Estimating an EQ-5D-3L Value Set for Romania Using Time Trade-Off
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Design and Sampling Framework
2.2. Interview Procedure
2.3. Valuation Protocol and Procedure
2.4. Selection of EQ-5D-3L Health States
2.5. Statistical Analyses
2.5.1. Exclusion Criteria
- Interviews of suspect quality, performed by interviewers that were excluded from the team of interviewers due to protocol noncompliance.
- Interviews performed by interviewers having more than 40% of the interviews performed flagged as interviews of suspect quality (as defined in the EQ-5D-5L valuation study).
- Interviews performed by interviewers not performing enough interviews to achieve a harmonized learning effect between interviewers [44]. The minimum number of interviews was set at 20.
- Interviews for which the interviewer had not shown the worse than dead example in the training part of the survey.
- Participants with a positive slope on the regression between their values and the misery index of the health states assessed for participants who gave the same value to all health states or did not trade time (non-traders).
- Set 1 (denoted V1) contained all valid responses and corresponded to the exclusion criterion 1.
- Set 2 (denoted V3) corresponded to the exclusion criteria.1, 2, 3, 4, 5.
2.5.2. Modeling
- Ordinary least squares (OLS)
- Robust OLS
- Models that account for the panel structure of the data (random intercept models with respondent and interviewer mixed effects; random coefficient models)
- Models that account for the censored nature of the data (tobit and interval regression models)
- Logical consistency and significance of parameters: All regression coefficients obtained need to be logically consistent between health states and, if possible, significant at the level of 0.05. This means that health states with severe problems on a dimension must have a lower predicted utility than health states with moderate or no problems. This holds true only for intensity levels of the same dimension. More precisely, we expected health state 11233 to be considered better than the state 11333, but we could not compare it with 21333, for example. This criterion was verified through the parameter estimates of the model, where the coefficients for level-3 states need to be higher than those for level-2 states, which, in turn, must be greater than zero.
- Theoretical considerations: As we expected the standard deviation of the observed values to increase with worsening severity of health states, models that accounted for heteroscedasticity were favored. If the percentage of observed values at −1 exceeded the normal range expected for valuation studies (2–10%), preference was given to censored models. For models meeting multiple criteria, preference was given to the model with the lowest number of independent variables (principle of parsimony).
- The goodness of fit. For all logically consistent models, we calculated the Akaike information criterion (AIC) and Bayesian information criterion (BIC). The smaller the values for AIC and BIC, the better the goodness of fit of the model. In case of different conclusions for the two indicators, BIC was preferred to also account for model parsimony.
- Prediction accuracy (Spearman’s correlation between predicted and observed utilities), value range, and the ranking of dimensions based on the size of the coefficient for the worst level on each dimension were also taken into account.
2.5.3. Comparison with Other Countries’ Value Sets
3. Results
4. Discussion
5. Limitations
Our Study Has a Certain Number of Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Health Order No. 318/2008 Regarding the Approval of Criteria for Inclusion, Noninclusion, and Exclusion of Drugs from the Reimbursement List, the Approval of the Documents to be Submitted by Solicitants Regarding Drug Inclusion on the Reimburse; Romanian Official Gazette: No. 210. 19 March 2008. Available online: https://www.monitoruloficial.ro/article--e-Monitor--297.html (accessed on 23 June 2020).
- Lopert, R.; Ruiz, F.; Chalkidou, K. Applying rapid ‘de-facto’ HTA in resource-limited settings: Experience from Romania. Health Policy 2013, 112, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, P.; Tesar, T.; Vostalova, L.; Draganic, P.; Manova, M.; Savova, A.; Petrova, G.; Rugaja, Z.; Männik, A.; Sowada, C.; et al. Pharmaceutical regulation in Central and Eastern European countries: A current review. Front. Pharmacol. 2017, 8, 18. [Google Scholar] [CrossRef]
- Radu, C.P.; Pana, B.C.; Furtunescu, F.L. Drug Policy in Romania. Value Health Reg. Issues 2018, 16, 28–32. [Google Scholar] [CrossRef]
- Lopert, R.; Ruiz, F.; Gheorghe, A.; Chanturidze, T. Deliverable 1: Situational Analysis of Romanian HTA. From “Technical Assistance for Institution Building of Health Technology Assessment Structure, Including Training for the National Agency for Medicines & Medical Devices”. March 2017. Available online: http://www.ms.ro/wp-content/uploads/2017/05/Inception-Report-en.pdf (accessed on 20 March 2019).
- EuroQol Research Foundation. EQ-5D-3L User Guide. 2018. Available online: https://euroqol.org/wp-content/uploads/2021/01/EQ-5D-3LUserguide-14-0421.pdf (accessed on 5 July 2021).
