Assessment of Unstimulated Whole Salivary Tumor Necrosis Factor Alpha (TNF-α) and Cellular Micronuclei Levels in Snuff (Naswar) Users and Non-Users for Early Diagnosis of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Study Design and Participants
2.3. Inclusion and Exclusion Criteria
2.4. Questionnaire
2.5. Unstimulated Whole Saliva (UWS) Collection
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
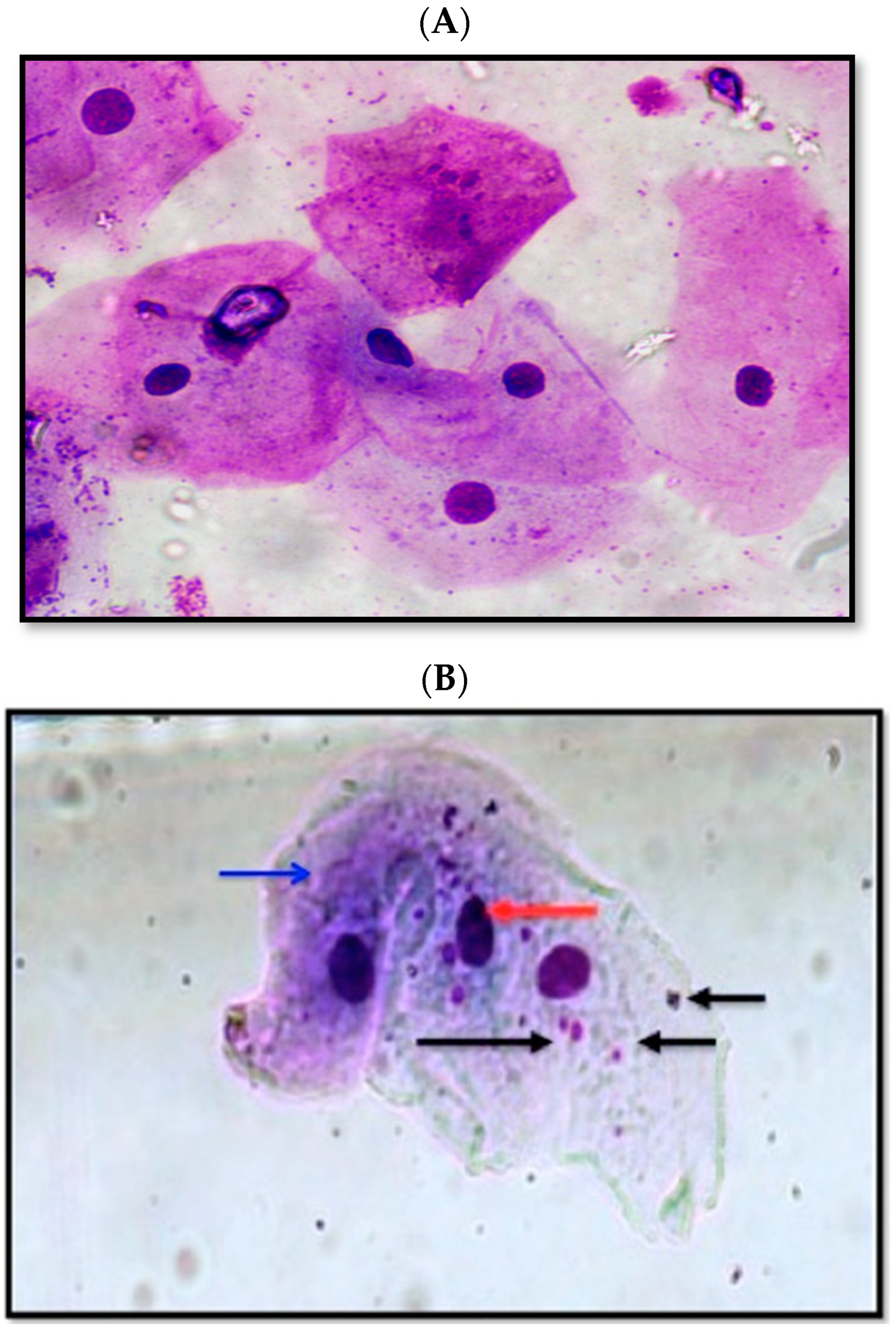
2.7. Cellular Micronuclei Assessment
2.8. Data Analysis
3. Results
3.1. General Characteristics of the Participants
3.2. Assessment of TNF-α and Micronuclei Cells in the Study Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Choi, S.; Myers, J.N. Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J. Dent. Res. 2008, 87, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Yadav, S. Oral squamous cell carcinoma: Etiology, pathogenesis and prognostic value of genomic alterations. Indian J. Cancer 2006, 43, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Sawair, F.A.; Al-Mutwakel, A.; Al-Eryani, K.; Al-Surhy, A.; Maruyama, S.; Cheng, J.; Al-Sharabi, A.; Saku, T. High relative frequency of oral squamous cell carcinoma in Yemen: Qat and tobacco chewing as its aetiological background. Int. J. Environ. Health Res. 2007, 17, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.G.; Chaturvedi, P. Smoking and other addictions related to cancer of the head and neck. Revista Médica Clínica Las Condes 2018, 29, 405–410. [Google Scholar] [CrossRef]
- Awan, K. Oral Cancer: Early Detection is Crucial. J. Int. Oral Health 2014, 6, i–ii. [Google Scholar]
- Saeed, M.; Muhammad, N.; Khan, S.A.; Gul, F.; Khuda, F.; Humayun, M.; Khan, H. Assessment of potential toxicity of a smokeless tobacco product (Naswar) available on the Pakistani market. Tob. Control 2012, 21, 396–401. [Google Scholar]
- Pérez-Ortuño, R.; Martínez-Sánchez, J.M.; Fu, M.; Ballbè, M.; Quirós, N.; Fernández, E.; Pascual, J.A. Assessment of tobacco specific nitrosamines (TSNAs) in oral fluid as biomarkers of cancer risk: A population-based study. Environ. Res. 2016, 151, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Nicotine and smokeless tobacco. CA Cancer J. Clin. 1988, 38, 244–247. [Google Scholar] [CrossRef]
- Fareed, M.; Afzal, M.; Siddique, Y. Micronucleus investigation in buccal mucosal cells among pan masala/gutkha chewers and its relevance for oral cancer. Biol. Med. 2011, 3, 8–15. [Google Scholar]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Zilic-Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Medicina Oral Patología Oral Cirugía Bucal 2012, 17, e10–e15. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lee, J.J.; Lai, T.C.; Lee, C.H.; Hsiao, Y.W.; Chen, P.S.; Liu, W.T.; Hong, C.Y.; Lin, S.K.; Ping Kuo, M.Y.; et al. Suppressive function of low-dose deguelin on the invasion of oral cancer cells by downregulating tumor necrosis factor alpha–induced nuclear factor-kappa B signaling. Head Neck 2016, 38 (Suppl. S1), E524–E534. [Google Scholar] [CrossRef]
