration on the horizontal and vertical components of furcation defects: A retrospective study. J. Periodontol. 2020, 91, 1148–1158. [Google Scholar] [CrossRef]
- Karring, T.L.J.; Cortellini, P. Regenerative periodontal therapy. In Clinical Peri-Odontology and Implant Dentistry; Lindhe, J., Karring, T., Lang, N.P., Eds.; Munskgaard: Copenhagen, Denmark, 1998; pp. 597–646. [Google Scholar]
- Tsao, Y.-P.; Neiva, R.; Al-Shammari, K.; Oh, T.-J.; Wang, H.-L. Factors Influencing Treatment Outcomes in Mandibular Class II Furcation Defects. J. Periodontol. 2006, 77, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.; Machtei, E.E.; Reitmeir, P.; Holle, R.; Kim, T.-S.; Eickholz, P. Radiographic parameters as prognostic indicators for healing of class II furcation defects. J. Clin. Periodontol. 2004, 31, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pruthi, V.K.; Gelskey, S.C.; Mirbod, S.M. Furcation therapy with bioabsorbable collagen membrane: A clinical trial. J. Can. Dent. Assoc. 2002, 68, 610–615. [Google Scholar]

| Baseline Characteristics | Upper Jaw (n = 30) | Lower Jaw (n = 30) |
|---|---|---|
| Demographic data | ||
| Male (n, %) | 20, 66.6% | 12, 40% |
| Female (n, %) | 10, 33.3% | 18, 60% |
| Age, mean ± SD | 50.03 ± 10.96 | 51.10 ± 10.38 |
| Grouping by PD (n, %) | ||
| A (1–3 mm) | 6, 20.0% | 9, 30.0% |
| B (4–6 mm) | 13, 43.3% | 13, 43.3% |
| C (>6 mm) | 11, 36.6% | 8, 26.6% |
| Parameters, mean ± SD (mm) | ||
| BL | 5.41 ± 1.88 | 4.87 ± 1.78 |
| PD | 5.46 ± 1.74 | 5.07 ± 1.61 |
| CAL | 5.80 ± 1.57 | 5.31 ± 1.69 |
| Groups | PD | CAL | ||||
|---|---|---|---|---|---|---|
| Baseline vs. 9 Months | p | Baseline vs. 9 Months | p | |||
| Upper jaw | ||||||
| A | 3.42 ± 0.34 | 2.18 ± 0.66 | <0.01 | 3.64 ± 0.51 | 2.43 ± 0.85 | <0.01 |
| B | 4.78 ± 0.45 | 2.57 ± 0.44 | <0.001 | 5.59 ± 0.90 | 3.08 ± 0.71 | <0.001 |
| C | 7.40 ± 1.08 | 3.56 ± 0.76 | <0.001 | 7.24 ± 0.96 | 3.54 ± 0.51 | <0.001 |
| Lower jaw | ||||||
| A | 3.49 ± 0.44 | 1.71 ± 0.32 | <0.001 | 4.18 ± 0.92 | 2.03 ± 0.44 | <0.001 |
| B | 4.75 ± 0.42 | 2.37 ± 0.45 | <0.001 | 5.03 ± 1.07 | 2.71 ± 0.57 | <0.001 |
| C | 7.35 ± 0.97 | 3.62 ± 0.50 | <0.001 | 7.02 ± 1.92 | 3.70 ± 1.46 | <0.001 |
| Groups | Bone Level Gain (%) | |||||
|---|---|---|---|---|---|---|
| 3 Months | 6 Months | p | 9 Months | p | ||
| 3 vs. 6 Months | 3 vs. 9 Months | |||||
| Upper jaw | A | 28.7 ± 7.78 | 31.1 ± 8.20 | 0.113 | 32.9 ± 8.54 | 0.022 |
| B | 24.3 ± 17.8 | 28.0 ± 15.1 | 0.363 | 32.3 ± 16.1 | 0.100 | |
| C | 13.7 ± 15.8 | 20.9 ± 21.4 | 0.146 | 23.0 ± 20.8 | 0.812 | |
| Lower jaw | A | 27.6 ± 13.3 | 36.8 ± 15.5 | 0.022 | 41.0 ± 14.2 | 0.003 |
| B | 28.2 ± 16.4 | 30.5 ± 22.2 | 0.428 | 33.3 ± 23.2 | 0.127 | |
| C | 20.3 ± 9.15 | 25.5 ± 8.65 | 0.101 | 28.8 ± 11.9 | 0.082 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorj, O.; Lee, W.-F.; Salamanca, E.; Pan, Y.-H.; Wu, Y.-F.; Hsu, Y.-S.; Lin, J.C.Y.; Lin, Y.-D.; Choy, C.-S.; Chang, W.-J. Guided Tissue Regeneration Treatment Yields Better Results in Class II Furcations in the Mandible Than in the Maxilla: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 7447. https://doi.org/10.3390/ijerph18147447
Dorj O, Lee W-F, Salamanca E, Pan Y-H, Wu Y-F, Hsu Y-S, Lin JCY, Lin Y-D, Choy C-S, Chang W-J. Guided Tissue Regeneration Treatment Yields Better Results in Class II Furcations in the Mandible Than in the Maxilla: A Retrospective Study. International Journal of Environmental Research and Public Health. 2021; 18(14):7447. https://doi.org/10.3390/ijerph18147447
Chicago/Turabian StyleDorj, Odontuya, Wei-Fang Lee, Eisner Salamanca, Yu-Hwa Pan, Yi-Fan Wu, Yung-Szu Hsu, Jerry C. Y. Lin, Yu-De Lin, Cheuk-Sing Choy, and Wei-Jen Chang. 2021. "Guided Tissue Regeneration Treatment Yields Better Results in Class II Furcations in the Mandible Than in the Maxilla: A Retrospective Study" International Journal of Environmental Research and Public Health 18, no. 14: 7447. https://doi.org/10.3390/ijerph18147447
APA StyleDorj, O., Lee, W.-F., Salamanca, E., Pan, Y.-H., Wu, Y.-F., Hsu, Y.-S., Lin, J. C. Y., Lin, Y.-D., Choy, C.-S., & Chang, W.-J. (2021). Guided Tissue Regeneration Treatment Yields Better Results in Class II Furcations in the Mandible Than in the Maxilla: A Retrospective Study. International Journal of Environmental Research and Public Health, 18(14), 7447. https://doi.org/10.3390/ijerph18147447







