Abstract
The objective was to assess the prevalence of anemia and nutritional status of mothers and children under five years among Syrian refugees in Lebanon and to identify nutritional deficiencies among pregnant, lactating, and non-pregnant non-lactating (NPNLM) mothers. A cross-sectional study was conducted among Syrian refugee mothers with children under five years in Greater Beirut, Lebanon (n = 433). Data on socio-economic status, maternal health, lifestyle characteristics, dietary intake, anthropometric measurements, and hemoglobin concentrations were collected. The prevalence of anemia was 21.7% among mothers and 30.5% among children. NPNLM with overweight/obesity and an at-risk waist circumference (WC) had 14.7-times and 10.9-times higher odds of anemia than mothers with normal WC and weight. Children of anemic mothers had 2.7-times and 4.4-times higher odds of total and mild anemia than those of non-anemic. Higher odds of mild anemia were found among children of lactating mothers than of NPNLM. A high percent energy intake of total fat and sugar was found among all mothers. Nutritional inadequacy was identified in higher proportions of lactating and pregnant mothers than NPNLM. Our findings highlighted the co-existence of overnutrition and anemia among Syrian refugee mothers and undernutrition among children from the same household. Culture-specific interventions are needed to support maternal nutrition, to ensure the health and wellbeing of their offspring.
1. Introduction
The burden of malnutrition remains a worldwide challenge [1]. Globally, nearly two billion adults are estimated to be overweight or obese and more than 140 million children to be stunted [1,2]. According to the World Health Organization (WHO), over half a billion women of reproductive age and 269 million children under five years suffer from anemia [3]. The coexistence of undernutrition (wasting, underweight, stunting, and micronutrient deficiencies), overnutrition (overweight and obesity), and non-communicable diseases within populations, households, and even individuals characterizes the double burden of malnutrition, mostly affecting low- and middle-income countries [4]. Poor economic development and rapid nutrition transition are exposing a growing proportion of the population to unhealthy environmental stressors and health consequences in low- and middle-income countries. Malnutrition in early life has long-lasting effects, such as chronic inflammation, dysbiosis, obesity, non-communicable diseases, stunting, and birth complications [5].
Maternal nutritional status at the time of conception and during pregnancy as well as early nutrition during the first two years of life determine to a great extent the health of the offspring, influencing more than a generation through intergenerational cycles [6,7,8]. Optimum maternal and child nutrition during the first 1000 days of life is crucial for brain development and for the prevention of chronic diseases and obesity at later stages of life [7,9,10]. Maternal malnutrition and micronutrient deficiencies may lead to permanent consequences on nutritional programming that can influence children’s growth, development, and survival [6,8,10]. Inadequate quantity and quality of nutrients in the maternal diet can expose women and children to various forms of malnutrition and to increased vulnerability to infections and deficiencies, especially anemia [5,7,10]. Serious health consequences may affect pregnant women and their offspring as a result of anemia, including prematurity, low birth weight, poor health and development, and reduced work productivity [11,12,13].
In the Middle East and North Africa region, food security is deteriorating rapidly, primarily driven by conflicts and political instability [14]. Poverty and food insecurity lead to both undernutrition and overnutrition concomitantly [15]. The Eastern Mediterranean Region has been witnessing rapid changes in food consumption habits coupled with a high burden of micronutrient deficiencies, increasing obesity rates, and a persistent burden of undernutrition. Women and girls are particularly prone to various forms of malnutrition in this region [16,17,18].
Syria has witnessed the longest and most intense conflict of the Arab Awakening resulting in the largest refugee crisis globally, exceeding 6.7 million by the end of 2018. It is estimated that more than 1.5 million Syrian displaced people live in Lebanon with nearly one million who were registered as refugees with the UN High Commission on Refugees (UNHCR) by November 2018, of whom more than half are women and children [19,20]. Lebanon continues to host the highest per capita concentration of refugees worldwide; one out of four people in Lebanon is a displaced person from Syria [21,22]. The conflict in Syria has exacerbated pre-existing development constraints and political instability in Lebanon, in response Syrian refugees have become even more vulnerable. Despite extensive humanitarian assistance, UN reports showed that more than half of Syrian refugee households were still unable to meet survival needs of food, health, and shelter; and one-third remained moderately to severely food insecure in 2018 [23].
Even though numerous assessments were conducted by international and national non-governmental organizations (NGOs) on Syrian refugees in Lebanon, there are limited studies measuring the prevalence of anemia and examining the nutritional status of refugees following a humanitarian crisis. Findings were relevant to the undernutrition and anemia status of children and women among Syrian refugees in Lebanon, yet overnutrition and nutritional inadequacies were overlooked [24,25]. Therefore, the objectives of this study were to (1) assess the prevalence of anemia and the nutritional status of Syrian refugee mothers and children under five years, (2) examine the dietary intake of pregnant, lactating, and non-pregnant non-lactating mothers of children below five years of age, and (3) examine associations between anemia, nutritional status, and socio-economic characteristics among Syrian refugee mothers living in Greater Beirut, Lebanon.
2. Materials and Methods
2.1. Study Design and Sampling Method
A cross-sectional survey was conducted among mothers and one of their children below five years in the Greater Beirut area in Lebanon. Mother–child dyads were recruited through the Primary Health Care Centers (PHCC), which are part of the National Primary Health Care (PHC) Network overseen by the Ministry of Public Health (MoPH), in 6 vulnerable localities of Greater Beirut, between July and September 2018. A two-step purposeful sampling was used to select recruitment sites located in the most vulnerable areas of Greater Beirut. The catchment area of PHCC located in Greater Beirut encompasses the urban agglomeration of the capital city of Beirut and adjacent districts of Mount Lebanon Governorate. Greater Beirut is the melting pot of the country as its inhabitants consist of more than half of the Lebanese population and over 305,000 refugees [22,26]. The districts were selected according to the highest vulnerability level of localities [27]. The survey areas included in this project are as follows: Baouchriyeh, Bourj Barajneh, Bourj Hammoud, Chiyah, Mazraa, and Mousaytbeh. The identification of PHCC and access to recruitment sites were carried out with the support of the Primary Health Care Department of the MoPH in Lebanon. Selected PHCC from these areas were approached with the approval letter from the MoPH.
Previous estimates of the anemia prevalence among Syrian refugee women (26.1%) and children under five in Lebanon (21.0%) were used for the calculations of the sample size [25]. Based on the highest prevalence found among women, a required sample of 296 mothers was estimated at a 95% confidence interval (95% CI) with a 5% margin of error. The sample size was adjusted with an estimated design effect of 1.5 for the sampling design (N = 444). Considering an estimated non-response rate of 15% and a dropout rate of 10%, a total of 555 mother–child dyads were targeted for the survey. Inclusion criteria for women of reproductive age to participate in this study were: (1) to hold the Syrian nationality, (2) to be between 15 and 49 years old, (3) to have a child aged between 0 and 59 months, (4) for the child to hold the Syrian nationality, and (5) for the child not to suffer from any inborn errors of metabolism or physical malformations. A convenience sample was used to recruit all eligible mothers at the selected centers. When multiple children under five years were eligible within one family, one child was selected randomly. Figure 1 represents the flowchart of the subjects’ recruitment into the survey depicting a non-response rate of 17.1% and a dropout rate of 11.4%. Of the completed interviews, all Syrian pregnant, lactating, and non-pregnant non-lactating mothers with one child below the age of five years were selected for this study (N = 433).
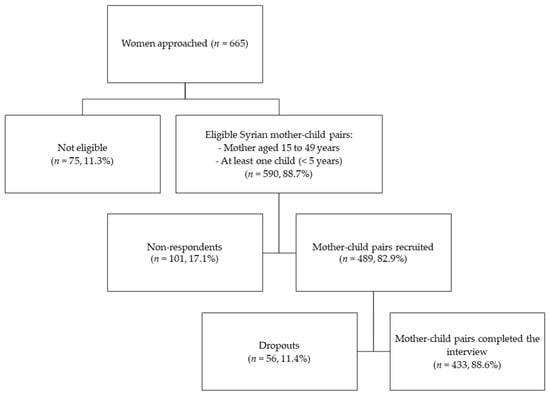
Figure 1.
Flowchart of the recruitment subjects in the survey.
2.2. Recruitment Strategy
The recruitment strategy included identifying mothers of children under five years through three approaches: (1) via the assistance of nurses at PHCC, (2) by direct approach from the research assistant in the waiting rooms, or (3) by posting flyers with a short description of the survey in the PHCC premises. The first approach occurred through the nurse or staff who has an established relationship with the mother. The second strategy was deemed necessary given that nurses could be overwhelmed with responsibilities and may not be able to approach and invite mothers to the survey on a regular basis. The third strategy allowed participants to reach out, if interested. Using a developed oral script, nurses and research assistants briefed the potential participant on the content of the survey, checked for the mother and child’s eligibility criteria, and obtained the mother’s informed written consent.