- Räsänen, P.; Roine, E.; Sintonen, H.; Semberg-Konttinen, V.; Ryynänen, O.P.; Roine, R. Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. Int. J. Technol. Assess. Health Care 2006, 22, 235–241. [Google Scholar] [CrossRef]
- Devlin, N.J.; Brooks, R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy 2017, 15, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Cleemput, I. A social preference valuations set for EQ-5D health states in Flanders, Belgium. Eur. J. Health Econ. 2010, 11, 205–213. [Google Scholar] [CrossRef]
- Wittrup-Jensen, K.U.; Lauridsen, J.; Pedersen, K.M. Modelling Danish EuroQol (EQ-5D) Tariffs by Applying the Time Trade-Off Method. 2008. Available online: https://www.sdu.dk/-/media/files/om_sdu/centre/cohere/working+papers/20084.pdf (accessed on 12 August 2018).
- Chevalier, J.; de Pouvourville, G. Valuing EQ-5D using Time Trade-Off in France. Eur. J. Health Econ. 2013, 14. [Google Scholar] [CrossRef]
- Greiner, W.; Claes, C.; Busschbach, J.; von der Schulenburg, J.-M.G. Validating the EQ-5D with time trade off for the German population. Eur. J. Health Econ. 2005, 6, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; von der Schulenburg, J.-M.G.; Greiner, W. Valuation of the EQ-5D-5L with composite time trade-off for the German population—An exploratory study. Health Qual. Life Outcomes 2017, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Kontodimopoulos, N.; Pappa, E.; Niakas, D.; Yfantopoulos, J.; Dimitrakaki, C.; Tountas, Y. Validity of the EuroQoL (EQ-5D) Instrument in a Greek General Population. Value Health 2008, 11. [Google Scholar] [CrossRef]
- Lamers, L.M.; McDonnell, J.; Stalmeier, P.F.M.; Krabbe, P.F.M.; Busschbach, J.J.V. The Dutch tariff: Results and arguments for an effective design for national EQ-5D valuation studies. Health Econ. 2006, 15, 1121–1132. [Google Scholar] [CrossRef]
- Scalone, L.; Cortesi, P.A.; Ciampichini, R.; Belisari, A.; D’Angiolella, L.S.; Cesana, G.; Mantovani, L.G. Italian Population-Based Values of EQ-5D Health States. Value Health 2013, 16, 814–822. [Google Scholar] [CrossRef]
- Golicki, D.; Niewada, M. General population reference values for 3-level EQ-5D (EQ-5D-3L) questionnaire in Poland. Pol. Arch. Intern. Med. 2015, 125, 18–26. [Google Scholar] [CrossRef]
- Golicki, D.; Niewada, M.; Hout, B.V.; Janssen, M.F.; Pickard, A.S.; van Hout, B.; Janssen, M.F.; Pickard, A.S. Interim EQ-5D-5L Value Set for Poland: First Crosswalk Value Set in Central and Eastern Europe. Value Health Reg. Issues 2014, 4, 19–23. [Google Scholar] [CrossRef]
- Ferreira, L.N.; Ferreira, P.L.; Pereira, L.N.; Oppe, M. The valuation of the EQ-5D in Portugal. Qual. Life Res. 2014, 2. [Google Scholar] [CrossRef]
- Badia, X.; Roset, M.; Herdman, M.; Kind, P. A Comparison of United Kingdom and Spanish General Population Time Trade-off Values for EQ-5D Health States. Med. Decis. Mak. 2001, 21, 7–16. [Google Scholar] [CrossRef]
- Prevolnik, O.M.; Rupel, V. The Eq-5d Health States Value Set for Slovenia. Zdrav. Var. 2012, 51, 128–140. [Google Scholar]
- Burström, K.; Sun, S.; Gerdtham, U.G.; Henriksson, M.; Johannesson, M.; Levin, L.Å.; Zethraeus, N. Swedish experience-based value sets for EQ-5D health states. Qual. Life Res. 2014, 23, 431–442. [Google Scholar] [CrossRef]
- Dolan, P.; Gudex, C.; Kind, P.; Williams, A. A Social Tariff for EuroQol: Results from a UK General Population Study; Discussion Paper 138; Centre for Health Economics, The University of York: York, UK, 1995. [Google Scholar]
- Dolan, P.; Gudex, C.; Kind, P.; Williams, A. The time trade-off method: Results from a general population study. Health Econ. 1996, 5, 141–154. [Google Scholar] [CrossRef]
- Kiadaliri, A.A. A comparison of Iran and UK EQ-5D-3L value sets based on visual analogue scale. Int. J. Health Policy Manag. 2016, 6, 267–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- König, H.H.; Bernert, S.; Angermeyer, M.C.; Matschinger, H.; Martinez, M.; Vilagut, G.; Haro, J.M.; de Girolamo, G.; de Graaf, R.; Kovess, V.; et al. Comparison of population health status in six European countries results of a representative survey using the EQ-5D questionnaire. Med. Care 2009, 47, 255–261. [Google Scholar] [CrossRef]
- Santos, M.; Cintra, M.A.; Monteiro, A.L.; Santos, B.; Gusmão-Filho, F.; Andrade, M.V.; Noronha, K.; Cruz, L.N.; Camey, S.; Tura, B.; et al. Brazilian valuation of EQ-5D-3L health states. Med. Decis. Mak. 2016, 36, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Herdman, M.; Kind, P. The influence of ill-health experience on the valuation of health. PharmacoEconomics 1998, 13, 687–696. [Google Scholar] [CrossRef]
- Dolan, P.; Kind, P. Inconsistency and health state valuations. Soc. Sci. Med. 1996, 42, 609–615. [Google Scholar] [CrossRef]
- Dolan, P. The effect of experience of illness on health state valuations. J. Clin. Epidemiol. 1996, 49, 551–564. [Google Scholar] [CrossRef]
- Commision, E. State of Health in the EU on Health Systems and Policies European a Partnership Hosted by WHO b. Health in Romania State of Health In The Eu: Country Profile Romania-2017. 2017. Available online: https://ec.europa.eu/health/sites/health/files/state/docs/chp_romania_english.pdf (accessed on 22 August 2018).