- Kashyap, B.; Reddy, P.S. Micronuclei assay of exfoliated oral buccal cells: Means to assess the nuclear abnormalities in different diseases. J. Cancer Res. Ther. 2012, 8, 184–191. [Google Scholar] [CrossRef]
- Evans, H.J. Neoplasia and Cytogenetic Abnormalities. In Aneuploidy; Springer: Boston, MA, USA, 1985; pp. 165–178. [Google Scholar]
- Jenssen, D.; Ramel, C. The micronucleus test as part of a short-term mutagenicity test program for the prediction of carcinogenicity evaluated by 143 agents tested. Mutat. Res. Rev. Genet. Toxicol. 1980, 75, 191–202. [Google Scholar] [CrossRef]
- Stich, H.F.; Rosin, M.P. Micronuclei in exfoliated human cells as a tool for studies in cancer risk and cancer intervention. Cancer Lett. 1984, 22, 241–253. [Google Scholar] [CrossRef]
- Krishnan, R.; Thayalan, D.K.; Padmanaban, R.; Ramadas, R.; Annasamy, R.K.; Anandan, N. Association of serum and salivary tumor necrosis factor-α with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac. J. Cancer Prev. 2014, 15, 7141–7148. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Dreger, S.; Shah, S.M.H.; Pohlabeln, H.; Khan, S.; Ullah, Z.; Rehman, B.; Zeeb, H. Oral cancer via the bargain bin: The risk of oral cancer associated with a smokeless tobacco product (Naswar). PLoS ONE 2017, 12, e0180445. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Suliankatchi, R.A.; Heise, T.L.; Dreger, S. Naswar (smokeless tobacco) use and the risk of oral cancer in Pakistan: A systematic review with meta-analysis. Nicotine Tob. Res. 2019, 21, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pilot, T.; Miyazaki, H. Global results: 15 years of CPITN epidemiology. Int. Dent. J. 1994, 44 (Suppl. 1), 553–560. [Google Scholar]
- Tolbert, P.E.; Shy, C.M.; Allen, J.W. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat. Res. Environ. Mutagenesis Relat. Subj. 1992, 271, 69–77. [Google Scholar] [CrossRef]
- Sarfaraz, A.; Rashid, S.; Irshad, M.; Khattak, M.T.; Shabir, H. Different tumour grading in smokeless tobacco (Naswar) user and non-user in squamous cell carcinoma. Prof. Med. J. 2020, 27, 1054–1058. [Google Scholar]
- Da Silva, V.H.P.; de Luna Antonio, R.; Pompeia, S.; Ribeiro, D.A. Cytogenetic biomonitoring in buccal mucosa cells from young smokers. Acta Cytol. 2015, 59, 474–478. [Google Scholar] [CrossRef]
- Ribeiro, D.A. Cytogenetic biomonitoring in oral mucosa cells following dental X-ray. Dentomaxillofac. Radiol. 2012, 41, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Chandirasekar, R.; Kumar, B.L.; Sasikala, K.; Jayakumar, R.; Suresh, K.; Venkatesan, R.; Jacob, R.; Krishnapriya, E.K.; Kavitha, H.; Ganesh, G.K. Assessment of genotoxic and molecular mechanisms of cancer risk in smoking and smokeless tobacco users. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2014, 767, 21–27. [Google Scholar] [CrossRef]
- Chakrobarty, B.; Roy, J.G.; Majumdar, S.; Uppala, D. Relationship among tobacco habits, human papilloma virus (HPV) infection, p53 polymorphism/mutation and the risk of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2014, 18, 211–216. [Google Scholar]