2.3. Data Collection
Face-to-face interviews took place in a private setting at the PHCC premises by Collaborative Institutional Training Initiative (CITI) certified and trained research assistants using a culture-specific multi-component questionnaire. Research assistants underwent an intensive and vigorous one-week training prior to the initiation of field work. This was necessary to obtain a nonjudgmental and neutral attitude, decrease potential social desirability bias, and minimize intra-observer measurement errors. Follow-up training sessions were conducted on a regular basis to maintain the quality of measurements among all enumerators. Data quality control, as well as random cross-checks during data entry, were conducted on questionnaires on a continuous basis, to reduce the risk of reporting bias and to increase the accuracy of the data. Information was collected on characteristics of the household, socio-economic status, maternal lifestyle, and health status. Household monthly income was classified as either being ≤750,000 Lebanese Pounds (LBP) or >750,000 LBP, which was the equivalent of 500 US dollars (USD) at the time of data collection. This classification was based on the legal minimum wage in Lebanon, approximately 675,000 LBP (equivalent to 450 USD in 2018) [27]. The crowding index was used as a proxy measure for the socio-economic status. It was computed as the total number of co-residents per household divided by the number of rooms excluding kitchens, bathrooms, hallways, balconies, and garage according to the American Crowding Index definition [28]. The International Physical Activity Questionnaire Short Form (IPAQ-SF) was used to measure physical activity levels [29].
2.4. Anthropometric Assessment
Anthropometric measurements were taken by trained research assistants using standardized protocols [30,31] and calibrated equipment. An average of two measurements was recorded to the nearest decimal. Height and weight were measured using a portable mechanical stadiometer (SECA 213) and electronic 2-in-1 weighing scale (SECA 876) with light clothing and bare feet or stockings. Measuring mats (SECA 417) were used to measure the length for children under two years. Mid-upper arm, waist, and hip circumferences were measured for mothers with light clothing using a non-elastic measuring tape (SECA 201). For all mothers, including pregnant mothers, the mid-upper arm circumference (MUAC) score was defined as: <23.0 cm (underweight), 23.0–27.9 cm (normal weight), 28.0–30.9 cm (overweight), and ≥31.0 cm (obese) [32,33,34,35]. Pre-pregnancy weight was not collected from pregnant mothers. For non-pregnant mothers, the nutritional status was evaluated using three indicators: body mass index (BMI), waist circumference (WC), and waist–hip ratio (WHR) as per the WHO classifications. BMI was calculated as the ratio of weight (kg) to height squared (m2). Nutritional status was defined as: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obese (BMI ≥ 30.0 kg/m2) [36]. WHR was computed as the ratio of waist circumference by hip circumference. The cut-off points of WC > 80 cm and WHR ≥ 0.85 cm were used to identify “at-risk WC” and “substantially increased risk WHR”, respectively [37]. WC and WHR were used as an indication of increased risk of metabolic complications and abdominal obesity among mothers. For children, the nutritional status was defined using the WHO child growth standards. Length/Height-for-age Z-scores (HAZ) were used to classify children as stunted (HAZ < −2) and not stunted (HAZ ≥ −2). Weight-for-age Z-scores (WAZ) were used to classify children as underweight (WAZ < −2) and not underweight (WAZ ≥ −2). Weight-for-length/height Z-scores (WHZ) were used to classify children as wasted (WHZ < −2) and not wasted (WHZ ≥ −2). BMI-for-age Z-scores (BAZ) were used to classify children as wasted (BAZ < −2), normal weight (−2 ≤ BAZ ≤ 2), and overweight or obese (BAZ > +2) [38]. The WHO Anthro Survey Analyzer was used to derive the z-scores [39].
2.5. Biochemical Assessment
Members of the research team underwent training on proper micro-technique blood collection for pediatrics and adults in order to measure hemoglobin concentrations (Hb) using the “HemoCue Hb301 System”. Control solutions were routinely used to ensure the accuracy of the measurement. Trained research team members conducted a finger prick on mothers and children (6–59 months) and a heel prick on infants (0–5 months) to collect a small drop of blood. The WHO cut-offs at sea level for determining total anemia were used for lactating and non-pregnant non-lactating mothers as Hb < 12.0 g/dL, and for pregnant mothers and children aged 6 to 59 months as Hb < 11.0 g/dL. Anemia was further classified as mild, moderate, and severe for non-pregnant mothers (11.0–11.9 g/dL, 8.0–10.9 g/dL, and <8.0 g/dL, respectively) and for pregnant mothers and children (6–59 months) (10.0–10.9 g/dL, 7.0–9.9 g/dL, and <7.0 g/dL, respectively) [40]. For infants aged zero to five months, total anemia was defined as Hb < 10.5g/dL, according to Marques et al. (2014) [41] and given the lack of WHO criteria for classifying the severity of anemia for this age group [40].
2.6. Dietary Assessment
Trained nutritionists within the research team collected data on dietary intake and daily meal patterns. The 2D food portion visuals [42] and standardized reference portions were used to facilitate and standardize the collection of the dietary data. The dietary intake of the mother was measured using the quantitative multiple-pass 24-h dietary recall method. The five-step multiple-pass method starts with the quick uninterrupted listing of foods by the interviewee, proceeds to probing for forgotten foods list and collecting the time and occasion, then a comprehensive description of foods and amounts eaten is gathered in the detailed cycle, and ends with a final probe review [43,44]. Dietary data from the 24-h recalls were analyzed with the NutriSurvey 2007 using the United States Department of Agriculture (USDA) database (SR 28, version: May 2016) [45]. Local single food items were added to the database from local food composition tables [46]. Standardized recipes were used to analyze composite and traditional dishes.
Dietary intake of the mothers was compared to the Dietary Reference Intakes (DRIs) for energy and nutrients, as recommended by the Institute of Medicine. DRIs refer to the Recommended Dietary Allowances (RDAs), Adequate Intakes (AIs), and Acceptable Macronutrient Distribution Ranges (AMDRs) for energy, macro- and micronutrients [47,48]. Intakes of monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), saturated fatty acids (SFA), and trans-fatty acids (TFA) were analyzed based on the FAO recommendations [49]. Cholesterol intake was assessed based on the criteria established by the National Institutes of Health [50]. The reference intake of total sugar was based on the WHO recommendation [51]. Appendix A displays the DRIs for macro- and micronutrients (Table A1) according to their reproductive status. Macronutrients’ intakes were expressed as percent total energy intake (%EI). In addition, the average energy and key macro- and micronutrient intakes were analyzed for mothers, according to their reproductive status. The nutritional inadequacy represents the proportion of mothers not meeting 2/3rd of the RDA or AI for key macro- and micronutrient according to their age group and reproductive status.
2.7. Statistical Analysis
Data were entered using KOBO Technology provided by Harvard Humanitarian Institute [52]. Data analysis was carried out using the Statistical Package for Social Sciences, version 27.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were performed for continuous and categorical variables. The number of subjects and percentages (n, %) represented nominal variables, whereas mean and standard deviation (mean ± SD) were used to represent continuous variables. The reproductive status of the mother was defined as pregnant mothers (PM), non-pregnant lactating mothers (LM), and non-pregnant non-lactating (NPNLM) mothers. Associations were investigated between the reproductive status of the mother and socio-economic and maternal health and lifestyle characteristics, nutritional status of mother and children as well as daily meal patterns of mothers using chi-square analysis for categorical variables and one-way ANOVA test to compare means across groups. Simple and multiple multinomial logistic regressions were used to examine associations of the mother’s reproductive status (dependent variable) with socio-economic and maternal health characteristics, anemia and nutritional status of mothers and children. Logistic regressions were also used to examine the association between maternal anemia (dependent variable) and the nutritional status of mothers and children. Results from the logistic regressions were expressed as OR for crude odds ratios and aOR for adjusted odds ratios with a 95% confidence interval. The significance of each regression model was evaluated using R-squared, the overall percentage, and Hosmer and Lemeshow test. Multicollinearity was measured between all the independent variables within regression models using correlation coefficients, tolerance, variance inflation factors (VIF), and the condition index. Statistical significance was defined as p-value < 0.05. Associations close to statistical significance were also reported to improve the interpretation of results [53,54].
3. Results
Nearly half of the study sample consisted of LM (47.1%) followed by NPNLM (35.1%) and PM (17.8%). The mean age of mothers was 27.4 years (±5.9) and of children 16.8 months (±14.3). On average, NPNLM were significantly older and LM had the youngest children. The majority of mothers in the study sample were married (98.8%), housewives with no paid job (97.2%) and nearly half of the fathers had no job or a part-time job (48.7%). More than half of the parents had completed primary or intermediate school (mothers: 58.1%; fathers: 68.4%), while 16% were found to be illiterate. Two-thirds of the households (65.5%) had a monthly income below or equal to 750,000 LBP, approximately equal to the legal minimum wage in Lebanon. The majority of the mothers were registered as refugees with the UNHCR (82.1%), of which only 8.9% received food assistance (e-vouchers) from the World Food Programme (WFP). One mother reported receiving food assistance from another source while she was not registered with the UNHCR. Overall, 13.5% of all mothers reported receiving cash or food assistance from the UNHCR or other sources. About half of the respondents lived in nuclear and extended families (52.7% and 47.3%, respectively). A high proportion of households had the father or family-in-law as head of the family (96.2%); in contrast, only 3.8% were headed by the mother or both parents. A mean crowding index score of 3.7 (±1.6) and a mean of three children under five years per household (2.7 ± 1.5) were recorded in our study sample. A significantly higher proportion of PM had one to two children under five years old as compared to LM and NPNLM (Table 1).

Table 1.