- Paveliu, M.S.; Lorenzovici, L.; Tudose, C. Oral anticoagulant treatment with antivitamin k in Romania. A cost-effectiveness analysis of using INR home testing devices. Farmacia 2018, 66, 358–364. [Google Scholar]
- Mogoşan, C.; Stoica, V.; Mihai, C.; Ciofu, C.; Bojincǎ, M.; Milicescu, M.; Crişan, V.; Mioara, B.; Bojincă, M.; Milicescu, M.; et al. Trends of rheumatoid arthritis monitorization in Romania. J. Med. Life 2010, 3, 330–337. [Google Scholar]
- de Smedt, D.; Clays, E.; Höfer, S.; Oldridge, N.; Kotseva, K.; Maggioni, A.P.; Janssen, B.; de Bacquer, D. Validity and reliability of the HeartQoL questionnaire in a large sample of stable coronary patients: The EUROASPIRE IV Study of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2016, 23, 714–721. [Google Scholar] [CrossRef]
- de Smedt, D.; Clays, E.; Doyle, F.; Kotseva, K.; Prugger, C.; Pająk, A.; Jennings, C.; Wood, D.; de Bacquer, D.; EUROASPIRE Study Group. Validity and reliability of three commonly used quality of life measures in a large European population of coronary heart disease patients. Int. J. Cardiol. 2013, 167, 2294–2299. [Google Scholar] [CrossRef]
- Salmon, C.T.; Nichols, J.S. The Next-Birthday Method of Respondent Selection. Public Opin. Q. 1983, 47, 270. [Google Scholar] [CrossRef]
- Olariu, E.; Paveliu, M.S.; Baican, E.; Oluboyede, Y.; Vale, L.; Niculescu-Aron, I.G. Measuring health-related quality of life in the general population and Roma communities in Romania: Study protocol for two cross-sectional studies. BMJ Open 2019, 9, e029067. [Google Scholar] [CrossRef] [PubMed]
- Stolk, E.; Ludwig, K.; Rand, K.; van Hout, B.; Ramos-Go, J.M. Overview, Update, and Lessons Learned from the International EQ-5D-5L Valuation Work: Version 2 of the EQ-5D-5L Valuation Protocol. Value Health 2019, 22, 23–30. [Google Scholar] [CrossRef]
- Ramos-Goñi, J.M.; Oppe, M.; Slaap, B.; Busschbach, J.J.; Stolk, E. Quality Control Process for EQ-5D-5L Valuation Studies. Value Health 2017, 20, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, N.; Bonsel, G.; Busschbach, J.; Stolk, E. Selecting Health States for EQ-5D-3L Valuation Studies: Statistical Considerations Matter. Value Health 2018, 21, 456–461. [Google Scholar] [CrossRef]
- ELaw, H.; Pickard, A.S.; Xie, F.; Walton, S.M.; Lee, T.A.; Schwartz, A. Parallel Valuation: A Direct Comparison of EQ-5D-3L and EQ-5D-5L Societal Value Sets. Med. Decis. Mak. 2018, 38, 968–982. [Google Scholar]
- Rencz, F.; Brodszky, V.; Gulácsi, L.; Golicki, D.; Ruzsa, G.; Pickard, A.S.; Law, E.H.; Péntek, M. Parallel Valuation of the EQ-5D-3L and EQ-5D-5L by Time Trade-Off in Hungary. Value Health 2020, 23, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Mulhern, B.; Longworth, L.; Janssen, M.F. An empirical study of two alternative comparators for use in time trade-off studies. Value Health 2016, 19, 53–59. [Google Scholar] [CrossRef]
- Dolan, P. Modeling Valuations for EuroQol Health States. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Bansback, N.; Tsuchiya, A.; Brazier, J.; Anis, A. Canadian Valuation of EQ-5D Health States: Preliminary Value Set and Considerations for Future Valuation Studies. PLoS ONE 2012, 7, e31115. [Google Scholar] [CrossRef]
- Roudijk, B.; Rogier, A.; Donders, T.; Stalmeier, P.F.M. Cultural Values: Can They Explain Differences in Health Utilities between Countries? Med. Decis. Mak. 2019, 39, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.; Kind, P. Preliminary findings of an investigation into the relationship between national culture and EQ-5D value sets. Qual. Life Res. 2010, 19, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.; Peytchev, A. Effect of Interviewer Experience on Interview Pace and Interviewer Attitudes. Public Opin. Q. 2007, 71, 273–286. [Google Scholar] [CrossRef]
- Engel, L.; Bansback, N.; Bryan, S.; Doyle-Waters, M.M.; Whitehurst, D.G. Exclusion Criteria in National Health State Valuation Studies: A Systematic Review. Med. Decis. Mak. 2016, 36, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Coordonate ale Nivelului de Trai in Romania. Veniturile si Consumul Populatiei, in Anul 2019, Institutul National de Statistica. 2020. Available online: https://insse.ro/cms/sites/default/files/field/publicatii/coordonate_ale_nivelului_de_trai_in_romania_2019-1_0.pdf (accessed on 20 August 2020).