- Motgi, A.A.; Chavan, M.S.; Diwan, N.N.; Chowdhery, A.; Channe, P.P.; Shete, M.V. Assessment of cytogenic damage in the form of micronuclei in oral epithelial cells in patients using smokeless and smoked form of tobacco and non-tobacco users and its relevance for oral cancer. J. Cancer Res. Ther. 2014, 10, 165. [Google Scholar] [CrossRef]
- Rai, A.; Kumar, A.; Hasan, S.; Saeed, S. Curcumin in oral mucosal lesions: An update. Asian J. Pharm. Clin. Res. 2019, 12, 32–43. [Google Scholar] [CrossRef]
- Pappas, R.S. Toxic elements in tobacco and in cigarette smoke: Inflammation and sensitization. Metallomics 2011, 3, 1181–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, A.; Chakraborty, T.; Mandal, K.; Gure, P.K.; Das, S.; Raychowdhury, R.; Ghosh, A.K.; De, M. Comparative study of exfoliated oral mucosal cell micronuclei frequency in normal, precancerous and malignant epithelium. Int. J. Hum. Genet. 2004, 4, 257–260. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dhar, S.; Sengupta, B.; Ghosh, A.; De, M.; Roy, S.; Raychowdhury, R.; Chakrabarti, S. Cytogenetic monitoring in human oral cancers and other oral pathology: The micronucleus test in exfoliated buccal cells. Toxicol. Mech. Methods 2009, 19, 427–433. [Google Scholar] [CrossRef]
- Amin, S.; Patel, N.; Chattoo, B. Oral cancers–Micronuclei as biomarker of genotoxicity a population-based study to establish usable dynamic cut off limits in tobacco users. Int. J. Mol. Immuno Oncol. 2019, 4, 9–12. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-α) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260. [Google Scholar] [CrossRef]
- Orta, T.; Günebakan, S. The effect of aging on micronuclei frequency and proliferation in human peripheral blood lymphocytes. Indian J. Hum. Genet. 2012, 18, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan-Mattos, J.; Anibal, F.; Perseguini, N.; Minatel, V.; Rehder-Santos, P.; Castro, C.; Vasilceac, F.A.; Mattiello, S.M.; Faccioli, L.H.; Catai, A.M. Effects of natural aging and gender on pro-inflammatory markers. Braz. J. Med. Biol. Res. 2019, 52, e8392. [Google Scholar] [CrossRef] [Green Version]
- De Gonzalo-Calvo, D.; Neitzert, K.; Fernández, M.; Vega-Naredo, I.; Caballero, B.; García-Macía, M.; Suárez, F.M.; Rodríguez-Colunga, M.J.; Solano, J.J.; Coto-Montes, A. Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic. Biol. Med. 2010, 49, 733–737. [Google Scholar] [CrossRef]
- Han, P.; Ivanovski, S. Effect of Saliva Collection Methods on the Detection of Periodontium-Related Genetic and Epigenetic Biomarkers—A Pilot Study. Int. J. Mol. Sci. 2019, 20, 4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justino, A.B.; Teixeira, R.R.; Peixoto, L.G.; Jaramillo, O.L.B.; Espindola, F.S. Effect of saliva collection methods and oral hygiene on salivary biomarkers. Scand. J. Clin. Lab. Investig. 2017, 77, 415–422. [Google Scholar] [CrossRef] [PubMed]
| Feature | Mean (SD)/(Frequency) |
|---|---|
| Age (years) | 38.85 (±11.5) |
| Duration of snuff use (years) | 20.43 (±12.7) |
| Site of dipping (n = 30) * | |
| Snuff use in lower vestibule | 19 (63.3%) |
| Snuff use in upper vestibule | 11 (36.6%) |
| Oral hygiene (n = 60) $ | |
| Good | 12 (20%) |
| Fair | 40 (66.6%) |
| Poor | 8 (13.3%) |
| Snuff Users | Non-Snuff Users | p-Value | |