Socio-economic and household characteristics by the reproductive status of Syrian refugee mothers.
Healthcare service utilization and maternal health characteristics are shown in Table 2. Most mothers were uninsured (93.7%) and about two-thirds of them usually visited public healthcare facilities (69.4%). Overall, 62.1% of the mothers had at least four antenatal care (ANC) visits while 16.6% of the mothers had zero ANC visits during their pregnancy with the child participating in the study. However, PM had the highest proportion of zero ANC visits than LM and NPNLM (21.1%, 16.1%, 15.1%, respectively). A small proportion of the mothers received health and nutrition messages from healthcare professionals exclusively (13.9%) or multiple sources (22.9%), while the majority relied mainly on family, friends, and/or a media platform (63.2%).

Table 2.
Healthcare service utilization and health characteristics by the reproductive status of Syrian refugee mothers.
In addition, self-reported previous diagnosis of anemia was highest among PM, who also suffered from higher incidences of flu as compared to other mothers (p < 0.05). Thirty percent of the mothers reported using nutritional supplements, of which the majority were PM (62.3%) followed by LM (27.1%) and NPNLM (17.8%). The most consumed supplements were iron and iron-folic acid (65.9%). Vitamin D was significantly more consumed by NPNLM as compared to LM and PM (p < 0.001). The reported compliance rate to the use of micronutrient supplements was overall high (80.2%), with the highest being among PM (89.3%).
As for daily meal patterns (Table 3), a significantly lower proportion of NPNLM had a daily breakfast as compared to PM and LM. On the other hand, LM consumed on average a significantly higher number of main meals per day as compared to the other groups of mothers. Coffee and tea consumption were higher during a meal versus between meals among mothers. It was also seen that more LM consumed coffee and tea during a meal compared to the other mothers. A significantly higher proportion of LM had a low-intensity physical activity level, while NPNLM had more high-intensity activity level and higher rates of smoking compared to LM and PM.

Table 3.
Maternal lifestyle characteristics by the reproductive status of Syrian refugee mothers.
The prevalence of anemia among mothers was 21.7%, with 15.4% being mild and 6.3% moderate cases (Table 4). Children aged zero to 59 months showed a higher prevalence of anemia (30.5%), with 24.2% being mild and 9.8% moderate cases. Severe anemia was not detected among both mothers and children. The highest prevalence of total anemia among mothers was observed with NPNLM (26.5%), followed by LM (19.4%) and PM (18.2%). Mild anemia was found the highest among NPNLM (18.5%) and moderate anemia among PM (10.4%). In contrast, the highest prevalence of anemia among children aged zero to 59 months was observed in households of LM (35.6%), followed by NPNLM (27.8%) and PM (22.1%). In addition, mild and moderate anemia among children aged 6 to 59 months were highest among children of LM (37.5% and 12.5%, respectively) as compared to children of PM and NPNLM. A significant association between the reproductive status and the classification of anemia among mothers and children was found, but not with total anemia. As for the nutritional status, overweight and obesity rates were found to be high according to the BMI of non-pregnant mothers (31.2% and 29.5%, respectively) and the MUAC classification among all mothers (26.9% and 34.6%, respectively). In addition, nearly two out of three non-pregnant mothers were found to have an at-risk waist circumference (65.2%). Yet, 9.0% of their children were found to be stunted (HAZ < −2), 4.4% underweight (WAZ < −2), 5.1% wasted (WHZ < −2), and 4.4% overweight or obese (BAZ > +2).

Table 4.
Anemia and nutritional status of Syrian refugee mothers and their children by the reproductive status.
Further analysis showed that moderate- and high-intensity activity levels of mothers were significantly associated with the age of the mother and child and having more children under the age of five years (p < 0.05). No association was found between overweight and obesity and their physical activity levels (data not shown).
Multinomial logistic regression analysis (Table 5) showed that PM were more likely to be younger compared to NPNLM, while LM were more likely to have younger children. Higher odds of self-reported anemia and suffering from the flu were found among PM as compared to NPNLM. PM and LM had 12-times and 2-times higher odds of using micronutrient supplements than NPNLM (p < 0.05). PM and LM also had higher odds of consuming a daily breakfast and lower odds of smoking were found when compared to NPNLM. Regression analysis showed that the risk of mild anemia was significantly lower among PM (aOR = 0.26, 95% CI: 0.08–0.82); in contrast, the risk of moderate anemia was greater among PM even though it did not reach statistical significance. As for children, the odds of mild anemia were three-times higher among children of LM in comparison to those of NPNLM (p < 0.05), while the odds of being wasted (BAZ z-score < −2) were higher for children of PM as compared to those of NPNLM (aOR = 5.90, 95% CI: 1.02–34.18). The odds of total anemia among children (zero to 59 months) were two-times higher among children of LM than those of NPNLM, only after adjusting for confounders.

Table 5.
Odds ratio of anemia, nutritional status, and age by the reproductive status of mothers.
Table 6 displays the descriptive characteristics of Syrian refugee mothers by anemia status and their associations with maternal anemia (anemic vs. not anemic) using simple and multiple logistic regressions. Significant associations were found between anemia and the crowding index, WFP food assistance (e-vouchers), and the total number of children using the simple logistic regressions. However, these associations lost their statistical significance after adjusting for confounders. As for the health characteristics, higher odds of self-reported previous diagnosis of anemia were found among anemic mothers as compared to non-anemic mothers (aOR = 2.27, 95% CI: 1.14–4.52). Receiving health and nutrition messages from family, friends, and/or media or from multiple sources was significantly associated with lower odds of anemia. Simple and multiple logistic regressions showed that the odds of total anemia among children aged zero to 59 months increased by nearly 3-folds (aOR = 2.67, 95% CI: 1.42–5.02) among anemic mothers as compared to non-anemic. Mild anemia among children was associated with anemia among mothers only after adjusting for confounders. As for the nutritional status of mothers, non-pregnant mothers with an at-risk waist circumference were 3-times more likely to be anemic (aOR = 3.05, 95% CI: 1.34–6.92) as compared to not anemic non-pregnant mothers. Remarkably, the odds of an at-risk WC and of being overweight or obese were 11-times and 15-times higher among anemic NPNLM compared to non-anemic NPNLM, respectively (p < 0.01). The odds of being underweight were 13-times higher among anemic NPNLM as compared to those with normal weight, only after adjusting for socio-economic characteristics. The additional analysis did not find any associations between the consumption of coffee and tea and anemia status among mothers.

Table 6.
Key characteristics and nutritional status indicators according to total anemia among Syrian refugee mothers.
Table 7 presents the dietary intake and nutritional inadequacy (<2/3rd of DRIs) of macro- and micronutrients of PM, LM, and NPNLM among Syrian refugees from vulnerable areas of Greater Beirut. The percent energy intake (%EI) of carbohydrates, protein, linoleic and linolenic acid, saturated and trans-fatty acids, and dietary intake of cholesterol were within the AMDRs, even though, on average, all mothers did not meet their energy intake requirement. On the other hand, the %EI of total fat, polyunsaturated fatty acids, and total sugar exceeded on average the recommendations for women. Significant differences in dietary intake and nutritional inadequacy were observed among mothers according to their reproductive status. Despite a significantly higher intake of carbohydrate (g/d) among PM and LM, a higher proportion of PM and LM (20.8% and 29.4%) did not meet 2/3rd of the RDA for carbohydrate intake as compared to NPNLM (13.8%). Similarly, a significantly larger proportion of PM and LM fell below 2/3rd of the RDA for protein than NPNLM (59.7%, 59.3%, and 38.2%, respectively; p < 0.05). On the other hand, LM had a higher proportion of nutritional inadequacy for dietary fibers as compared to PM and NPNLM (72.5%, 66.2%, and 70.9%, respectively; p < 0.05).

Table 7.
Dietary intake and nutritional inadequacy (<2/3rd of DRIs) of energy and macro- and micronutrients by the reproductive status of Syrian mothers.
With regards to micronutrients, at least three out of four mothers (75.0% to 93.5%) did not meet 2/3rd of the RDA or AI for potassium, vitamin A, calcium, vitamin E, folate, pantothenic acid, vitamin B12, and vitamin B6. Furthermore, at least half of mothers had intakes of zinc (71.8%), magnesium (69.2%), iron (63.9%), vitamin C (63.0%), and riboflavin (50.5%) below 2/3rd of the RDAs. Interestingly, all mothers did not consume at least 2/3rd of the RDA for vitamin D with an average intake of 0.64 µg/d. A significantly higher proportion of LM did not meet 2/3rd of the RDAs or AIs for potassium, vitamin A, vitamin E, vitamin B6, pantothenic acid, zinc, copper, and manganese, compared to PM and NPNLM. On the other hand, a higher proportion of PM, followed by NPNLM, did not reach 2/3rd of the RDA for iron as compared to LM (81.8%, 80.1%. and 45.1%, respectively; p < 0.05) despite the highest intake of total iron being observed among PM (PW: 10.2 ± 9.8; LW: 8.2 ± 6.2; NPNLM: 8.5 ± 7.0 mg/d).