| Variable | Definition |
|---|---|
| TTO | Time Trade-Off |
| MO2 | 1 if mobility at level 2; 0 otherwise |
| MO3 | 1 if mobility at level 3; 0 otherwise |
| SC2 | 1 if self-care at level 2; 0 otherwise |
| SC3 | 1 if self-care at level 3; 0 otherwise |
| UA2 | 1 if usual activities at level 2; 0 otherwise |
| UA3 | 1 if usual activities at level 3; 0 otherwise |
| PD2 | 1 if pain/discomfort at level 2; 0 otherwise |
| PD3 | 1 if pain/discomfort at level 3; 0 otherwise |
| AD2 | 1 if anxiety/depression at level 2; 0 otherwise |
| AD3 | 1 if anxiety/depression at level 3; 0 otherwise |
| Model | Method/f(x) |
| OLS | Ordinary least squares: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| ROLS | Robust ordinary least squares: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| RME | Respondent-level mixed effects: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| IME | Interviewer-level mixed effects: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| RCM | Random coefficient model: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| TOB | Tobit model: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| IRM | Interval regression model: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| IRMC | Interval regression model censored at −1: f(MO2,MO3,SC2,SC3,UA2,UA3,PD2,PD3,AD2,AD3) |
| Variable | Category | V3 (n = 1556) | Weighted V3 (n = 1556) | General Population | ||
|---|---|---|---|---|---|---|
| N | % | N | % | % | ||
| MO | No problems | 1288 | 82.8 | 1249 | 80.28 | |
| Some problems | 264 | 16.97 | 302 | 19.42 | N/A | |
| Confined to bed | 4 | 0.26 | 5 | 0.29 | ||
| SC | No problems | 1423 | 91.45 | 1386 | 89.07 | N/A |
| Some problems | 129 | 8.29 | 165 | 10.63 | ||
| Unable | 4 | 0.26 | 5 | 0.29 | ||
| UA | No problems | 1310 | 84.19 | 1262 | 81.08 | |
| Some problems | 236 | 15.17 | 282 | 18.12 | N/A | |
| Unable | 10 | 0.64 | 13 | 0.80 | ||
| PD | No problems | 1136 | 73.01 | 1106 | 71.07 | |
| Some problems | 416 | 26.74 | 445 | 28.58 | N/A | |
| Extreme problems | 4 | 0.26 | 6 | 0.35 | ||
| AD | No problems | 1288 | 82.78 | 1273 | 81.82 | |