|---|---|---|---|
| Age (years) | 41.9 (±12.2) | 35.8 (±10.4) | 0.84 § |
| Duration of snuff (years) | 20.43 (±12.79) | - | - |
| TNF-α (pg/mL) | 9.6 (±3.3) | 5.2 (±3.0) | 0.01 § |
| No of micronuclei (per 1000 cells counted) | 30.7 (±7.8) | 9.2 (±3.3) | 0.008 § |
| Age | TNF-α Levels | ||
|---|---|---|---|
| Age | Pearson correlation | 1 | 0.099 * |
| Sig. (2-tailed) | 0.454 | ||
| TNF-α levels | Pearson correlation | 0.099 * | |
| Sig. (2-tailed) | 0.454 | ||
| Age | Micronuclei Score | ||
|---|---|---|---|
| Age | Pearson correlation | 1 | 0.215 * |
| Sig. (2-tailed) | 0.099 | ||
| Micronuclei score | Pearson correlation | 0.215 * | |
| Sig. (2-tailed) | 0.099 | ||
| Duration of Snuff Use | TNF-α Levels | ||
|---|---|---|---|
| Duration of snuff use | Pearson correlation | 1 | 0.048 * |
| Sig. (2-tailed) | 0.801 | ||
| TNF-α levels | Pearson correlation | 0.048 * | |
| Sig. (2-tailed) | 0.801 | ||
| Duration of Snuff Use | Micronuclei Score | ||
|---|---|---|---|
| Duration of snuff use | Pearson correlation | 1 | 0.007 * |
| Sig. (2-tailed) | 0.973 | ||
| Micronuclei score | Pearson correlation | 0.007 * | |
| Sig. (2-tailed) | 0.973 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, W.; Khan, M.M.; Zafar, S.; Alqutub, M.N.; AlMubarak, A.M.; Mokeem, S.; Khan, Z.A.; Usman, M.K.; Ahmed, N.; Aldahiyan, N.; et al. Assessment of Unstimulated Whole Salivary Tumor Necrosis Factor Alpha (TNF-α) and Cellular Micronuclei Levels in Snuff (Naswar) Users and Non-Users for Early Diagnosis of Oral Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2021, 18, 7230. https://doi.org/10.3390/ijerph18147230
Muhammad W, Khan MM, Zafar S, Alqutub MN, AlMubarak AM, Mokeem S, Khan ZA, Usman MK, Ahmed N, Aldahiyan N, et al. Assessment of Unstimulated Whole Salivary Tumor Necrosis Factor Alpha (TNF-α) and Cellular Micronuclei Levels in Snuff (Naswar) Users and Non-Users for Early Diagnosis of Oral Squamous Cell Carcinoma. International Journal of Environmental Research and Public Health. 2021; 18(14):7230. https://doi.org/10.3390/ijerph18147230
Chicago/Turabian StyleMuhammad, Waqar, Muhammad M. Khan, Shafaq Zafar, Montaser N. Alqutub, Abdulrahman M. AlMubarak, Sameer Mokeem, Zafar A. Khan, Muhammad K. Usman, Naseer Ahmed, Nada Aldahiyan, and et al. 2021. "Assessment of Unstimulated Whole Salivary Tumor Necrosis Factor Alpha (TNF-α) and Cellular Micronuclei Levels in Snuff (Naswar) Users and Non-Users for Early Diagnosis of Oral Squamous Cell Carcinoma" International Journal of Environmental Research and Public Health 18, no. 14: 7230. https://doi.org/10.3390/ijerph18147230
APA StyleMuhammad, W., Khan, M. M., Zafar, S., Alqutub, M. N., AlMubarak, A. M., Mokeem, S., Khan, Z. A., Usman, M. K., Ahmed, N., Aldahiyan, N., Vohra, F., & Abduljabbar, T. (2021). Assessment of Unstimulated Whole Salivary Tumor Necrosis Factor Alpha (TNF-α) and Cellular Micronuclei Levels in Snuff (Naswar) Users and Non-Users for Early Diagnosis of Oral Squamous Cell Carcinoma. International Journal of Environmental Research and Public Health, 18(14), 7230. https://doi.org/10.3390/ijerph18147230