4. Discussion
To our knowledge, this is the first study to examine the prevalence and key determinants of anemia and the nutritional status of Syrian refugee mothers and their children under five years according to the mother’s reproductive status. Overall, the prevalence of anemia was 21.7% among mothers and 30.5% among children under five years, this being categorized as a moderate public health significance according to the WHO classification [55]. Our findings remained below the global prevalence of anemia estimated at 29.9% for women of reproductive age and 39.8% for children under five years in 2019. Nevertheless, an increasing trend of anemia has been recorded by the WHO between 2012 and 2019 among women of reproductive age and children under five years in Lebanon and Syria [3]. Anemia levels reported in the present study were higher than those in previous reports among Syrian refugees in Lebanon, but lower than Iraqi refugees in Lebanon and Syrian refugees in the Za’atari refugee camp in Jordan [24,25,56]. According to a survey conducted in Syria in 2015 and 2016, almost a quarter of women of reproductive age (24.5%) and children under five years (25.9%) had anemia [57]. Our findings are more consistent with anemia levels of the Lebanese host population for women of reproductive age and children under five years about two decades ago [58,59,60,61].
The highest prevalence of total and mild anemia among mothers was noted among non-pregnant non-lactating mothers (NPNLM), followed by lactating mothers (LM) and last by pregnant mothers (PM). Concomitantly, NPNLM were also found to be significantly older than LM and PM. An interpretation of these findings suggests that the occurrence of anemia may increase as women are getting older and move forward in the stages of the life cycle [62], even though maternal age and the number of children under five years were not significantly associated with maternal anemia in our survey. Similar findings were reported among pregnant women in rural Jordan [63]. In contrast, other studies in the literature indicated that the anemia prevalence among pregnant women increased with age and the number of children under five years in the household [62,64]. This is due to increased nutrient requirements during pregnancy and lactation and gradual depletion of the iron stores with repeated pregnancies, although lactation amenorrhea compensates for the loss of iron [10,65,66]. Low maternal iron stores at conception are a strong predictor of the risk of iron deficiency and anemia during pregnancy, but also mirror the infant’s iron endowment after birth [67,68]. In fact, our findings showed that child anemia was strongly associated with maternal anemia. Particularly, children of LM suffered the most from total, mild, and moderate anemia and had 3-times higher odds of mild anemia than those of NPNLM. Possible explanations for the increased risk of child anemia include: (1) an inadequate iron intake of women during pregnancy resulting in a minimized accumulation of iron in the fetus during the last trimester [67,68] and (2) a suboptimal maternal and child nutrition due to increased nutrient requirements leading to greater risks of malnutrition and micronutrient deficiencies [9,10]. Birth spacing and family planning play an important role in replenishing depleted maternal iron stores following a pregnancy [66,69].
The prevalence of anemia among PM in our survey was much lower than the WHO Global and Eastern Mediterranean Region estimates as well as other reported anemia rates from neighboring countries [3,62,63]. Indeed, a significantly lower risk of mild anemia was found among PM in our study (aOR = 0.26, 95%CI: 0.08–0.82). This may be explained by the fact that PM and LM had 12-folds and 2-folds higher odds of using micronutrient supplements than NPNLM in our study, despite an overall low usage of micronutrient supplements by mothers in the study sample. The benefits of multiple ANC visits and micronutrient supplementation, including iron and iron-folic acid, on anemia levels during pregnancy and birth outcomes have been well documented [64,65,68,69]. The access to free or subsidized healthcare assistance in place for UNHCR-registered and unregistered refugees in Lebanon could have played a role in reducing the mild anemia prevalence among PM. As a matter of fact, the majority of Syrian refugees across the nation and in our survey reported having access to primary healthcare services, despite main barriers such as economic vulnerability, area of residence, and household composition [70]. Albeit the high proportion of mothers who attended at least four ANC visits (62.1%), slightly less than a quarter of PM had zero visits in our survey. One explanation can be related to their UNHCR registration status as refugees. It was observed that nearly a quarter of PM were not registered as refugees and had a shorter length of stay in Lebanon as compared to LM and NPNLM, suggesting these women arrived in Lebanon after the suspension of UNHCR registrations by the government in 2015 [70,71]. As a consequence, unregistered PM, with higher nutritional needs and requirements, may not have had access to any cash or food assistance and may have faced additional barriers to access and utilize the free or subsidized healthcare assistance in place for registered and unregistered refugees in Lebanon, such as the lack of awareness of service availability [72]. Multiple sources of health messages, including family, friends, and social media, had a protective impact on maternal anemia. These findings shed light on the crucial role of counselling approaches and awareness-raising sensitive to cultural norms which need to target mothers and older members of the household to improve the health and nutritional status of women of reproductive age. For instance, women’s groups at a community level can be developed to promote the engagement of women in discussions and provide maternal social support [73,74,75].
Medium levels of wasting and low levels of stunting were identified among children under five years as per the WHO classification for malnutrition [76]. These levels were also within observed ranges in the Eastern Mediterranean Region. In contrast, the prevalence of overweight and obesity among children in our study was far below those of countries of the region [77]. Stunting rates have dropped by nearly 40% in the Eastern Mediterranean Region from 1990, despite the milder reduction rate observed since 2012 [78]. This trend is also reflected among the Syrian refugee pediatric population in Lebanon. When comparing our findings to the nutritional assessment conducted by UNICEF in 2013, stunting levels dropped by half, yet wasting and underweight increased sharply [25]. A significantly higher risk of wasting was also found among children of PM in our survey. As stunting indicates chronic malnutrition [79], it suggests that poor dietary habits and food-related coping strategies have been in place for a longer period of time [80].
On the other hand, overnutrition was predominant among non-pregnant mothers with more than 60% being overweight or obese. Our findings are in accordance with the 2016 age-standardized estimates of overweight and obesity in Lebanon and Syria [81] and with previous studies conducted among Lebanese women [82,83]. Even though two-thirds of the mothers appeared to have moderate or high activity levels, no association was found with the nutritional status of mothers in the context of our study. According to Lee et al., the IPAQ-SF typically overestimated physical activity levels by 84% [84]. Moderate to high activity was shown to have a protective role against the risk of obesity among Lebanese women, however, this was in the setting of a better socio-economic status and higher education enabling them to buy food with available resources and the knowledge to make better food and lifestyle choices [85]. With two-thirds of the participants reporting a monthly household income below the legal minimum wage in Lebanon, we project that excess overweight and obesity may be due not only to the shift in dietary patterns, but also to increased food-coping strategies due to food insecurity, thus leading to inadequate diet quality that may be energy-dense yet poor in micronutrients [5,86]. Lebanon has been undergoing the nutrition transition with increasing rates of obesity and non-communicable diseases with a shift in dietary patterns characterized by an elevated intake of foods with high energy density and rich in fat and added sugar [87,88]. The prices of food influence to a great extent the choices of food, and lower socio-economic groups tend to resort to cheaper lower-quality diets [89]. In addition, the interrelationship between economic vulnerabilities, food insecurity, and psychological distress has been well documented and can negatively impact health status [28,90].
Findings from this study demonstrated a significant association between total anemia and overweight or obesity (BMI ≥ 25.0 kg/m2) and an at-risk waist circumference among mothers, suggesting the existence of a double burden of malnutrition at the individual level [91]. Controversial findings regarding the relationship of anemia with overweight and obesity, central obesity, and obesity-associated inflammation were found in the literature [92,93]. For instance, Wirth et al. found associations between obesity and inflammation, but showed that the risk of anemia was lower among overweight and obese women [92]. However, obesity is generally linked to micronutrient deficiencies interceded by poor nutrition and chronic inflammation [5,66,94]. Another explanation comes into play linking poverty, food insecurity, and malnutrition including anemia [95] and obesity [82,96]. These findings point towards a poor diet leading to the gradual accumulation of malnutrition, nutrient deficiencies, and anemia in the long term [94]. Maternal obesity, associated with poor micronutrient status, may also impair offspring development and early growth as a consequence of the intergenerational effects of malnutrition [5].
Looking at the dietary intake of mothers, overall low energy intakes were reported by our study population. The latter may be explained by the commonly identified underreporting among women [97]. Misreporting may be biased towards reporting lower intakes of fat, carbohydrates, and alcohol [98] and may be influenced by several psychosocial factors such as being overweight, body image concerns, demographics, depression, anxiety, and social desirability [99]. Nevertheless, the diet of women of reproductive age in our study was characterized by a percent energy intake of total fat and total sugar exceeding their respective recommendations, in addition to inadequate intakes of protein and fibers. Protein-rich foods include meat, poultry, seafood, eggs, milk, and dairy products from animal sources and beans, peas, nuts, and seeds from plant sources. These sources also contribute to the dietary intake of B vitamins, vitamins D and E, iron, zinc, calcium, and magnesium among others. In addition, dietary fibers naturally occur in fruits, vegetables, beans, and nuts as well as whole-grain products. These foods are also known to be the primary contributors to a wide range of vitamins and minerals, namely vitamins A and C, folate, and potassium [100]. As a result, it is not surprising that a widespread inadequate dietary intake of the abovementioned vitamins and minerals was simultaneously observed in the diet of the mothers in our survey. Our findings corroborate with those of Nasreddine et al. showing that Lebanese women had a high intake of total fat and higher consumption of sweets, while a lower micronutrient intake was observed [18]. We suggest that similar dietary patterns, that mimic those of the local population, were a sign of “dietary acculturation”. The latter refers to the adoption and integration of new eating patterns and food choices of the host country after migration [101]. Studies among asylum seekers in Germany, including Syrian refugees, established similar conclusions [102]. Multidimensional factors led to dietary adaptations and a remarkable shift towards Western/urban eating habits among asylum seekers in Germany [103].