| Some problems | 226 | 14.52 | 244 | 15.70 | N/A | |
| Extreme problems | 42 | 2.70 | 39 | 2.48 | ||
| Gender | Female | 1020 | 65.55 | 809 | 51.99 | 52% |
| Residence area | Urban | 1139 | 73.20 | 843 | 54.21 | 55.20% |
| Education level | No formal education | 6 | 0.39 | 11 | 0.72 | 2% |
| Low | 181 | 11.63 | 242 | 15.57 | 36.90% | |
| Medium | 778 | 50.00 | 826 | 53.11 | 45.20% | |
| Tertiary | 583 | 37.47 | 468 | 30.07 | 15.90% | |
| No response | 8 | 0.51 | 8 | 0.53 | ||
| Occupation | Employed | 935 | 60.09 | 829 | 53.28 | 52.10% |
| Unemployed | 33 | 2.12 | 50 | 3.23 | 3.90% | |
| Retired | 386 | 24.81 | 415 | 26.67 | 26.20% | |
| Stay at home/domestic | 107 | 6.88 | 137 | 8.80 | 7.10% | |
| In education | 79 | 5.08 | 98 | 6.30 | 4.80% | |
| No response | 16 | 1.03 | 27 | 1.72 | ||
| Income | Below the average | 664 | 42.67 | 734 | 47.18 | 41.39% |
| Average | 271 | 17.42 | 252 | 16.19 | 30.75% | |
| Above the average | 497 | 31.94 | 427 | 27.43 | 27.86% | |
| No response | 124 | 7.97 | 143 | 9.20 | ||
| Health State | N | Mean | SD | Median | 25th Percentile | 75th Percentile | Negative Values (%) |
|---|---|---|---|---|---|---|---|
| 11112 | 162 | 0.899 | 0.106 | 0.95 | 0.85 | 0.95 | 0.00 |
| 11113 | 162 | 0.721 | 0.281 | 0.8 | 0.65 | 0.9 | 2.47 |
| 11121 | 154 | 0.906 | 0.089 | 0.95 | 0.85 | 0.95 | 0.00 |
| 11122 | 164 | 0.848 | 0.123 | 0.9 | 0.8 | 0.95 | 0.00 |
| 11211 | 155 | 0.941 | 0.061 | 0.95 | 0.9 | 1 | 0.00 |
| 11313 | 155 | 0.578 | 0.306 | 0.65 | 0.45 | 0.8 | 3.87 |
| 12111 | 151 | 0.937 | 0.069 | 0.95 | 0.9 | 1 | 0.00 |
| 12212 | 154 | 0.828 | 0.103 | 0.85 | 0.8 | 0.9 | 0.00 |
| 12222 | 157 | 0.745 | 0.151 | 0.75 | 0.675 | 0.85 | 0.64 |
| 12331 | 149 | 0.344 | 0.325 | 0.4 | 0.2 | 0.55 | 8.05 |
| 13133 | 151 | 0.151 | 0.431 | 0.25 | 0.05 | 0.4 | 23.18 |
| 13221 | 149 | 0.639 | 0.264 | 0.7 | 0.55 | 0.8 | 2.68 |
| 21111 | 152 | 0.942 | 0.058 | 0.95 | 0.9 | 1 | 0.00 |
| 21133 | 162 | 0.346 | 0.355 | 0.4 | 0.2 | 0.55 | 9.88 |
| 21211 | 158 | 0.866 | 0.099 | 0.9 | 0.8 | 0.95 | 0.00 |
| 21323 | 157 | 0.516 | 0.224 | 0.5 | 0.4 | 0.675 | 1.91 |
| 21332 | 152 | 0.329 | 0.338 | 0.35 | 0.2 | 0.5375 | 9.21 |
| 22121 | 149 | 0.800 | 0.117 | 0.8 | 0.75 | 0.9 | 0.00 |
| 22222 | 154 | 0.730 | 0.152 | 0.75 | 0.65 | 0.85 | 0.00 |
| 22233 | 154 | 0.321 | 0.367 | 0.375 | 0.15 | 0.5625 | 11.04 |
| 23112 | 152 | 0.688 | 0.209 | 0.7 | 0.6 | 0.8 | 0.66 |
| 23323 | 154 | 0.227 | 0.388 | 0.3 | 0.1 | 0.5 | 17.53 |
| 31131 | 155 | 0.167 | 0.417 | 0.25 | 0.05 | 0.45 | 20.00 |
| 31223 | 158 | 0.270 | 0.392 | 0.35 | 0.1 | 0.5 | 12.66 |
| 32113 | 164 | 0.369 | 0.346 | 0.4 | 0.25 | 0.6 | 10.98 |
| 32232 | 157 | 0.075 | 0.414 | 0.15 | −0.2 | 0.35 | 28.66 |
| 32322 | 151 | 0.203 | 0.408 | 0.3 | 0.05 | 0.5 | 21.19 |
| 33232 | 164 | −0.047 | 0.451 | 0.1 | −0.4375 | 0.3 | 40.85 |
| 33311 | 158 | 0.143 | 0.479 | 0.25 | 0 | 0.5 | 24.05 |
| 33333 | 154 | −0.510 | 0.421 | −0.6 | −0.9 | −0.175 | 78.57 |
| ROLS | IRM | IRMC | ||||
|---|---|---|---|---|---|---|
| Variable | Coefficient | SE | Coefficient | SE | Coefficient | SE |
| (Constant) | 0.034 * | 0.007 | 0.032 * | 0.005 | 0.032 * | 0.005 |
| MO2 | 0.037 * | 0.007 | 0.038 * | 0.005 | 0.038 * | 0.005 |
| MO3 | 0.304 * | 0.008 | 0.394 * | 0.013 | 0.397 * | 0.013 |
| SC2 | 0.039 * | 0.007 | 0.040 * | 0.006 | 0.040 * | 0.006 |
| SC3 | 0.168 * | 0.008 | 0.206 * | 0.010 | 0.208 * | 0.010 |
| UA2 | 0.037 * | 0.007 | 0.044 * | 0.005 | 0.045 * | 0.005 |
| UA3 | 0.172 * | 0.007 | 0.189 * | 0.011 | 0.190 * | 0.012 |
| PD2 | 0.073 * | 0.007 | 0.072 * | 0.006 | 0.072 * | 0.006 |
| PD3 | 0.326 * | 0.007 | 0.371 * | 0.012 | 0.372 * | 0.012 |
| AD2 | 0.043 * | 0.007 | 0.054 * | 0.006 | 0.054 * | 0.006 |
| AD3 | 0.167 * | 0.007 | 0.206 * | 0.010 | 0.206 * | 0.010 |
| AIC | 7222 | −154 | −21 | |||
| BIC | 7297 | −12 | 121 | |||
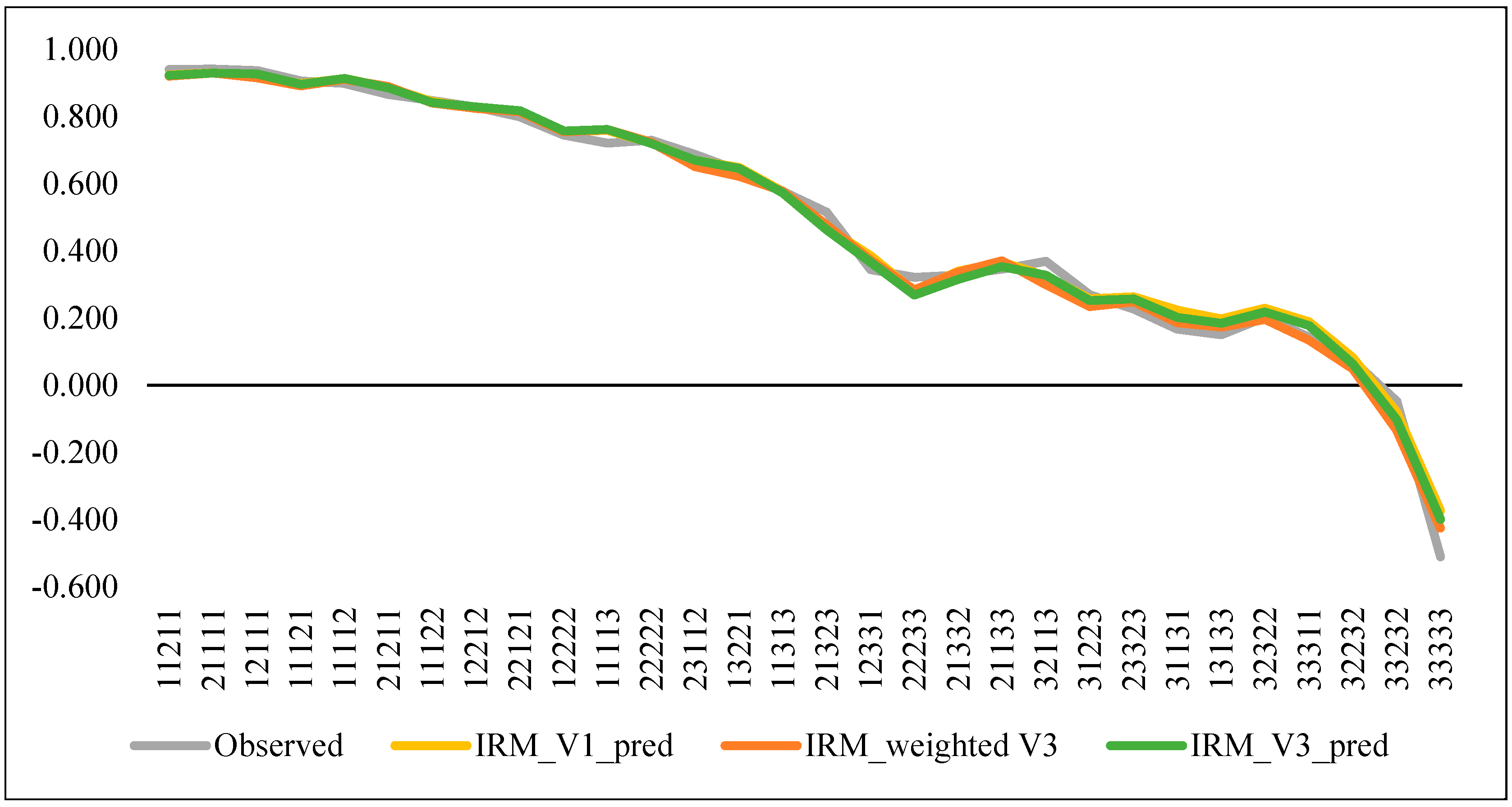
| Spearman’s correlation (predicted vs. observed) | 0.9968 | 0.9954 | 0.9954 | |||
| Pearson’s correlation (predicted vs. observed) | 0.9947 | 0.9959 | 0.9959 | |||
| U(11111) | 1.000 | 1.000 | 1.000 | |||
| U(22222) | 0.737 | 0.720 | 0.719 | |||
| U(33333) | −0.173 | −0.399 | −0.405 | |||
| No. (%) of WTD health states (%) | 7 (2.9%) | 22 (9%) | 22 (9%) | |||