Poor nutritional adequacies were also found among mothers according to their reproductive status. Of particular interest are the essential hemopoietic nutrients recognized for their role in erythropoiesis, including protein, iron, vitamins A, B12, B6, C, D, E, folate, riboflavin, copper, and zinc [65,94,104]. Higher proportions of PM and LM did not meet 2/3rd RDA for protein than NPNLM. This is mainly due to their higher requirements, even though no significant differences were found in their daily intake of protein. Concomitantly, the majority of PM and NPNLM fell short of 2/3rd RDA for iron as compared to LM. Since no significant differences were observed in the dietary intake of iron among mothers, the advantage was rather the result of lower needs of iron for LM justified by the lactation amenorrhea [66]. Additionally, LM were the least to reach 2/3rd of the RDA for dietary fibers due to higher requirements compared to PM and NPNLM, even though LM consumed a significantly higher number of meals on average per day. In fact, significantly higher proportions of nutritional inadequacies were found among LM, followed by NPNLM and PM, particularly for vitamin A, vitamin B6, zinc, copper, potassium, and manganese. A suboptimal maternal diet during lactation compromises the human milk quality as the content of certain micronutrients are affected, namely the water-soluble vitamins and fat content [105,106]. Breastfeeding has a vital role in the prevention of all forms of malnutrition and micronutrient deficiencies, indicating the need to improve maternal nutrition and women’s nutritional status as early as possible [107]. Similar to our findings, studies in Lebanon found that women were at risk of micronutrient inadequacies especially for hematinic nutrients including iron, folate, zinc, vitamin B12, and calcium [18,58]. Our findings are also in line with the literature showing low intakes of micronutrients among pregnant women and women of reproductive age in low- and middle-income countries [108,109] and in developed countries [110].
Healthier lifestyle patterns were present significantly more among PM and LM with higher odds of consuming daily breakfast and lower odds of smoking. Our findings are less pronounced compared to a previous assessment conducted among Lebanese pregnant women. These women were more likely to have low birth weight babies and reported a lower duration of breastfeeding [111]. The consumption of coffee and tea during a meal was found to be common among mothers in our survey, especially among LM. However, no association with anemia was found in our study despite that they are potent inhibitors of iron absorption. It can be explained by the multifactorial nature of the causes of and contributors to anemia, such as poor dietary practices, infections and inflammation, and socio-economic factors [65]. In addition, no associations between anemia and socio-economic characteristics were found in our study. This is in accordance with previous studies among women in Lebanon and rural Jordan [58,61,63] and the NHANES study among pregnant women [112].
Shifts towards unhealthy foods and lifestyle, with an escalating burden of overweight and obesity, coexisting with undernutrition and anemia, reflect the nutrition situation of most low- and middle-income countries and countries in the Eastern Mediterranean Region [16]. Despite the extensive humanitarian assistance provided to Syrian refugees in Lebanon since the beginning of the conflict in 2011 [23], Beirut and Mount Lebanon governorates have the fewest number of beneficiaries as compared to other regions in Lebanon [70]. The aforementioned was also reflected in our survey as 82.1% of the Syrian households in Greater Beirut were registered as refugees with the UNHCR, of which only 8.9% were receiving food assistance from the World Food Programme. The nutritional status of vulnerable population groups can be further exposed to acute and chronic malnutrition due to the severe financial crisis in Lebanon since October 2019, resulting in hyperinflation of food prices, soaring unemployment rates, and reduced incomes, combined with containment measures for COVID-19 pandemic and the aftermath of the Beirut port explosion in August 2020 [78,113,114].
The findings of this study should be considered in light of a few limitations. The cross-sectional design allows for the investigation of associations rather than causal relationships. Furthermore, dietary data were explored using one 24-h dietary recall which may face potential limitations due to reliance on memory and day-to-day variations; however, every attempt was exerted to minimize misreporting. The five-step multiple pass recall method may reduce bias and provide accurate estimates of food intake at the population level; however, underreporting under field conditions and its accuracy in overweight and obese persons remain underexplored [43,115]. Nevertheless, the 24-h dietary recall presents several strengths such as capturing all eating patterns and preparation methods, not relying on the literacy of the respondent, not affecting food choices, and having a low burden on the memory [116]. The IPAQ-SF was shown to overestimate levels of physical activity by 84% on average and its use as an indicator of relative or absolute physical activity is weak [84]. Lastly, our study sample consists of Syrian mothers and children under five years attending primary healthcare centers in the most vulnerable areas of Greater Beirut and thus the results cannot be generalized to all Syrian refugees in Lebanon. In the context of this study, a randomized recruitment strategy through the UNCHR could have been more representative of Greater Beirut, yet a convenience sample was selected in order to avoid coercion due to offered benefits to registered refugees and fear related to their legal residency status. One of the strengths of this study is the inclusion of unregistered Syrian refugees and displaced persons in the study population. The number of unregistered Syrian refugees may have increased in Lebanon due to the governmental decision in 2015 to suspend registration of Syrian refugees by the UHNCR or to other associated barriers to obtain legal residency [70,71]. Recruitment took place in primary healthcare centers where patients come freely, without expectations, to participate in vaccination campaigns and/or benefit from other health services. Therefore, our sample population is representative of Syrian refugee mothers attending primary healthcare centers in Greater Beirut. Our population group, mothers with children under five years in a highly vulnerable setting, represents a group of interest for international humanitarian assistance and policy makers at national and regional levels, especially as the number of refugees and displaced people might increase rapidly with current economic crises.
5. Conclusions
This study highlights the co-existence of overweight, obesity, and anemia among women of reproductive age as well as undernutrition among children under five years within the same households. Evidence on poor dietary intake and nutritional inadequacies of Syrian refugee mothers during pregnancy and lactation is also presented. Findings from this study can serve as a basis for culture-specific interventions, long-term strategies, and policies to protect and promote maternal and child nutrition for refugees in Lebanon and neighboring countries. The burden of malnutrition and nutritional inadequacies calls for immediate double-duty actions and reforms in order to alleviate poverty and food insecurity and promote more decent and sustainable livelihoods for Syrian refugees [117]. Culture-specific interventions to improve the nutritional status of women of reproductive age and children are crucial, in addition to efforts to protect and promote breastfeeding [16,17]. Family planning services should be intensified to allow the replenishment of nutrient stores by the promotion of birth spacing, reduction of unplanned pregnancies, and receiving adequate nutrition during pregnancy that is essential for the mothers and their offspring’s health and wellbeing [69,109]. Further research is still needed to explore the determinants of the double burden of malnutrition and anemia among refugee women and children in countries affected by protracted conflicts and displacement.
Author Contributions
J.A.-R., T.J., and V.S. conceptualized the research design and led the parent study. L.N., L.J., and N.H. provided support in designing the parent study. J.A.-R., T.J., V.S., and L.N. sought after ethics approval. J.A.-R. and T.J. organized, implemented, and supervised the study during data collection and entry. H.T. provided support in biostatistics and assistance through the MRVP program during data collection. J.A.-R. conducted data analysis, conceptualized, and wrote the original draft of the manuscript. T.J., V.S., L.N., L.J., and N.H., and J.F. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The publication is an output of a PhD scholarship from the Food Security Center from the University of Hohenheim which is part of the DAAD (German Academic Exchange Service) program “exceed” and is supported by DAAD and the German Federal Ministry for Economic Cooperation and Development (BMZ) and in cooperation with the hosting Institute of Nutritional Sciences (140). This research was also funded by the Fiat Panis Foundation, the BCFN YES! 2017 Research Grant Award from the Barilla Center for Food and Nutrition (BCFN) Foundation, and the University Research Board at the American University of Beirut (award number: 103366).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of the Freiburger Independent Ethics Committee (FEKI) in Germany (FEKI code: 017/1434) and the IRB for Social and Behavioral studies at the American University of Beirut (AUB) in Lebanon (IRB ID: SBS-2017-0294) prior to the onset of the study. The Primary Health Care Department of the MoPH in Lebanon granted a letter of approval to access to the PHCC which are part of the National PHC Network overseen by the MoPH in Greater Beirut. Approval letters were also obtained from the directors of PHCC selected for the survey prior accessing their premises.
Informed Consent Statement
Written informed consent was obtained from all participants’ prior enrollment in the study. When mothers below 18 years were identified, a parental consent and an informed assent were sought. In case the mother was illiterate, a witness or the nurse signed on her behalf after reading and explaining the consent form to the participant. Confidentiality was assured to the participants by assigning random identifiers and allowing access to the data only to the investigators.