| Mean (SD) | 0.510 (0.232) | 0.430 (0.282) | 0.428 (0.284) | |||
| Ranking of dimensions | PD-MO-UA-SC-AD | MO-PD-SC-AD-UA | MO-PD-SC-AD-UA | |||
| IRM | V3 (n = 1556) | Weighted V3 (n = 1556) | V1 (n = 1649) | |||
|---|---|---|---|---|---|---|
| Variable | Coefficient | SE | Coefficient | SE | Coefficient | SE |
| (Constant) | 0.032 * | 0.005 | 0.039 * | 0.006 | 0.032 * | 0.005 |
| MO2 | 0.038 * | 0.005 | 0.031 * | 0.007 | 0.037 * | 0.006 |
| MO3 | 0.394 * | 0.013 | 0.416 * | 0.019 | 0.389 * | 0.013 |
| SC2 | 0.040 * | 0.006 | 0.046 * | 0.007 | 0.044 * | 0.006 |
| SC3 | 0.206 * | 0.010 | 0.228 * | 0.017 | 0.207 * | 0.010 |
| UA2 | 0.044 * | 0.005 | 0.041 * | 0.006 | 0.043 * | 0.005 |
| UA3 | 0.189 * | 0.011 | 0.183 * | 0.015 | 0.182 * | 0.011 |
| PD2 | 0.072 * | 0.006 | 0.070 * | 0.007 | 0.069 * | 0.006 |
| PD3 | 0.371 * | 0.012 | 0.359 * | 0.016 | 0.355 * | 0.012 |
| AD2 | 0.054 * | 0.006 | 0.051 * | 0.007 | 0.054 * | 0.006 |
| AD3 | 0.206 * | 0.010 | 0.200 * | 0.012 | 0.209 * | 0.010 |
| AIC | −154 | −100 | 62 | |||
| BIC | −12 | 42 | 205 | |||
| Spearman’s correlation (predicted vs. observed) | 0.9954 | 0.9944 | 0.9963 | |||
| Pearson’s correlation (predicted vs. observed) | 0.9959 | 0.9956 | 0.9962 | |||
| U(11111) | 1.000 | 1.000 | 1.000 | |||
| U(22222) | 0.720 | 0.723 | 0.720 | |||
| U(33333) | −0.399 | −0.425 | −0.374 | |||
| No. (%) of WTD health states (%) | 22 (9%) | 22 (9%) | 19 (7.82%) | |||
| Mean (SD) | 0.430 (0.282) | 0.420 (0.289) | 0.438 (0.276) | |||
| Ranking of dimensions | MO-PD-SC-AD-UA | MO-PD-SC-AD-UA | MO-PD-AD-SC-UA | |||
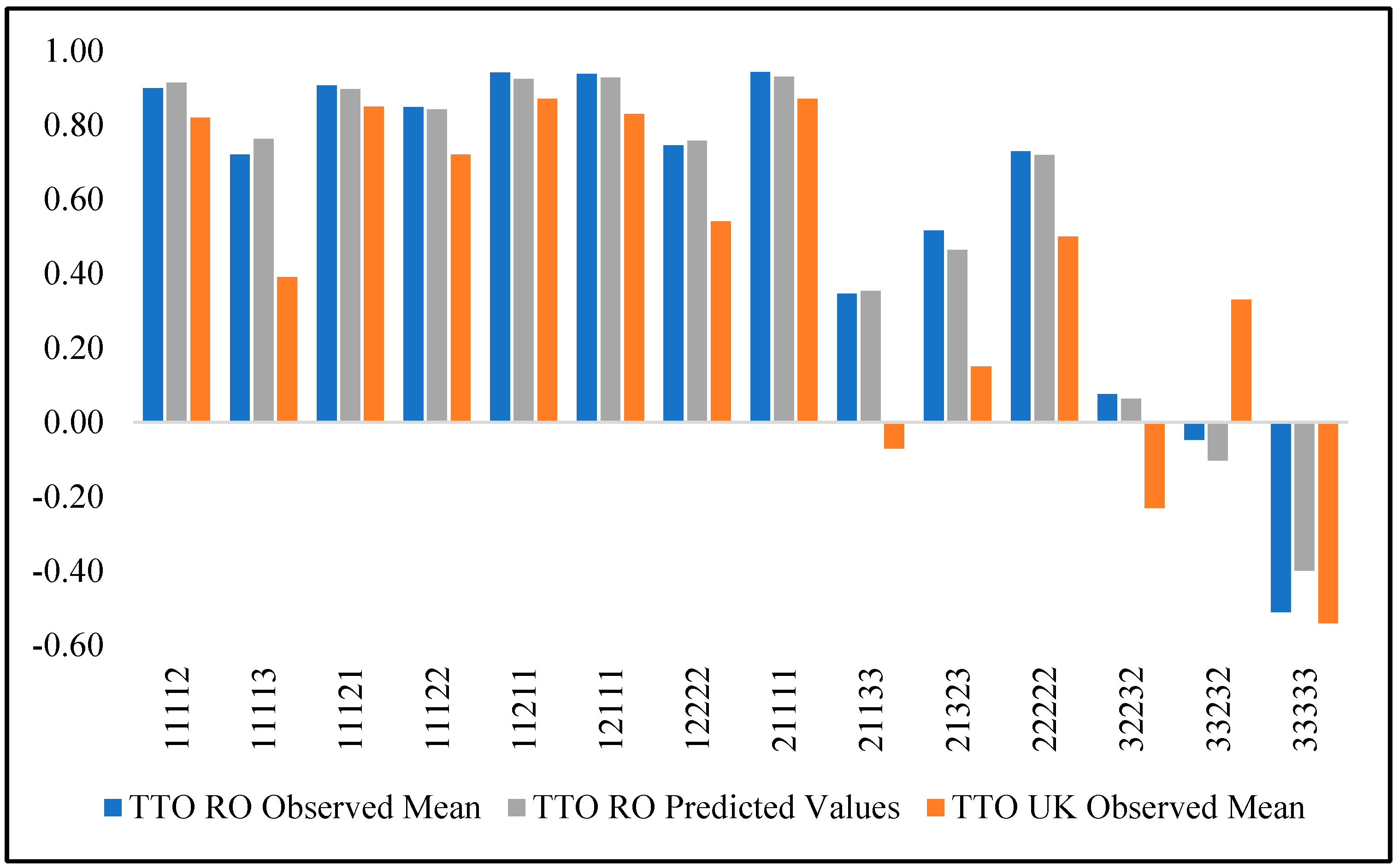
| Health | TTO UK | TTO Romania | Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| State | Observed | SD | Predicted | N | Observed | SD | Predicted | Diff. | Z | p-Value |
| 11112 | 0.82 | 0.29 | 0.85 | 162 | 0.90 | 0.11 | 0.91 | −0.08 | 9.48 | 0.0000 |
| 11113 | 0.39 | 0.56 | 0.41 | 162 | 0.72 | 0.28 | 0.76 | −0.33 | 14.97 | 0.0000 |
| 11121 | 0.85 | 0.25 | 0.80 | 154 | 0.91 | 0.09 | 0.90 | −0.06 | 7.87 | 0.0000 |
| 11122 | 0.72 | 0.37 | 0.73 | 164 | 0.85 | 0.12 | 0.84 | −0.13 | 13.36 | 0.0000 |
| 11211 | 0.87 | 0.23 | 0.88 | 155 | 0.94 | 0.06 | 0.92 | −0.07 | 14.34 | 0.0000 |
| 12111 | 0.83 | 0.30 | 0.82 | 151 | 0.94 | 0.07 | 0.93 | −0.11 | 19.04 | 0.0000 |
| 12222 | 0.54 | 0.47 | 0.59 | 157 | 0.75 | 0.15 | 0.76 | −0.21 | 17.05 | 0.0000 |
| 21111 | 0.87 | 0.24 | 0.85 | 152 | 0.94 | 0.06 | 0.93 | −0.07 | 15.44 | 0.0000 |
| 21133 | −0.07 | 0.59 | −0.04 | 162 | 0.35 | 0.35 | 0.35 | −0.42 | 14.92 | 0.0000 |
| 21323 | 0.15 | 0.59 | 0.13 | 157 | 0.52 | 0.22 | 0.46 | −0.37 | 20.46 | 0.0000 |
| 22222 | 0.50 | 0.47 | 0.52 | 154 | 0.73 | 0.15 | 0.72 | −0.23 | 18.75 | 0.0000 |
| 32232 | −0.23 | 0.57 | −0.26 | 157 | 0.07 | 0.41 | 0.06 | −0.30 | 9.22 | 0.0000 |
| 33232 | 0.33 | 0.51 | −0.37 | 164 | −0.05 | 0.45 | −0.10 | 0.38 | −10.69 | 0.0000 |
| 33333 | −0.54 | 0.41 | −0.59 | 154 | −0.51 | 0.42 | −0.40 | −0.03 | 0.87 | 0.1913 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paveliu, M.S.; Olariu, E.; Caplescu, R.; Oluboyede, Y.; Niculescu-Aron, I.-G.; Ernu, S.; Vale, L. Estimating an EQ-5D-3L Value Set for Romania Using Time Trade-Off. Int. J. Environ. Res. Public Health 2021, 18, 7415. https://doi.org/10.3390/ijerph18147415
Paveliu MS, Olariu E, Caplescu R, Oluboyede Y, Niculescu-Aron I-G, Ernu S, Vale L. Estimating an EQ-5D-3L Value Set for Romania Using Time Trade-Off. International Journal of Environmental Research and Public Health. 2021; 18(14):7415. https://doi.org/10.3390/ijerph18147415
Chicago/Turabian StylePaveliu, Marian Sorin, Elena Olariu, Raluca Caplescu, Yemi Oluboyede, Ileana-Gabriela Niculescu-Aron, Simona Ernu, and Luke Vale. 2021. "Estimating an EQ-5D-3L Value Set for Romania Using Time Trade-Off" International Journal of Environmental Research and Public Health 18, no. 14: 7415. https://doi.org/10.3390/ijerph18147415
APA StylePaveliu, M. S., Olariu, E., Caplescu, R., Oluboyede, Y., Niculescu-Aron, I.-G., Ernu, S., & Vale, L. (2021). Estimating an EQ-5D-3L Value Set for Romania Using Time Trade-Off. International Journal of Environmental Research and Public Health, 18(14), 7415. https://doi.org/10.3390/ijerph18147415