Data Availability Statement
Dataset analyzed during this study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank all participants for taking part of the survey and we are grateful to their time and patience. We would like to extend our thanks to the directors of the primary health care centers for allowing us to use their premises. The centers included Howard Karagheusian Commemorative Corporation and Armenian Relief Cross of Lebanon in Bourj Hammoud, Makhzoumi Foundation PHC in Mazraa, Child & Mother Welfare Hospital in Msaytbeh, Mar Antonious PHC in Baouchriyeh, and Maternal Childhood Center in Chiyah and Bourj Barajneh. We appreciate and acknowledge the efforts of our volunteers, field workers, and research assistants for their hard work during data collection. We would like to express our sincere gratitude to the Medical Research Volunteer Program (MRVP) at AUB for their supply of assistance during data collection and entry and to the Ambulatory Clinical Laboratory in the Department of Pathology and Laboratory Medicine at AUB for facilitating the trainings on proper micro-technique blood collection. We would like to extend our deep appreciation and gratitude to the Department of Nutrition and Food Sciences at AUB for providing their support and technical expertise and helping in recruiting the research team and volunteers. Last but not least, we would like to acknowledge the help of Judith Lauvai for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Dietary Reference Intakes (DRIs) for macro- and micronutrients women of reproductive age according to their reproductive status.
Table A1.
Dietary Reference Intakes (DRIs) for macro- and micronutrients women of reproductive age according to their reproductive status.
| Pregnant Mothers | Lactating Mothers | Non-Pregnant Non-Lactating Mothers | |
|---|---|---|---|
| Energy and macronutrients | |||
| Energy (kcal/d) | 2200–2652 | 2600–2700 | 2200 |
| Carbohydrates (%EI/d) | 45–65 | 45–65 | 45–65 |
| Protein (%EI/d) | 10–35 | 10–35 | 10–35 |
| Total fat (%EI/d) | 20–35 | 20–35 | 20–35 |
| MUFA (%EI/d) | 15–20 | 15–20 | 15–20 |
| Linoleic acid (%EI/d) | 5–10 | 5–10 | 5–10 |
| Linolenic acid (%EI/d) | 0.6–1.2 | 0.6–1.2 | 0.6–1.2 |
| PUFA (%EI/d) | 6–11 | 6–11 | 6–11 |
| SFA (%EI/d) | 8–10 | 8–10 | 8–10 |
| TFA (%EI/d) | <1 | <1 | <1 |
| Cholesterol (mg/d) | <200 | <200 | <200 |
| Total sugar (%EI/d) | <10 | <10 | <10 |
| Macronutrients | |||
| Carbohydrates (g/d) | 175 | 210 | 130 |
| Protein (g/d) | 71 | 71 | 46 |
| Linoleic acid (g/d) | 13 * | 13 * | 11–12 * |
| Linolenic acid (g/d) | 1.4 * | 1.3 * | 1.1 * |
| Fibers (g/d) | 28 * | 29 * | 25–26 * |
| Micronutrients | |||
| Iron (mg/d) | 27 | 9–10 | 15–18 |
| Folate (µg/d) | 600 | 500 | 400 |
| Vitamin B12 (µg/d) | 2.6 | 2.8 | 2.4 |
| Vitamin C (mg/d) | 80–85 | 115–120 | 65–75 |
| Vitamin A (µg/d) | 750–770 | 1200–1300 | 700 |
| Vitamin D (µg/d) | 15 | 15 | 15 |
| Vitamin E (mg/d) | 15 | 19 | 15 |
| Vitamin K (µg/d) | 75–90 * | 75–90 * | 75–90 * |
| Thiamin (mg/d) | 1.4 | 1.4 | 1–1.1 |
| Riboflavin (mg/d) | 1.4 | 1.6 | 1–1.1 |
| Niacin (mg/d) | 18 | 17 | 14 |
| Pantothenic acid (mg/d) | 6 * | 7 * | 5 * |
| Vitamin B6 (mg/d) | 1.9 | 2 | 1.2–1.3 |
| Zinc (mg/d) | 11–12 | 12–13 | 8–9 |
| Copper (mg/d) | 1 | 1.3 | 0.9 |
| Calcium (mg/d) | 1000–1300 | 1000–1300 | 1000–1300 |
| Magnesium (mg/d) | 350–400 | 310–360 | 310–360 |
| Sodium (mg/d) | 1500 * | 1500 * | 1500 * |
| Potassium (mg/d) | 4700 * | 5100 * | 4700 * |
| Phosphorus (mg/d) | 700–1250 | 700–1250 | 700–1250 |
| Manganese (mg/d) | 2.0 * | 2.6 * | 1.6–1.8 * |
| Selenium (µg/d) | 60 | 55 | 70 |
d: day; %EI: percent energy intake; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids; TFA: trans-fatty acids. Dietary Reference Intakes (DRIs) refers to the Recommended Dietary Allowances (RDAs), Adequate Intakes (AIs), and Acceptable Macronutrient Distribution Ranges (AMDRs) for energy and macro- and micronutrients as recommended by the Institute of Medicine [47,48]. RDAs are presented in bold type and AIs in ordinary types followed by an asterisk (*). Intakes of MUFA, PUFA, SFA, and TFA were analyzed based on the FAO recommendations [49]. Cholesterol intake was assessed based on the criteria of the National Institute of Health [50]. The reference intake of total sugar was based on the WHO recommendation [51].
References
- Food and Agriculture Organization; International Fund for Agricultural Development; United Nations Children’s Fund; World Food Programme; World Health Organization. In Brief to The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020; p. 320. [Google Scholar]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 January 2021).
- World Health Organization. WHO Global Anaemia Estimates, 2021 Edition. Available online: https://www.who.int/data/maternal-newborn-child-adolescent-ageing/advisory-groups/gama/gama-advisory-group-members (accessed on 5 June 2021).
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the Double Burden of Malnutrition and the Changing Nutrition Reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The Double Burden of Malnutrition: Aetiological Pathways and Consequences for Health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Nutrition in Early Life and the Programming of Adult Disease: A Review. J. Hum. Nutr. Diet. 2015, 28, 1–14. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Christian, P.; Stewart, C.P. Maternal Micronutrient Deficiency, Fetal Development, and the Risk of Chronic Disease. J. Nutr. 2010, 140, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K. The 1,000-Day Window and Cognitive Development. Hidden Hunger 2016, 115, 1–15. [Google Scholar] [CrossRef]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current Concepts of Maternal Nutrition. Obstet. Gynecol. Surv. 2016, 71, 413–426. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron Deficiency in Pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Juul, S.E.; Derman, R.J.; Auerbach, M. Perinatal Iron Deficiency: Implications for Mothers and Infants. Neonatology 2019, 115, 269–274. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Low, M.; Thompson, J.; Farrell, A.; De-Regil, L.-M. Iron Supplementation Benefits Physical Performance in Women of Reproductive Age: A Systematic Review and Meta-Analysis. J. Nutr. 2014, 144, 906–914. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Regional Overview of Food Security and Nutrition—Building Resilience for in Times of Conflict and Crisis: Food Security and Nutrition a Perspective from the Near East and North Africa (NENA) Region; FAO: Cairo, Egypt, 2017; p. 62. [Google Scholar]
- Tanumihardjo, S.A.; Anderson, C.; Kaufer-Horwitz, M.; Bode, L.; Emenaker, N.J.; Haqq, A.M.; Satia, J.A.; Silver, H.J.; Stadler, D.D. Poverty, Obesity, and Malnutrition: An International Perspective Recognizing the Paradox. J. Am. Diet. Assoc. 2007, 107, 1966–1972. [Google Scholar] [CrossRef]
- Nasreddine, L.; Ayoub, J.J.; Al Jawaldeh, A. Review of the Nutrition Situation in the Eastern Mediterranean Region. East. Mediterr. Health J. 2018, 24, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The Prevalence of Micronutrient Deficiencies and Inadequacies in the Middle East and Approaches to Interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef]
- Nasreddine, L.; Chamieh, M.C.; Ayoub, J.; Hwalla, N.; Sibai, A.-M.; Naja, F. Sex Disparities in Dietary Intake across the Lifespan: The Case of Lebanon. Nutr. J. 2020, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Goverment of Lebanon. The United Nations Lebanon Crisis Response Plan 2017–2020 (2020 Update); Goverment of Lebanon: Beirut, Lebanon, 2020.
- United Nations Office for the Coordination of Humanitarian Affairs. Humanitarian Bulletin Lebanon (Issue 33, 1 August–31 October 2018); OCHA: Beirut, Lebanon, 2018. [Google Scholar]
- United Nations High Commissioner for Refugees. Refugees from Syria: Lebanon; UNHCR: Beirut, Lebanon, 2015. [Google Scholar]
- United Nations Office for the Coordination of Humanitarian Affairs. Lebanon Overview (May 2016); OCHA: Beirut, Lebanon, 2016. [Google Scholar]
- United Nations High Commissioner for Refugees; United Nations Children’s Fund; World Food Programme. VASYR 2018—Vulnerability Assessment of Syrian Refugees in Lebanon—Lebanon; UNHCR: Beirut, Lebanon, 2018. [Google Scholar]
- Hossain, S.M.M.; Leidman, E.; Kingori, J.; Al Harun, A.; Bilukha, O.O. Nutritional Situation among Syrian Refugees Hosted in Iraq, Jordan, and Lebanon: Cross Sectional Surveys. Confl. Health 2016, 10. [Google Scholar] [CrossRef]
- United Nations Children’s Fund. 2013 Joint Nutrition Assessment Syrian Refugees in Lebanon—Lebanon; UNICEF: Beirut, Lebanon, 2014. [Google Scholar]
- United Nations Office for the Coordination of Humanitarian Affairs. Lebanon: Beirut and Mount Lebanon Governorates Profile (May 2016); OCHA: Beirut, Lebanon, 2016. [Google Scholar]
- International Labour Organization. Syrian Refugees in Lebanon Face Harsh Working Conditions. Available online: http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_240126/lang--en/index.htm (accessed on 7 August 2020).
- World Health Organization. WHO Housing and Health Guidelines; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- International Physical Activity Questionnaire Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms; YouthREX: Toronto, ON, Canada, 2005.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES)—Anthropometry Procedures Manual; CDC: Hyattsville, MD, USA, 2017; p. 95.
- World Health Organization. Training Course on Child Growth Assessment, WHO Child Growth Standards—B. Measuring a Child’s Growth; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Fakier, A.; Petro, G.; Fawcus, S. Mid-Upper Arm Circumference: A Surrogate for Body Mass Index in Pregnant Women. S. Afr. Med. J. 2017, 107, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Tumilowicz, A. Guide to Screening for Food and Nutrition Services Among Adolescents and Adults Living with HIV; Food and Nutrition Technical Assistance III Project (FANTA); FHI 360: Washington, DC, USA, 2010; p. 20. [Google Scholar]
- Food and Nutrition Technical Assistance; United States Agency for International Development; FHI 360. Global MUAC Cutoffs for Adults: A Technical Consultation; FANTA Project: Washington, DC, USA, 2018; p. 6. [Google Scholar]
- United Nations High Commissioner for Refugees. World Food Programme Guidelines for Selective Feeding: The Management of Malnutrition in Emergencies; UNHCR: Geneva, Switzerland, 2011. [Google Scholar]
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S. [Google Scholar]
- World Health Organization. Waist Circumference and Waist–Hip Ratio Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Training Course on Child Growth Assessment, WHO Child Growth Standards—C. Intepreting Growth Indicators; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- World Health Organization. WHO Anthro for Personal Computers, Version 3.2.2, 2011: Software for Assessing Growth and Development of the World’s Children; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Marques, R.F.S.V.; Taddei, J.A.A.C.; Lopez, F.A.; Braga, J.A.P. Breastfeeding Exclusively and Iron Deficiency Anemia during the First 6 Months of Age. Rev. Assoc. Med. Bras. 2014, 60, 18–22. [Google Scholar] [CrossRef]
- Millen, B.E.; Morgan, J.L. The 2D Food Portion Visual; Nutrition Consulting Enterprises: Framingham, MA, USA, 1996. [Google Scholar]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of Dietary Recall Using the USDA Five-Step Multiple-Pass Method in Men: An Observational Validation Study. J. Am. Diet. Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef]
- Johnson, R.K. Dietary Intake—How Do We Measure What People Are Really Eating? Obes. Res. 2002, 10 (Suppl. S1), 63S–68S. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, J. Nutrition Surveys and Assessment—NutriSurvey Software 2007. Available online: http://www.nutrisurvey.de/index.html (accessed on 7 August 2020).
- Pellett, P.L.; Shadarevian, S. Food Composition: Tables for Use in the Middle East; American University of Beirut: Beirut, Lebanon, 2013; ISBN 978-9953-586-02-1. [Google Scholar]
- National Research Council. Recommended Dietary Allowances, 10th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1989; ISBN 978-0-309-04633-6. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Food and Agriculture Organization. WHO|Fats and Fatty Acids in Human Nutrition; FAO: Rome, Italy, 2010. [Google Scholar]
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Harvard Humanitarian Initiative. KoBoToolbox—Data Collection Tools for Challenging Environments. Available online: https://kobotoolbox.org/ (accessed on 7 August 2020).
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical Tests, P Values, Confidence Intervals, and Power: A Guide to Misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Moyé, L.; Cohen, M. Liberation From the p Value’s Tyranny. Circ. Res. 2018, 122, 1046–1048. [Google Scholar] [CrossRef]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Ghattas, H.; Sassine, A.J.; Seyfert, K.; Nord, M.; Sahyoun, N.R. Food Insecurity among Iraqi Refugees Living in Lebanon, 10 Years after the Invasion of Iraq: Data from a Household Survey. Br. J. Nutr. 2014, 112, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kern, J. Nutrition Interventions Syria 2017; WFP: Aleppo, Syria, 2017. [Google Scholar]
- Al Khatib, L.; Obeid, O.; Sibai, A.-M.; Batal, M.; Adra, N.; Hwalla, N. Folate Deficiency Is Associated with Nutritional Anaemia in Lebanese Women of Childbearing Age. Public Health Nutr. 2006, 9, 921–927. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of Food and Agriculture: Food Systems for Better Nutrition; The State of Food and Agriculture Series; FAO: Rome, Italy, 2013; ISBN 978-92-5-107671-2. [Google Scholar]
- Hwalla, N. Nutrition Country Profile: Lebanese Republic; FAO: Rome, Italy, 2007; p. 32. [Google Scholar]
- Hwalla, N.; Adra, N.; Jackson, R.T. Iron Deficiency Is an Important Contributor to Anemia Among Reproductive Age Women in Lebanon. Ecol. Food Nutr. 2004, 43, 77–92. [Google Scholar] [CrossRef]
- Khader, A.; Madi, H.; Riccardo, F.; Sabatinelli, G. Anaemia among Pregnant Palestinian Women in the Occupied Palestinian Territory. Public Health Nutr. 2009, 12, 2416–2420. [Google Scholar] [CrossRef]
- Al-Mehaisen, L.; Khader, Y.; Al-Kuran, O.; Abu Issa, F.; Amarin, Z. Maternal Anemia in Rural Jordan: Room for Improvement. Anemia 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Saaka, M.; Oladele, J.; Larbi, A.; Hoeschle-Zeledon, I. Dietary Diversity Is Not Associated with Haematological Status of Pregnant Women Resident in Rural Areas of Northern Ghana. J. Nutr. Metab. 2017, 2017. [Google Scholar] [CrossRef]
- World Health Organization. Global Anaemia Reduction Efforts among Women of Reproductive Age: Impact, Achievement of Targets and the Way Forward for Optimizing Efforts; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Mawani, M.; Ali, S.A.; Bano, G.; Ali, S.A. Iron Deficiency Anemia among Women of Reproductive Age, an Important Public Health Problem: Situation Analysis. Reprod. Syst. Sex. Disord. 2016, 5, 6. [Google Scholar] [CrossRef]
- Burke, R.M.; Leon, J.S.; Suchdev, P.S. Identification, Prevention and Treatment of Iron Deficiency during the First 1000 Days. Nutrients 2014, 6, 4093–4114. [Google Scholar] [CrossRef]
- Scholl, T.O. Maternal Iron Status: Relation to Fetal Growth, Length of Gestation, and Iron Endowment of the Neonate. Nutr. Rev. 2011, 69, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Agbozo, F.; Abubakari, A.; Der, J.; Jahn, A. Maternal Dietary Intakes, Red Blood Cell Indices and Risk for Anemia in the First, Second and Third Trimesters of Pregnancy and at Predelivery. Nutrients 2020, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- United Nations High Commissioner for Refugees; United Nations Children’s Fund; World Food Programme. VASYR 2019—Vulnerability Assessment of Syrian Refugees in Lebanon—Lebanon; UNHCR: Beirut, Lebanon, 2019. [Google Scholar]
- United Nations High Commissioner for Refugees. Protection—UNHCR Lebanon; UNHCR: Beirut, Lebanon, 2017. [Google Scholar]
- Truppa, C.; Leresche, E.; Fuller, A.F.; Marnicio, A.S.; Abisaab, J.; El Hayek, N.; Zmeter, C.; Toma, W.S.; Harb, H.; Hamadeh, R.S.; et al. Utilization of Primary Health Care Services among Syrian Refugee and Lebanese Women Targeted by the ICRC Program in Lebanon: A Cross-Sectional Study. Confl. Health 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.K.; Marquis, G.S.; Colecraft, E.K.; Lartey, A.; Sakyi-Dawson, O.; Ahunu, B.K.; Butler, L.M. Caregivers’ Nutrition Knowledge and Attitudes Are Associated with Household Food Diversity and Children’s Animal Source Food Intake across Different Agro-Ecological Zones in Ghana. Br. J. Nutr. 2016, 115, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.H.; Paw, M.K.; Nosten, S.; Darakamon, M.C.; Gilder, M.E.; Charunwatthana, P.; Carrara, V.I.; Wickramasinghe, K.; Angkurawaranon, C.; Plugge, E.; et al. “Because the Baby Asks for It”: A Mixed-Methods Study on Local Perceptions toward Nutrition during Pregnancy among Marginalised Migrant Women along the Myanmar-Thailand Border. Glob. Health Action 2018, 11, 1473104. [Google Scholar] [CrossRef]
- Schmeer, K.K.; Piperata, B.A.; Herrera Rodríguez, A.; Salazar Torres, V.M.; Centeno Cárdenas, F.J. Maternal Resources and Household Food Security: Evidence from Nicaragua. Public Health Nutr. 2015, 18, 2915–2924. [Google Scholar] [CrossRef]
- De Onis, M.; Borghi, E.; Arimond, M.; Webb, P.; Croft, T.; Saha, K.; De-Regil, L.M.; Thuita, F.; Heidkamp, R.; Krasevec, J.; et al. Prevalence Thresholds for Wasting, Overweight and Stunting in Children under 5 Years. Public Health Nutr. 2019, 22, 175–179. [Google Scholar] [CrossRef]
- Nasreddine, L.M.; Kassis, A.N.; Ayoub, J.J.; Naja, F.A.; Hwalla, N.C. Nutritional Status and Dietary Intakes of Children amid the Nutrition Transition: The Case of the Eastern Mediterranean Region. Nutr. Res. 2018, 57, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Jawaldeh, A.A.; Doggui, R.; Borghi, E.; Aguenaou, H.; Ammari, L.E.; Abul-Fadl, A.; McColl, K. Tackling Childhood Stunting in the Eastern Mediterranean Region in the Context of COVID-19. Children 2020, 7, 239. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and Child Undernutrition: Global and Regional Exposures and Health Consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Hasan, M.; Islam, M.M.; Mubarak, E.; Haque, M.A.; Choudhury, N.; Ahmed, T. Mother’s Dietary Diversity and Association with Stunting among Children <2 Years Old in a Low Socio-Economic Environment: A Case-Control Study in an Urban Care Setting in Dhaka, Bangladesh. Matern. Child. Nutr. 2019, 15, e12665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory Data Repository. Body Mass Index (BMI). Available online: https://www.who.int/data/maternal-newborn-child-adolescent/monitor (accessed on 28 November 2020).
- Jomaa, L.; Naja, F.; Cheaib, R.; Hwalla, N. Household Food Insecurity Is Associated with a Higher Burden of Obesity and Risk of Dietary Inadequacies among Mothers in Beirut, Lebanon. BMC Public Health 2017, 17, 567. [Google Scholar] [CrossRef]
- Naja, F.; Nasreddine, L.; Itani, L.; Chamieh, M.C.; Adra, N.; Sibai, A.M.; Hwalla, N. Dietary Patterns and Their Association with Obesity and Sociodemographic Factors in a National Sample of Lebanese Adults. Public Health Nutr. 2011, 14, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, M.C.; Moore, H.J.; Summerbell, C.; Tamim, H.; Sibai, A.M.; Hwalla, N. Diet, Physical Activity and Socio-Economic Disparities of Obesity in Lebanese Adults: Findings from a National Study. BMC Public Health 2015, 15, 279. [Google Scholar] [CrossRef]
- Ghattas, H. Food Security and Nutrition in the Context of the Global Nutrition Transition; FAO: Rome, Italy, 2014; p. 21. [Google Scholar]
- Nasreddine, L.; Naja, F.; Chamieh, M.C.; Adra, N.; Sibai, A.-M.; Hwalla, N. Trends in Overweight and Obesity in Lebanon: Evidence from Two National Cross-Sectional Surveys (1997 and 2009). BMC Public Health 2012, 12, 798. [Google Scholar] [CrossRef]
- Naja, F.; Nasreddine, L.; Itani, L.; Adra, N.; Sibai, A.M.; Hwalla, N. Association between Dietary Patterns and the Risk of Metabolic Syndrome among Lebanese Adults. Eur. J. Nutr. 2013, 52, 97–105. [Google Scholar] [CrossRef]
- Darmon, N.; Drewnowski, A. Contribution of Food Prices and Diet Cost to Socioeconomic Disparities in Diet Quality and Health: A Systematic Review and Analysis. Nutr. Rev. 2015, 73, 643–660. [Google Scholar] [CrossRef]
- Emerson, J.A.; Tol, W.; Caulfield, L.E.; Doocy, S. Maternal Psychological Distress and Perceived Impact on Child Feeding Practices in South Kivu, DR Congo. Food Nutr. Bull. 2017, 38, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Mahmudiono, T.; Segalita, C.; Rosenkranz, R.R. Socio-Ecological Model of Correlates of Double Burden of Malnutrition in Developing Countries: A Narrative Review. Int. J. Environ. Res. Public Health 2019, 16, 3730. [Google Scholar] [CrossRef]
- Wirth, J.P.; Rajabov, T.; Petry, N.; Woodruff, B.A.; Shafique, N.B.; Mustafa, R.; Tyler, V.Q.; Rohner, F. Micronutrient Deficiencies, Over- and Undernutrition, and Their Contribution to Anemia in Azerbaijani Preschool Children and Non-Pregnant Women of Reproductive Age. Nutrients 2018, 10, 1483. [Google Scholar] [CrossRef]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an Emerging Risk Factor for Iron Deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, C.M.; Suchdev, P.S. Anemia Epidemiology, Pathophysiology, and Etiology in Low- and Middle-Income Countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Ghose, B.; Tang, S.; Yaya, S.; Feng, Z. Association between Food Insecurity and Anemia among Women of Reproductive Age. PeerJ 2016, 4, e1945. [Google Scholar] [CrossRef]
- Ford, N.D.; Patel, S.A.; Narayan, K.M.V. Obesity in Low- and Middle-Income Countries: Burden, Drivers, and Emerging Challenges. Annu. Rev. Public Health 2017, 38, 145–164. [Google Scholar] [CrossRef]
- Scagliusi, F.B.; Polacow, V.O.; Artioli, G.G.; Benatti, F.B.; Lancha, A.H. Selective Underreporting of Energy Intake in Women: Magnitude, Determinants, and Effect of Training. J. Am. Diet. Assoc. 2003, 103, 1306–1313. [Google Scholar] [CrossRef]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using Intake Biomarkers to Evaluate the Extent of Dietary Misreporting in a Large Sample of Adults: The OPEN Study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef]
- Maurer, J.; Taren, D.L.; Teixeira, P.J.; Thomson, C.A.; Lohman, T.G.; Going, S.B.; Houtkooper, L.B. The Psychosocial and Behavioral Characteristics Related to Energy Misreporting. Nutr. Rev. 2006, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services: Washinton, DC, USA, 2015.
- Satia-Abouta, J. Dietary Acculturation: Definition, Process, Assessment, and Implications. Int. J. Hum. Ecol. 2003, 4, 71–86. [Google Scholar]
- Khan, S.; Fischer, L.; Ghaziani, S.; Jeremias, T.; Scherbaum, V. Nutritional Habits of Asylum Seekers Living in Communal Accomodation in Stuttgart, Germany. Ernahr. Umsch. 2019, 18–25. [Google Scholar] [CrossRef]
- Schmitt, R.; Fülle, J.; Abou-Rizk, J.; Al-Sayed, L.; Masserrat, N.; Schüle, E.; Scherbaum, V. Nutritional Habits of Female Asylum Seekers—From Tradition to Adaptation. Ernahr. Umsch. 2019, 45–51. [Google Scholar] [CrossRef]
- Vilter, R.W. Essential Nutrients in the Management of Hematopoietic Disorders of Human Beings: A Résumé. Am. J. Clin. Nutr. 1955, 3, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Bzikowska, A.; Czerwonogrodzka-Senczyna, A.; Weker, H.; Wesołowska, A. Correlation between Human Milk Composition and Maternal Nutritional Status. Rocz. Panstw. Zakl. Hig. 2018, 69, 363–367. [Google Scholar] [CrossRef]
- Scherbaum, V.; Srour, M.L. The Role of Breastfeeding in the Prevention of Childhood Malnutrition. World Rev. Nutr. Diet. 2016, 115, 82–97. [Google Scholar] [CrossRef]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary Intakes of Women during Pregnancy in Low- and Middle-Income Countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef]
- Harika, R.; Faber, M.; Samuel, F.; Kimiywe, J.; Mulugeta, A.; Eilander, A. Micronutrient Status and Dietary Intake of Iron, Vitamin A, Iodine, Folate and Zinc in Women of Reproductive Age and Pregnant Women in Ethiopia, Kenya, Nigeria and South Africa: A Systematic Review of Data from 2005 to 2015. Nutrients 2017, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.; Hure, A.; Macdonald-Wicks, L.; Smith, R.; Collins, C. A Systematic Review and Meta-Analysis of Micronutrient Intakes during Pregnancy in Developed Countries. Nutr. Rev. 2013, 71. [Google Scholar] [CrossRef]
- Bachir, R.; Chaaya, M. Maternal Smoking: Determinants and Associated Morbidity in Two Areas in Lebanon. Matern. Child. Health J. 2008, 12. [Google Scholar] [CrossRef]
- Mei, Z.; Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cusick, S.E.; Lacher, D.A.; Grummer-Strawn, L.M. Assessment of Iron Status in US Pregnant Women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am. J. Clin. Nutr. 2011, 93, 1312–1320. [Google Scholar] [CrossRef]
- World Food Programme. Assessing the Impact of the Economic and COVID-19 Crises in Lebanon—June 2020—Lebanon; WFP: Beirut, Lebanon, 2020. [Google Scholar]
- World Food Programme. Beirut Port Explosion: Impact on Key Economic and Food Security Indicators—August 2020—Lebanon; WFP: Beirut, Lebanon, 2020. [Google Scholar]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method Reduces Bias in the Collection of Energy Intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings; FAO: Rome, Italy, 2018. [Google Scholar]
- Hawkes, C.; Ruel, M.T.; Salm, L.; Sinclair, B.; Branca, F. Double-Duty Actions: Seizing Programme and Policy Opportunities to Address Malnutrition in All Its Forms. Lancet 2020, 395, 142–155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).